Abstract
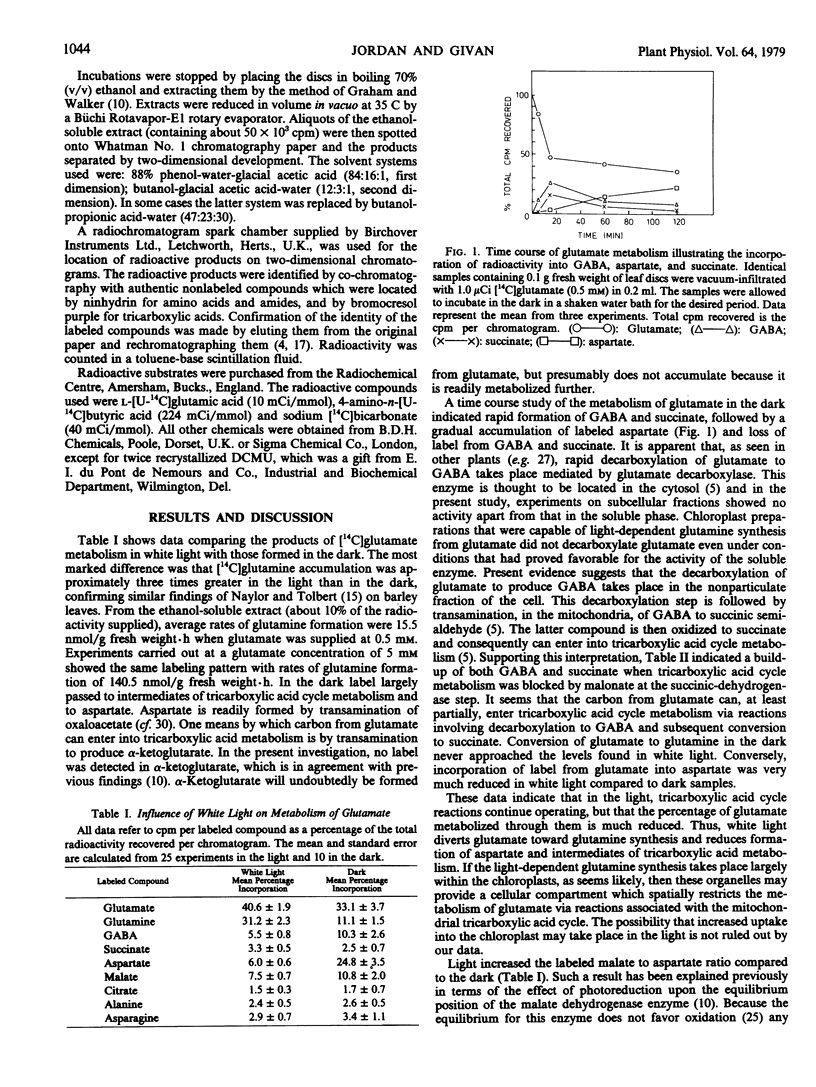
Metabolism of [14C]glutamate was studied in leaf discs of Vicia faba L. in light and in darkness. In white light glutamine was the main labeled product. In the dark label was principally in compounds closely associated with tricarboxylic acid cycle metabolism, predominantly aspartate. Entry of label from glutamate into tricarboxylic acid metabolism appeared to be at least partially by decarboxylation of glutamate to γ-amino butyric acid, followed by conversion to succinate. 3-(3,4-dichlorophenyl)-1, 1-Dimethylurea inhibited light-enhanced synthesis of glutamine and caused reversion toward the dark pattern of metabolism. Methionine sulfoximine severely inhibited glutamine synthesis and caused accumulation of labeled malate.
Monochromatic 650 nanometer light gave similar results to white light. Monochromatic light of 710 nanometers had a much smaller effect on glutamine synthesis but did significantly raise the ratio of labeled malate to aspartate. γ-Amino [14C]butyric acid was metabolized entirely via tricarboxylic acid cycle metabolism in light or dark, and in the light the ratio of labeled malate to aspartate was raised.
These results suggest that illuminated leaves metabolize glutamate to glutamine mainly in the chloroplasts. When chloroplastic glutamine synthesis fails to take place, either in darkness or in the presence of inhibitors, glutamate is apparently metabolized outside the chloroplast. Light lowers the NAD+ to NADH ratio outside the chloroplast, consequently altering the equilibrium of the malate dehydrogenase reaction. Alteration of the malate to aspartate ratio by 710 nanometer light suggests that ATP generated by photosystem I-dependent cyclic photophosphorylation may affect extrachloroplastic NAD+ to NADH ratios.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Chain R. K. Regulation of ferredoxin-catalyzed photosynthetic phosphorylations. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4961–4965. doi: 10.1073/pnas.72.12.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan C. V. Glutamine Synthesis and Its Relation to Photophosphorylation in Pisum Chloroplasts: Effects of 3-(3,4-Dichlorophenyl)-1,1-dimethylurea and Antimycin A. Plant Physiol. 1976 Apr;57(4):623–627. doi: 10.1104/pp.57.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretovich V. L., Kariakina T. I., Liubimova N. V., Neronova A. N. Iantarnyi polual'degid--predshestvennik gliutamina v rastenii. Dokl Akad Nauk SSSR. 1966 Oct 11;170(5):1212–1215. [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Localisation of glutamine synthetase in chloroplasts. Nat New Biol. 1973 Nov 14;246(150):61–62. doi: 10.1038/newbio246061a0. [DOI] [PubMed] [Google Scholar]
- STERN J. R., OCHOA S., LYNEN F. Enzymatic synthesis of citric acid. V. Reaction of acetyl coenzyme A. J Biol Chem. 1952 Sep;198(1):313–321. [PubMed] [Google Scholar]
- Santarius K. A., Stocking C. R. Intracellular localization of enzymes in leaves and chloroplast membrane permeability to compounds involved in amino acid syntheses. Z Naturforsch B. 1969 Sep;24(9):1170–1179. doi: 10.1515/znb-1969-0915. [DOI] [PubMed] [Google Scholar]
- Skokut T. A., Wolk C. P., Thomas J., Meeks J. C., Shaffer P. W. Initial organic products of assimilation of [N]ammonium and [N]nitrate by tobacco cells cultured on different sources of nitrogen. Plant Physiol. 1978 Aug;62(2):299–304. doi: 10.1104/pp.62.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic Z. S., Walker D. A. Photosynthesis by isolated pea chloroplasts: some effects of adenylates and inorganic pyrophosphate. Plant Physiol. 1977 Mar;59(3):428–432. doi: 10.1104/pp.59.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G., Thompson J. F. Anaerobic Accumulation of gamma-Aminobutyric Acid and Alanine in Radish Leaves (Raphanus sativus, L.). Plant Physiol. 1972 Apr;49(4):572–578. doi: 10.1104/pp.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]


