Abstract
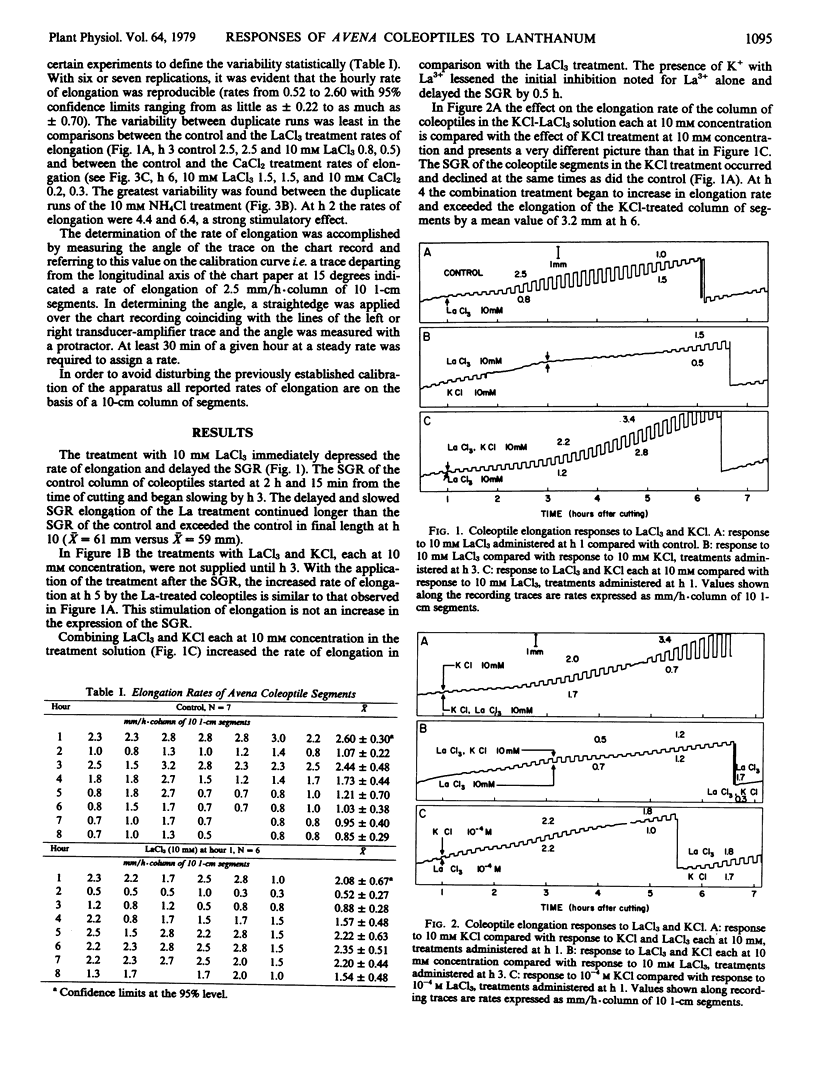
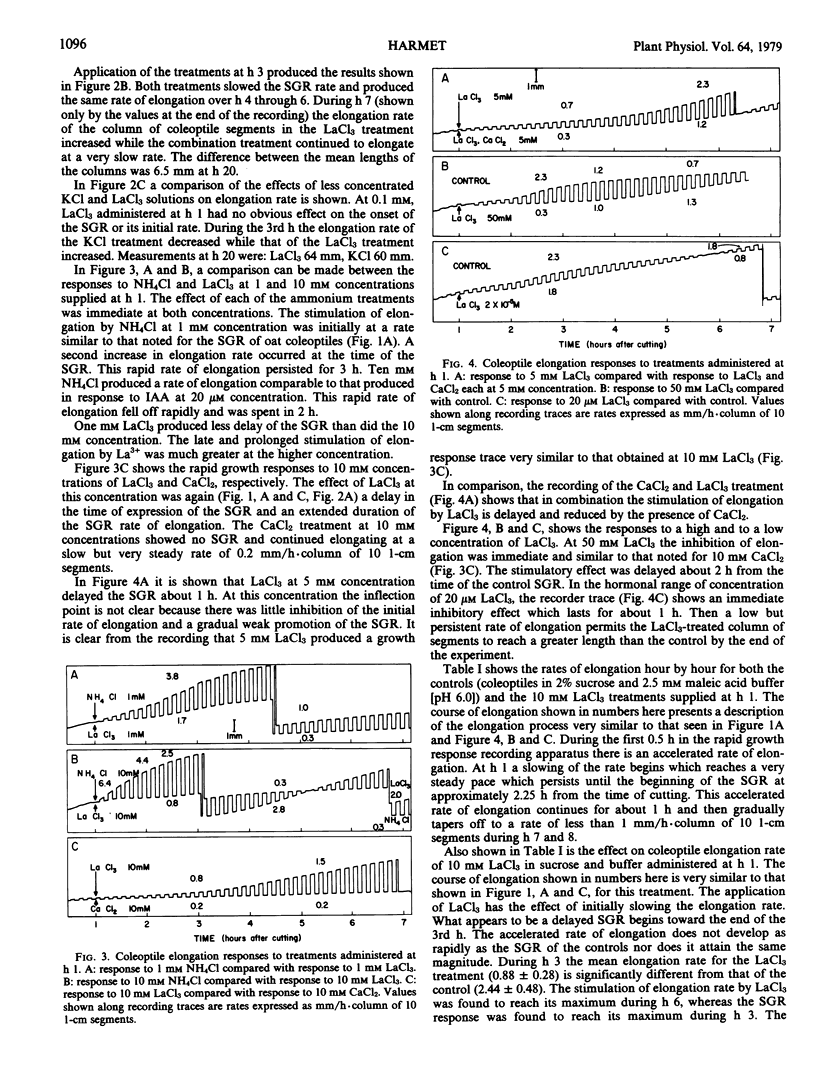
The rapid growth responses of oat (var. Victory) coleoptile segments treated with millimolar concentrations of the chlorides of La3+, Ca2+, K+, and NH4+, respectively, have been measured. La3+ and Ca2+ initially depressed the endogenous elongation rate. In the case of La3+ a prolonged stimulatory effect on the rate of elongation was produced by concentrations of 50 millimolar down to 20 micromolar after an initial depression of elongation rate. The effect of K+ was slightly stimulatory and showed a synergistic effect in combination with La3+. NH4+ produced an immediate rapid increase in elongation rate. La3+ did not behave as a “super calcium” in its action upon the spontaneous growth response. The prolonged elongation of the La3+-treated segments exhibiting the spontaneous growth response is apparently a newly observed effect. These rapid growth responses are interpreted as an interaction between anionic lipid-protein complexes in the plasmalemma and the respective ions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell N. A., Thomson W. W. Effects of lanthanum and ethylenediaminetetraacetate on leaf movements of mimosa. Plant Physiol. 1977 Oct;60(4):635–639. doi: 10.1104/pp.60.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Properties of a Solubilized Microsomal Auxin-binding Protein from Coleoptiles and Primary Leaves of Zea mays. Plant Physiol. 1978 Jul;62(1):152–157. doi: 10.1104/pp.62.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Fuente R. K., Leopold A. C. Time course of auxin stimulations of growth. Plant Physiol. 1970 Aug;46(2):186–189. doi: 10.1104/pp.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Schmitt M. R. The nature of spontaneous changes in growth rate in isolated coleoptile segments. Plant Physiol. 1975 Apr;55(4):757–762. doi: 10.1104/pp.55.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast P. W., Hubbard N. J. Abundance of alkali metals, alkaline and rare earths, and strontium-87/ strontium-86 ratios in lunar samples. Science. 1970 Jan 30;167(3918):485–487. doi: 10.1126/science.167.3918.485. [DOI] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Nagahashi G., Thomson W. W. Effect of lanthanum on ion absorption in corn roots. Plant Physiol. 1975 Mar;55(3):542–546. doi: 10.1104/pp.55.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Bracker C. E. Ultrastructural alteration of plant plasma membranes induced by auxin and calcium ions. Plant Physiol. 1976 Oct;58(4):544–547. doi: 10.1104/pp.58.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic salts on tissue permeability. Plant Physiol. 1976 Aug;58(2):182–185. doi: 10.1104/pp.58.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic solutes on the binding of auxin. Plant Physiol. 1976 Dec;58(6):783–785. doi: 10.1104/pp.58.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson W. W., Platt K. A., Campbell N. The use of lanthanum to delineate the apoplastic continuum in plants. Cytobios. 1973 Sep-Oct;8(29):57–62. [PubMed] [Google Scholar]
- VONHIPPEL P. H., WONG K. Y. NEUTRAL SALTS: THE GENERALITY OF THEIR EFFECTS ON THE STABILITY OF MACROMOLECULAR CONFORMATIONS. Science. 1964 Aug 7;145(3632):577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]
- Zegers P. V., Harmet K. H., Hanzely L. Inhibition of IAA-induced elongation in Avena coleoptile segments by lead: a physiological and an electron microscopic study. Cytobios. 1976;15(57):23–35. [PubMed] [Google Scholar]


