Letter to the Editor
Conditions involving severe immune dysregulation are strong risk factors for non-Hodgkin lymphoma (NHL).1 However, it is unclear whether subclinical immunologic perturbations influence NHL risk. We and other investigators have recently reported NHL associations with elevated pre-diagnostic serum concentrations of the immune markers soluble CD23 (sCD23), sCD27, sCD30, B cell attracting chemokine-1 (BCA1), soluble tumor necrosis factor receptor-2 (sTNFR2), and soluble vascular endothelial growth factor receptor-2 (sVEGFR2).2–7 While these findings support a role for immunologic effects in lymphomagenesis, the relatively short duration of follow-up after phlebotomy in these studies (10–13 years) limits the ability to rule out reverse causation as an explanation, given the potential for developing lymphomas to express these markers or indirectly affect their expression. Investigations within cohorts with longer follow-up time are needed to clarify the temporal nature of these associations. To that end, we conducted a nested case-control study investigating serum levels of these analytes and NHL diagnosed up to 23 years after phlebotomy within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study, a cohort with banked fasting serum from 29 133 male Finnish smokers.
The ATBC Study was approved by institutional review boards of the National Cancer Institute and the National Public Health Institute of Finland; written informed consent was obtained from all participants.8 We identified 272 first-primary NHL cases diagnosed 2+ years after phlebotomy through 2009 via the Finnish Cancer Registry. Fifty-three multiple myeloma cases were also selected but excluded from this report. We individually matched controls (N=325) to cases by baseline age (+/− 2 years), phlebotomy date (+/− 30 days), and number of previous specimen thaws from among participants alive and cancer-free at case diagnosis. Serum concentrations of the analytes were measured by ELISA (sCD23, sCD27, sCD30; Bender Medsystems, Burlingame, CA, USA) and Luminex-based assay (BCA1, sTNFR2, sVEGFR2; EMD Millipore, Billerica, MA, USA). For sCD23, a 1:2 dilution was performed for batches 3 onward to account for low serum volumes for many of the test samples; statistical analyses of this analyte were restricted to these batches. Sera from case-control pairs were assayed consecutively within batches along with blinded quality-control pool replicates (n = 32). Measurements were highly reproducible, with coefficients of variation for log-transformed concentrations <5%.
Odds ratios (ORs) and 95% confidence intervals (CIs) for analyte concentrations (grouped into three categories using control tertiles as cutpoints unless otherwise specified; see Table 1) were computed using conditional logistic regression adjusting for cigarette pack-years. Trend tests were conducted by modeling intra-category medians as a continuous parameter. Analyses were conducted both overall and stratified across three follow-up periods: 2-<8 years (85 cases, median follow-up 5.4 years), 8-<15 years (101, 11.3), and 15-<24 years (86, 19.8). Polytomous regression models were used to compute associations with the two most common histologic subtypes, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL; N=74) and diffuse large B-cell lymphoma (DLBCL; N=60), adjusting for age, year of phlebotomy, number of prior controlled thaws, and cigarette pack-years. Analyses of other NHL subtypes were limited by sparse numbers and are not reported. Analyses stratified by pack-years were conducted using unconditional logistic regression modeling with adjustment for age, year of phlebotomy, and number of prior controlled thaws. The statistical analysis was performed using SAS version 9.2. All statistical tests were two-sided.
Table 1.
Associations between serum sCD23, sCD27, sCD30, and sTNFR2 and future NHL risk (overall and by follow-up period) in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study
| Analyte, Units | Categorya | Stratified by Follow-up Time From Phlebotomy To Case Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| All Cases and Controls | 2–<8 Years | 8–<15 Years | 15–23 Years | ||||||
|
|
|
|
|
||||||
| (272 Cases, 325 Controls) | (85 Cases, 105 Controls) | (101 Cases, 118 Controls) | (86 Cases, 102 Controls) | ||||||
| NCab/NCont | ORc (95% CI) | NCa/NCont | OR (95% CI) | NCa/NCont | OR (95% CI) | NCa/NCont | OR (95% CI) | ||
| sCD23d, U/ml | ≤ 3.125 | 87/139 | 1.0 | 24/35 | 1.0 | 35/48 | 1.0 | 28/56 | 1.0 |
| 3.126 – 53.561 | 65/68 | 1.5 (0.9–2.3) | 20/21 | 1.8 (0.8–4.3) | 26/26 | 1.3 (0.6–2.7) | 19/21 | 1.7 (0.7–4.0) | |
| > 53.561 | 84/69 | 2.4 (1.5–3.8) | 28/27 | 2.1 (0.9–5.1) | 28/27 | 1.5 (0.7–3.2) | 28/15 | 5.1 (1.8–14.6) | |
| Ptrend = 0.0005 | Ptrend = 0.12 | Ptrend = 0.30 | Ptrend = 0.002 | ||||||
| sCD27, U/ml | ≤ 44 | 56/111 | 1.0 | 10/31 | 1.0 | 24/44 | 1.0 | 22/36 | 1.0 |
| 45 – 61 | 94/112 | 2.0 (1.2–3.1) | 23/37 | 3.9 (1.2–12.4) | 27/33 | 1.3 (0.6–2.9) | 44/42 | 2.0 (0.9–4.1) | |
| > 61 | 121/102 | 3.0 (1.8–4.9) | 52/37 | 8.4 (2.6–27.8) | 50/41 | 2.7 (1.3–5.7) | 19/24 | 1.4 (0.6–3.5) | |
| Ptrend < 0.0001 | Ptrend = 0.0005 | Ptrend = 0.007 | Ptrend = 0.46 | ||||||
| sCD30, pg/ml | ≤ 19 295 | 52/107 | 1.0 | 13/29 | 1.0 | 20/36 | 1.0 | 19/42 | 1.0 |
| 19 296 – 29 775 | 105/111 | 2.5 (1.5–4.2) | 31/33 | 3.5 (1.3–9.5) | 38/43 | 2.1 (0.9–4.7) | 36/35 | 2.5 (1.1–5.7) | |
| > 29 775 | 114/106 | 3.3 (1.9–5.5) | 41/43 | 4.1 (1.4–11.8) | 43/38 | 2.6 (1.1–6.1) | 30/25 | 3.7 (1.5–9.3) | |
| Ptrend < 0.0001 | Ptrend = 0.03 | Ptrend = 0.04 | Ptrend = 0.01 | ||||||
| sTNFR2, pg/ml | ≤ 5 634.40 | 67/108 | 1.0 | 11/28 | 1.0 | 24/36 | 1.0 | 32/44 | 1.0 |
| 5 634.41 – 7 841.82 | 93/110 | 1.6 (1.0–2.6) | 31/32 | 3.2 (1.2–8.4) | 36/42 | 1.3 (0.6–2.8) | 26/36 | 1.1 (0.4–2.5) | |
| > 7 841.82 | 111/107 | 2.0 (1.2–3.3) | 43/45 | 3.6 (1.2–10.4) | 41/40 | 1.8 (0.8–4.1) | 27/22 | 1.5 (0.6–3.4) | |
| Ptrend = 0.008 | Ptrend = 0.04 | Ptrend = 0.13 | Ptrend = 0.33 | ||||||
NHL indicates non-Hodgkin lymphoma; NCa, number of cases; NCont, number of controls; OR, odds ratio; CI, confidence interval.
sCD23 categorized as ≤ assay limit of detection, ≤ the median among controls, and > control median. Other analytes categorized using tertiles among controls as cutpoints.
Case frequencies do not include one case without assay data for the four analytes shown in this table.
ORs computed by conditional logistic regression with adjustment for cigarette pack-years.
sCD23 analyses restricted to samples in batches 3 onward.
Cases had higher cigarette pack-years than controls (37.0 vs. 33.5 respectively; P=0.02), but were otherwise comparable for age, body mass index, and education level (Supplemental Table 1). sCD27, sCD30, BCA1, sTNFR2, and sVEGFR2 were moderately correlated with one another among controls, with Spearman rank coefficients ranging from 0.21 (BCA1-sVEGFR2) to 0.70 (sTNFR2-sVEGFR2), whereas sCD23 levels were not correlated with other analytes (r = −0.14 to 0.09).
As summarized in Table 1, cases had higher serum levels than controls of sCD23 (high category vs. low: OR = 2.4, 95% CI = 1.5–3.8, Ptrend = 0.0005), sCD27 (3.0, 1.8–4.9, Ptrend < 0.0001), sCD30 (3.3, 1.9–5.5, Ptrend < 0.0001), and sTNFR2 (2.0, 1.2–3.3, Ptrend = 0.008). When these analytes were modeled simultaneously, associations remained for sCD23 (OR = 1.8, Ptrend = 0.03), sCD27 (2.1, 0.006), and sCD30 (2.2, 0.02). Associations were apparent for cases diagnosed 15–23 years post-phlebotomy for sCD23 (5.1, 1.8–14.6, Ptrend = 0.002) and sCD30 (3.7, 1.5–9.3, Ptrend = 0.01), whereas associations with sCD27 and sTNFR2 were strongest for cases diagnosed 2-<8 years post-phlebotomy, and weaker or null in later follow-up periods. The NHL associations with sCD23 and sCD30 did not significantly differ across categories of cigarette pack-years (Supplemental Table 2). We observed little evidence of association with BCA1 or sVEGFR2 (Supplemental Table 3).
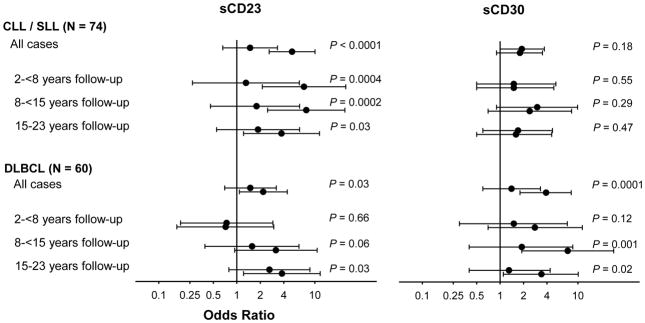
sCD23 and sCD30 were associated with DLBCL, both overall (sCD23: OR = 2.2, 95% = 1.1–4.4, Ptrend = 0.03; sCD30: 3.9, 1.8–8.2, Ptrend = 0.0001) and in later follow-up periods (Figure 1; results for all analytes in Supplemental Table 4). sCD23 was strongly associated with CLL/SLL (5.1, 2.6–10.1, Ptrend = 0.0001), particularly for cases diagnosed within 2–<8 years (7.3, 2.1–24.9, Ptrend = 0.0004) and 8–<15 years (7.8, 2.5–24.3, Ptrend = 0.0002) post-phlebotomy. sCD30 was not significantly associated with CLL/SLL.
Figure 1. Associations between serum sCD23 and sCD30 and future risk of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and diffuse large B cell lymphoma (DLBCL), overall and by period of follow-up.
Odds ratios (represented by circles) and corresponding 95% confidence limits (represented by vertical bars) are shown from comparisons of medium (middle circle) and high (bottom circle) vs. low (reference; top circle) analyte concentrations. The analyte category cutpoints are provided in Table 1. Odds ratios computed through polytomous regression with adjustment for age, year of phlebotomy, number of prior controlled thaws, and cigarette pack-years. P-values are from test of trend.
Our findings are consistent with the hypothesis that circulating sCD23 and sCD30, associated with NHL risk as many as 15–23 years after phlebotomy, are biomarkers reflective of immunologic mechanisms contributing to lymphomagenesis. sCD23 and sCD30, the soluble forms of the surface receptors CD23 and CD30, have been proposed to be informative surrogate markers of B cell activation.9 Both CD23, a low-affinity IgE receptor, and sCD23 have been shown to induce B cell stimulatory effects.10,11 CD30 is preferentially expressed by activated type-2 T cells, which produce cytokines enhancing B cell activation.12,13 Sustained B cell activation may contribute to NHL development by increasing the potential for unrepaired lymphomagenic genetic errors.
We cannot rule out the possibility of occult disease influencing some of our findings, particularly for indolent malignancies such as CLL. Indeed, we observed strong sCD23 associations for CLL/SLL diagnosed closer in time to phlebotomy which may be at least partly disease-induced, as circulating sCD23 in CLL patients has been associated with tumor burden.14 However, our sCD23 findings for the more aggressive DLBCL are consistent with etiologic effects, given the consistency in associations across follow-up periods and especially the strong risk present even among cases diagnosed 15–23 years after phlebotomy. The weaker NHL associations for sCD27 and sTNFR2 ≥8 years post-phlebotomy may reflect disease-induced effects, odds ratio attenuation due to a decline in representativeness of one-time analyte measurements as surrogates for usual levels, or both.
The generalizability of these findings is questionable, given that the study population consisted entirely of male Finnish heavy smokers. However, similar NHL and subtype associations with sCD23 and sCD30 have previously been reported (although with fewer years of follow-up) in population-based cohorts that include women (including one all-female cohort) and non-smokers.3,4,6
Our findings raise the possibility that serum sCD23 and sCD30 may hold promise as surrogate endpoints for cross-sectional studies of suspected B-cell-stimulative risk factors. It is also possible, though speculative, that these markers may have future clinical value in developing NHL risk prediction models. In particular, given the strong association with CLL/SLL risk observed for elevated sCD23 in serum collected ≤15 years before diagnosis, there is a need to investigate whether elevated levels of this marker are predictive of future progression to CLL among patients with monoclonal B cell lymphocytosis (MBL), an asymptomatic precursor condition to this malignancy.15 Studies addressing these questions, particularly using serially collected specimens, are warranted.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORSHIP CONTRIBUTIONS
M.P.P. led the study design and statistical analysis, and prepared the manuscript; Q.L., S.J.W., J.V., D.A., and N.R. contributed to the study design; T.J.K. conducted the assays; L.A.P. supervised this laboratory work; A.H. oversaw the data formatting and quality control checks of the assay data; J.N.H. contributed to the statistical analysis; all authors provided intellectual input into preparation of the manuscript.
References
- 1.Hartge P, Wang SS, Bracci PM, Devesa SS, Holly EA. Non-Hodgkin Lymphoma. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 3. New York: Oxford University Press, New York, NY, USA; 2006. pp. 898–918. [Google Scholar]
- 2.Gu Y, Shore RE, Arslan AA, Koenig KL, Liu M, Ibrahim S, et al. Circulating cytokines and risk of B-cell non-Hodgkin lymphoma: a prospective study. Cancer Causes Control. 2010;21:1323–1333. doi: 10.1007/s10552-010-9560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purdue MP, Lan Q, Martinez-Maza O, Oken MM, Hocking W, Huang WY, et al. A prospective study of serum soluble CD30 concentration and risk of non-Hodgkin lymphoma. Blood. 2009;114:2730–2732. doi: 10.1182/blood-2009-04-217521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeulen R, Hosnijeh FS, Portengen L, Krogh V, Palli D, Panico S, et al. Circulating soluble CD30 and future risk of lymphoma; evidence from two prospective studies in the general population. Cancer Epidemiol Biomarkers Prev. 2011;20:1925–1927. doi: 10.1158/1055-9965.EPI-11-0396. [DOI] [PubMed] [Google Scholar]
- 5.Purdue MP, Lan Q, Bagni R, Hocking WG, Baris D, Reding DJ, et al. Prediagnostic serum levels of cytokines and other immune markers and risk of non-hodgkin lymphoma. Cancer Res. 2011;71:4898–4907. doi: 10.1158/0008-5472.CAN-11-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Roos AJ, Mirick DK, Edlefsen KL, LaCroix AZ, Kopecky KJ, Madeleine MM, et al. Markers of B-Cell Activation in Relation to Risk of Non-Hodgkin Lymphoma. Cancer Res. 2012;72:4733–4743. doi: 10.1158/0008-5472.CAN-12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purdue MP, Hofmann JN, Kemp TJ, Chaturvedi AK, Lan Q, Park JH, et al. A prospective study of 67 serum immune and inflammation markers and risk of non-Hodgkin lymphoma. Blood. 2013;122:951–957. doi: 10.1182/blood-2013-01-481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ATBC Cancer Prevention Study. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 9.Vendrame E, Martinez-Maza O. Assessment of pre-diagnosis biomarkers of immune activation and inflammation: insights on the etiology of lymphoma. J Proteome Res. 2011;10:113–119. doi: 10.1021/pr100729z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon J. CD23 and B cell activation. Clin Exp Allergy. 1992;22:199–204. doi: 10.1111/j.1365-2222.1992.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 11.Fournier S, Rubio M, Delespesse G, Sarfati M. Role for low-affinity receptor for IgE (CD23) in normal and leukemic B-cell proliferation. Blood. 1994;84:1881–1886. [PubMed] [Google Scholar]
- 12.Del PG, De CM, Almerigogna F, Daniel CK, D’Elios MM, Zancuoghi G, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 13.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 14.Reinisch W, Willheim M, Hilgarth M, Gasché C, Mader R, Szepfalusi S, et al. Soluble CD23 reliably reflects disease activity in B-cell chronic lymphocytic leukemia. J Clin Oncol. 1994;12:2146–2152. doi: 10.1200/JCO.1994.12.10.2146. [DOI] [PubMed] [Google Scholar]
- 15.Mowery YM, Lanasa MC. Clinical aspects of monoclonal B-cell lymphocytosis. Cancer Control. 2012;19:8–17. doi: 10.1177/107327481201900102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.