Abstract
The Zika virus (ZIKV) is a newly emerging pathogen that has resulted in a worldwide epidemic. It primarily spreads either through infected Aedes aegypti or Aedes albopictus mosquitos leading to severe neurological disorders such as microcephaly and Guillain-Barré syndrome in susceptible individuals. The mode of ZIKV entry into specific cell types such as: epidermal keratinocytes, fibroblasts, immature dendritic cells (iDCs), and stem-cell-derived human neural progenitors has been determined through its major surface envelope glycoprotein. It has been known that oligosaccharides that are covalently linked to viral envelope proteins are crucial in defining host-virus interactions. However, the role of sugars/glycans in exploiting host-immune mechanisms and aiding receptor-mediated virus entry is not well defined. Therefore, this review focuses on host-pathogen-interactions to better understand ZIKV pathogenesis.
Keywords: Host-virus interactions, Zika virus, virus glycosylation, immunity, neuropathogenesis
Introduction
The Zika virus (ZIKV) is a major emerging pathogen (Jamil et al., 2016) that has recently caused sporadic but large epidemics world-wide. This includes countries in South and Southeast Asia, Oceania, the Americas, and Africa. ZIKV belongs to a member of the genus Flavivirus in the Flaviviridae family. These are single-stranded, positive sense RNA viruses typically transmitted by arthropod vectors (Malone et al., 2016). This genus also includes dengue virus (DENV) 1–4, West Nile virus (WNV) 1 and 2, Yellow Fever virus (YFV), and Japanese encephalitis virus (JEV) I–V (Lindenbach and Rice, 2003). The flaviviruses infect a wide range of hosts such as rodents, pigs, birds, non-human primates, and humans (Daep et al., 2014).
ZIKV was first isolated from a rhesus monkey in Zika forest, Uganda in 1947 (Dick et al., 1952). Thereafter it was isolated from the mosquito (Aedes africanus) in 1948 (Dick et al, 1952), and humans in 1952 (Macnamara, 1954). ZIKV is mainly spread through Aedes aegypti and Aedes albopictus mosquitoes which comprise two major lineages: African and Asian (Ladner et al., 2016; Weaver et al., 2016). Due to the increase in ZIKV infections in recent years, in May 2015 the Pan American Health Organization issued an epidemiological alert about Zika virus infection. In February 2016, the World Health Organization stated “Zika virus is a public health emergency of international concern” due to extensive travel of infected humans across the world (Lupton, 2016) (Table 1).
Table 1.
Key events in Zika virus disease. Table representing crucial events that are taken place from the Zika virus (ZIKV) identification to the therapeutics development.
| Year | Incident | References |
|---|---|---|
| 1947 | Identified in the Zika forest of Uganda in a rhesus monkey. | (Dick, Kitchen et al. 1952) |
| 1948 | Isolated from infected Aedes africanus, in the Zika forest. | (Dick 1952) |
| 1952 | Serum analysis revealed presence of neutralizing antibodies in infected individuals in East Africa. | (Smithburn 1952) |
| 1964 | A researcher infected with Zika, presented mild illness. | (Macnamara 1954) |
| 2007 | Zika first large human infection outbreak in the Pacific Island of Yap. Three quarters of yap residents got infected with Zika virus during the outbreak. | (Duffy, Chen et al. 2009) |
| 2008 | First documented case of sexual transmission. | (Foy, Kobylinski et al. 2011) |
| 2012 | Phylogenetic analysis indicating the geographic expansion of African MR766 to Asian lineage EC Yap (Yap State, Micronesia, 2007). | (Haddow, Schuh et al. 2012) |
| 2014 | Outbreaks on Easter Island, French Polynesia (FP), the Cook Islands, and New Caledonia. | (Cao-Lormeau and Musso 2014; Musso 2015) |
| 1,505 asymptomatic blood samples were analyzed during FP outbreak and 42 donors have shown Zika virus infection. | (Musso, Nhan et al. 2014; Musso, Roche et al. 2015) | |
| Zika virus can be transmitted sexually and through blood transfusions. | ||
| 2015 | Many places including Brazil, Cabo Verde, Colombia, Suriname, EI Salvador, French Polynesia, Mexico, Paraguay and Panama reported the Zika viral infections. | (Kindhauser, Allen et al. 2016) |
| Brazil’s National Reference Laboratory confirmed that Zika virus is circulating in the country and is correlating with the increase in neurological disorders (Guillain-Barre syndrome and microcephaly). | ||
| 2016 | Brazil reports 3,893 suspected microcephaly cases, and 1,708 cases of Guillain-Barre syndrome cases, including 49 deaths. | (Kindhauser, Allen et al. 2016) |
| The Vector Control Advisory Group recommend two new tools to control the Zika virus transmitting Aedes mosquitoes. | (WHO 2016) | |
| Revised Zika Strategic Response Plan launched by WHO/PAHO and partners, with major importance given to preventing Zika disease and managing Zika associated medical complications. | (WHO 2016) | |
| WHO generates a Target Product Profile for Zika vaccines to use in emergency conditions against congenital Zika syndrome and more than 55 diagnostic kits have been developed. | (Hombach, Friede et al. 2016) |
Zika virus genome organization and polyprotein processing
ZIKV contains a single-stranded positive sense RNA genome that is approximately 10 kb in size with a single open reading frame (ORF). The ORF encodes a large polyprotein that is processed to produce the structural proteins (capsid [C], precursor membrane [prM], envelope [E]), and non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) that assist in virus replication and in pathogenesis (Fig. 1). The non-structural proteins are important for entry, translation, replication, morphogenesis, and pathogenesis (Lindenbach and Rice, 2003; Olagnier et al., 2016; Sirohi et al., 2016). The ORF is flanked by highly conserved secondary structures at the 5′ and 3′ untranslated regions that help in translation and replication of the genome (Zhu et al., 2016). ZIKV replicates in the virus-induced membranous vesicles that are derived from the endoplasmic reticulum (ER) and Golgi complex by exploiting membrane trafficking (Miller and Krijnse-Locker, 2008). After release of the genome into the cytoplasm, ZIKV replicates through a negative strand intermediate (Lindenbach and Rice, 2003). Due to its limited coding capacity, ZIKV largely depends on host machinery for virus replication, translation, and viral morphogenesis (Gale et al., 2000).
Figure 1.
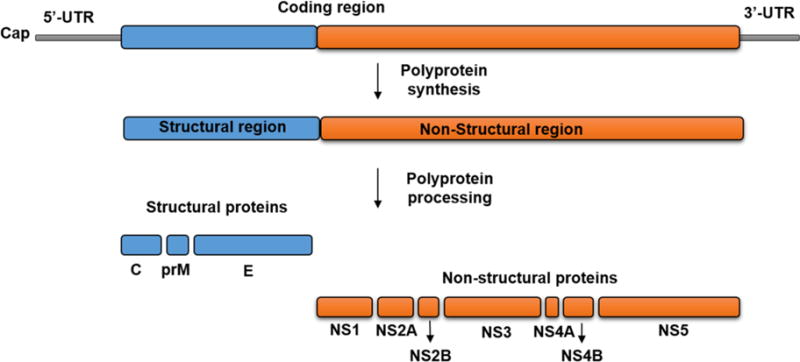
Schematic representation of Flaviviruses genome organization, and polyprotein processing. The genome of flaviviruses contains single open reading frame, flanked by 5′-and 3′-UTRs. The ORF translated into a polyprotein is cleaved into three structural proteins -C, prM and E (blue color) and seven non-structural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (orange color).
The non-structural protein NS1 is a 46 KDa glycoprotein containing 2–3 glycosylation sites and 12 conserved cysteine residues that can form disulphide bonds (Muller and Young, 2013; Edeling et al., 2014). Mutations in glycosylation sites Asn130 and Asn207 drastically affect virus replication and virus production (Pryor et al., 1998; Crabtree et al., 2005). Although the NS1 protein has no hydrophobic transmembrane domain, it associates with the membrane through a glycosylphosphatidylinositol (GPI) anchor (Jacobs et al., 2000). It is located inside the cell and is also found in foci on the cell surface. Upon proteolytic separation from the envelope protein, it is secreted out of the cell (Alcon-LePoder et al., 2005). Cell surface expression of NS1 could elicit a strong humoral immune response, which further aids in antibody-mediated killing of virus infected cells (Crook et al., 2014). The NS1 and NS4 non-structural proteins interact with each other and co-localize in the viral replicase complex to help negative strand synthesis during the viral genome replication (Lindenbach and Rice, 1999).
The NS2A is a 22 kDa hydrophobic protein and plays an important role in infectious viral particle production (Xie et al., 2013). Cleavage of NS2A upstream of the C-terminus by viral serine protease produces a smaller 20 kDa protein, which is essential in viral replication (Leung et al., 2008). The NS2A non-structural protein interacts with the NS3, NS5, and Kunjin virus (KUN) 3′ Non-coding-region and co-localizes with the replication complex (Liu et al., 2002). These interactions are essential in managing the shift between RNA packaging and RNA replication in virus particle production (Mackenzie et al., 1998; Yu et al., 2013). The NS2B is a 14 kDa membrane-associated protein that mediates membrane interaction by its conserved central hydrophobic region (Bollati et al., 2009). These interactions increase the host cell membrane permeability (Chang et al., 1999). Furthermore, NS2B interacts with and acts like a cofactor for NS3 trypsin-like serine protease function (Luo et al., 2008).
NS3 is a multifunctional 70 kDa protein that has multiple enzymatic activities, such as trypsin-like serine protease activity, (Chambers et al., 1990; Brinkworth et al., 1999; Droll et al., 2000; Tautz et al., 2000; Yusof et al., 2000; Luo et al., 2008), Mg2+ inhibited and poly(A)-stimulated NTPase activity (Feito et al., 2008). In addition, it also has Mg2+ stimulated, poly(A)-inhibited RNA triphosphatase (RTPase) activity and ATP-driven RNA duplex unwinding activity at its C-terminus. Therefore, mutations in the putative NTP-binding site (Lys199) destroy the NTPase and RTPase activities (Gebhard et al., 2012). The NS3 localizes to the membranes by interacting with the membrane-localizing transmembrane viral protein NS2B and helps in viral polyprotein processing and viral replication (Falgout et al., 1991; Markoff et al., 1997; Yu et al., 2013). The trypsin-like serine protease activity has been mapped to His53, Asp77, and Ser138 (catalytic triad) (Chambers et al., 1990; Gupta et al., 2015).
NS4A is a 16 kDa multifunctional, hydrophobic protein. It regulates membrane proliferation, localizes the replication complex to the membrane, aids in polyprotein processing, and is crucial for immune evasion (Mackenzie et al., 1998; Miller et al., 2007). The NS4A C-terminus interacts and translocates NS4B to the ER lumen (Miller et al., 2007). The NS4B is a 27 KDa transmembrane protein present at the replication site with double-stranded RNA, an intermediate of viral replication and NS3 protein (Miller et al., 2007).
NS5 is a highly conserved 103 kDa protein and plays a crucial role in viral replication (Lu and Gong, 2013; Li et al., 2014; Klema et al., 2016). It has N-terminal RNA cap-processing activity (homology with the S-adenosyl-methionine (SAM)-dependent methyltransferases) (Klema et al., 2016), GTP-binding activity (Benarroch et al., 2004), and C-terminal RdRP activity (Malet et al., 2007; Zhang et al., 2008). The interaction of NS5 protein stimulates NS3 NTPase activity (Feito et al., 2008) and creates an importin binding site. As a result, this helps in nuclear localization (Vasudevan et al., 2001; Klema et al., 2016).
Zika virus cellular entry
Compared to other flaviviruses, little is known about the morphology, replication, and pathogenesis of ZIKV. Recently, the structure of the thermo-stable French Polynesian ZIKV strain H/PF/2013 was determined by cryoelectronmicroscopy. The overall structural architecture of ZIKV is similar to that of DENV and WNV, except for approximately 10 amino acids. These ammino acids surround the Asn154 glycosylation site in each of the 180 envelope glycoproteins that make up the icosahedral shell (Kostyuchenko et al., 2016; Sirohi et al., 2016). Sequence comparisons of the glycoprotein (E) of ZIKV with the other members of the flaviviruses family indicating unusual degree of variability within the ZIKV strains and between the family (Fig. 2 and Table 2).
Figure 2.

Multiple sequence alignment of glycoprotein E of Flaviviruses. The multiple sequence alignment of flaviviruses glycoprotein E sequences from Zika virus (MR766, PRVABC59, CDC259359, and FLR), WNV (lineage 1 and 2), and Dengue (Serotype 1, 2, 3 and 4) were analyzed. and The common residues between sequences were represented as dot and was analysed using bioEdit tool.
Table 2.
Percentage of sequence similarities between Zika virus (Asian and African lineages) and other flaviviruses glycoprotein E sequences were calculated using BioEdit tool.
| Strain | MR766 | PRVABC59 | CDC259359 | FLR | WNV1 | WNV2 | DENV1 | DENV2 | DENV3 | DENV4 |
|---|---|---|---|---|---|---|---|---|---|---|
| MR766 | 100 | |||||||||
| PRVABC59 | 96.6 | 100 | ||||||||
| CDC259359 | 96.8 | 99.8 | 100 | |||||||
| FLR | 96.8 | 99.8 | 100 | 100 | ||||||
| WNV1 | 53.7 | 54 | 53.8 | 53.8 | 100 | |||||
| WNV2 | 54.5 | 54.8 | 54.6 | 54.6 | 94 | 100 | ||||
| DENV1 | 56.5 | 56.5 | 56.5 | 56.5 | 49.1 | 49.8 | 100 | |||
| DENV2 | 53.3 | 52.5 | 52.7 | 52.7 | 46.3 | 47.4 | 68.4 | 100 | ||
| DENV3 | 56.6 | 56.7 | 56.9 | 56.9 | 45.3 | 45.6 | 77.9 | 67.6 | 100 | |
| DENV4 | 54.7 | 54.5 | 54.7 | 54.7 | 48.3 | 48.4 | 63.8 | 63.6 | 63.2 | 100 |
ZIKV is an enveloped, icosahedral virus approximately 40 nm in diameter. The capsid of ZIKV is made up of three structural proteins: the capsid protein, prM, and the envelope protein (Zhang et al., 2012; Kostyuchenko et al., 2016; Sirohi et al., 2016). The outer shell is composed of 180 copies of both the envelope (E) glycoprotein (53 kDa) and the membrane (M) protein (21 kDa), and is surrounded by a lipid envelope (Kostyuchenko et al., 2016). The capsid protein is attached to the viral RNA genome. The envelope protein is a surface protein that is involved in viral attachment, fusion, penetration, hemagglutination, host range, and cell tropism.
The envelope protein contains three domains (I, II, III) and two transmembrane helices. Domain I contains a β-barrel central structure. Domain II comprises the fusion loop, the segment directly involved in the fusion of viral and cellular membranes during virus entry. Domain III is located on the surface of the viron. It has an immunoglobulin-like β-barrel structure that helps in cellular-binding (Modis et al., 2005). The Zika virus envelope protein EDI, EDII, and EDIII domains share 35%, 51%, and 29% amino acid identity with the DENV ED I-III domains, respectively (Stettler et al., 2016). Maturation of the virion requires cleavage of the membrane protein by the trans-Golgi network and cellular protein (furin) to pr and M protein. This is followed by the release of the newly formed viral particles from the cell (Zhang et al., 2012; Sirohi et al., 2016)
The ZIKV envelope protein has one N-linked glycosylation site (N154). Whereas, the DENV envelope protein contains two glycosylation sites (N67 and N153) that are involved in the interaction with attachment factors on the cell surface (Hacker et al., 2009; Dai et al., 2016; Zhu et al., 2016) such as receptor tyrosine kinases like TYRO3 and AXL (Hamel et al., 2015; Dang et al., 2016; Hanners et al., 2016; Tang et al., 2016) (Fig. 3). In addition, other flaviviridae members (DENV and WNV) use dendritic cell-specific intercellular adhesion molecule 3-grabbing non integrin (DC-SIGN) (Tassaneetrithep et al., 2003; Wang et al., 2015), liver/lymph node-specific ICAM-3 grabbing non integrin (L-SIGN) (Perera-Lecoin et al., 2014; Wang et al., 2015), proteins of the transmembrane immunoglobulin and mucin domain 1 (TIM1) (Jemielity et al., 2013), and claudin-1 (Che et al., 2013) for cell entry (Fig. 3). However, it is unclear whether ZIKV uses such cell-specific adhesion molecules and whether they play critical roles in virus budding and transmission, thereby warranting further studies.
Figure 3.
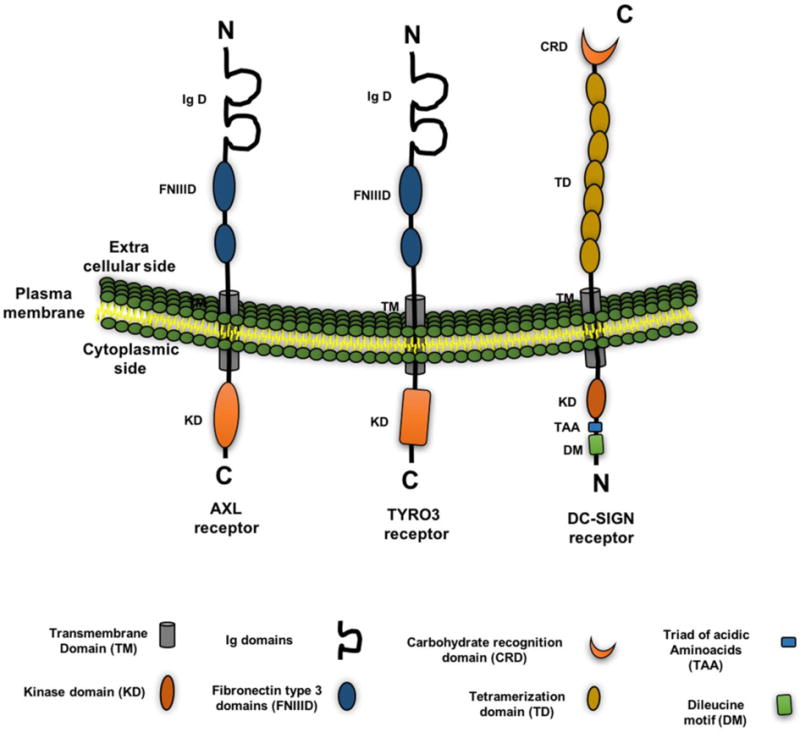
Flavivirus receptors and their domain organization. Figure represents various receptor(s) expressed on the cell such as TIM (T-cell immunoglobulin and mucin domain), TAM (TYRO3, and AXL), and C-type lectin (DC-SIGN) that are known to interact with the Zika virus and other flaviviruses and to facilitate their entry. The domain organization of each receptor(s) such as transmembrane domain (TM), kinase domain (KD), Ig domain, Fibronectin type 3 domains (FNIIID), carbohydrate recognition domain (CRD), triad of acidic aminoacids (TAA), tetramerization domain (TD), and dileucine motif (DM) are represented in the figure with color and a particular shape.
Role of glycosylation in Zika virus-host interactions
Recently, Zhu et al (2016) analysed potential O-glycosylation and N-glycosylation sites in 24 representative pre-epidemic and epidemic ZIKV using NetOGlyc 4.0 (http://www.cbs.dtu.dk/services/NetOGlyc/) and NetNGlyc 1.0(http://www.cbs.dtu.dk/services/NetNGlyc/) and found that the O-glycosylation and N-glycosylation sites are highly conserved (Zhu et al., 2016). Virus particles contain one strand of RNA genome, surrounded by a lipid bilayer and three distinct types of viral structural proteins (E, M/prM, and C). The glycoprotein E is responsible for targeting virus to host cell receptors, and it facilitates virus entry. As such, it is a major target for antibodies (Dai et al., 2016; Kostyuchenko et al., 2016; Sirohi et al., 2016)(Fig. 4). However, ZIKV is able to mediate antibody escape due to highly variable amino acids (approximately 10) that surround the Asn154 glycosylation site in its envelope protein (Kostyuchenko et al., 2016; Sirohi et al., 2016). Sequence analysis (Fig. 2) and glycosylation site prediction (Table 3) studies indicate that DENV has two (Asn67 and Asn153) glycosylation motifs; whereas, ZIKV has one (Asn154) glycosylation site (Zhu et al., 2016). The connection between glycosylation, structure, tropism, immune recognition/evasion mechanisms, and pathogenesis of ZIKV remains largely unknown and addressing these will aid in the rapid development of vaccines and therapeutics.
Figure 4.
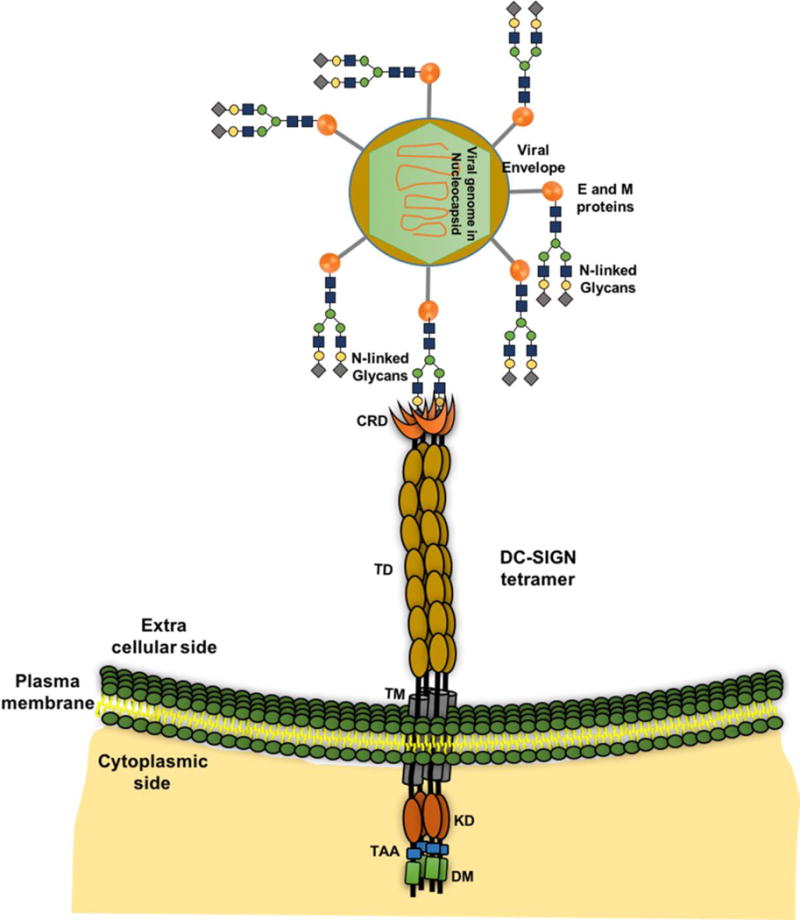
Interaction between glycans (glycoprotein E) and receptor (DC-SIGN). Representation of the host-virus interactions through DC-SIGN tetramer on cell surface and N-linked glycans on glycoprotein of flavivirus are shown.
Table 3.
Predicted potential glycosylation sites in the glycoprotein E of Flaviviruses. Table represents the potential glycosylation sites in the glycoprotein E sequences of -ZIKV strains (MR766, PRVABC59, CDC259359, and FLR), WNV (lineage 1 and 2), and Dengue (Serotype 1, 2, 3 and 4) and predicted using NetNGlyc tool. The default threshold of 0.5, represents a predicted glycosylated site in Asn-X-Ser/Thr, X is any amino acid except proline. The ‘potential’ score is the averaged output of nine neural networks. The jury agreement column indicates how many of the nine networks support the prediction. The N-Glyc Result column shows one of the following outputs for predictions indicating glycosylated sites: + Potential > 0.5; ++ Potential > 0.5 and Jury agreement (9/9).
| S.no | Strain | Position | Potential | Jury agreement | N-Glyc results |
|---|---|---|---|---|---|
| 1 | Zika MR766 | – | – | – | − |
| 2 | Zika PRVABC59 | 154 NDTG | 0.63 | (6/9) | + |
| 481 NGSI | 0.36 | (8/9) | − | ||
| 3 | Zika CDC259359 | 154 NDTG | 0.63 | (6/9) | + |
| 481 NGSI | 0.36 | (8/9) | |||
| 4 | Zika FLR | 154 NDTG | 0.63 | (6/9) | + |
| 481 NGSI | 0.36 | (8/9) | − | ||
| 5 | WNV1 | 154 NYST | 0.68 | (9/9) | ++ |
| 6 | WNV2 | – | – | – | − |
| 7 | DENV1 | 67 NTTT | 0.66 | (7/9) | + |
| 153 NEST | 0.54 | (6/9) | + | ||
| 8 | DENV2 | 67 NTTT | 0.65 | (9/9) | ++ |
| 153 NDTG | 0.55 | (6/9) | + | ||
| 9 | DENV3 | 67 NITT | 0.69 | (8/9) | + |
| 153 NETQ | 0.63 | (9/9) | ++ | ||
| 470 NTSM | 0.26 | (8/9) | −− | ||
| 10 | DENV4 | 67 NITT | 0.7 | (8/9) | + |
| 153 NDTS | 0.65 | (8/9) | + | ||
| 472 NTSM | 0.32 | (8/9) | − |
As described above, the first ZIKV was isolated in the Zika forest in 1947. Later strains with Asian and African lineages did not cause significant numbers of human infections until 2007 (Dick et al., 1952). The Yap Island epidemic in 2007 in Micronesia brought ZIKV into notice, along with the alarming increase from 2007 to 2016 in epidemics in New Caledonia, French Polynesia, Brazil, and South and Central America, and its association with the severe birth defects (microcephaly) and neurological diseases Guillain-Barré syndrome (GBS) (Haddow et al., 2012; Al-Qahtani et al., 2016; Nishiura et al., 2016). ZIKV attained many sequence and structural changes from 1947 to 2016, which increased virulence and became responsible for the many epidemics across the globe (Haddow et al., 2012; Zhu et al., 2016). These studies suggested that ZIKV pathogenicity has gradually evolved during inter-species transmission via the intra-cerebral route making a relatively non-pathogenic viral strain to a “pathogenic” strain. The ZIKV glycoprotein E is the major surface protein that makes initial contact with the cell-surface receptor to gain entry into the host cell. However, the glycoprotein E-linked glycan structure and composition have not yet been examined. Therefore, analysing the structure and composition of the glycans at each site across the family will aid in developing a signature that would further assists in elucidating emerging ZIKV pathogenesis and developing drug targets.
ZIKV infection is associated with rashes, headache, nausea, diarrhea and fever, including serious neuroimmunological diseases such as microcephaly in neonates and GBS in adults (Oehler et al., 2014; Araujo et al., 2016b; Cao-Lormeau et al., 2016; Watrin et al., 2016). The association between GBS is clearly revealed in French Polynesia in a case study between October 2013 and April 2014 (Kindhauser et al., 2016). The presence of Zika viral RNA and antigens in two congenitally infected new-borns’ placenta and brain tissues from Brazil strengthen the connection between Zika virus infections in infants (2015) (de Oliveira Poersch et al., 2005; Oehler et al., 2014; Sarno et al., 2016).
Several modes of ZIKV infection has been studied such as antibody-dependent enhancement of infection and receptor mediated entry (DC-SIGN, Tyro3, and AXL and TIM-1) to facilitate the virus entry to macrophages, monocytes, neuronal progenitor cells, and fetal cells (Hamel et al., 2015; Li et al., 2016b; Tang et al., 2016). However, the correlation between the increased number of ZIKV infections and microcephaly cases remains to be defined. Furthermore, the analysis of tissue samples for viral RNA and proteins during the different stages of gestation from expectant mothers with ZIKV infection indicate the virus can cross the placenta and cause chronic placentitis and microcalcifications (Noronha et al., 2016). This observation was further supported by viral RNA and protein detection in amniotic fluids, placental tissues, and fetal brain tissues (Martines et al., 2016; Mlakar et al., 2016). Recent studies have demonstrated that the receptors (Tyro3, Axl, and TIM1) expressed on primary human placental cells, endothelial cells, fibroblast cells, amniotic epithelial cells, trophoblast progenitors, and macrophages (Hofbauer cells) facilitate ZIKV (PRVABC59 and MR766) maternal-fetal transmission (Bayer et al., 2016; Noronha et al., 2016; Tabata et al., 2016). The ZIKV infects the primary human placental macrophages and placental cytotrophoblasts, thereby induces the production of type I interferon (IFN) IFN-α, pro-inflammatory cytokines IL-6, chemokine MCP-1, and antiviral genes such as RIG-I, MDA5, and LGP2 (Quicke et al., 2016). During infection, ZIKV stimulates cell death, induces type I interferon (IFN) response and pro-inflammatory cytokines that disrupt the placental barrier leading to neurological disorders such as microcephaly (Miner et al., 2016). However, another study shows that trophoblast-derived type III IFN encodes resistance to ZIKV infections in barrier cells of the placenta and primary human trophoblasts (PHTs). The IFNλ1 protects placental cells and non-placental cells from ZIKV infection (Bayer et al., 2016). The ZIKVBR infected mice, human cortical progenitor cells, and brain organoids presented microcephaly related symptoms, like cell death, disrupted cortical layers, and reduced proliferative zones (Wu et al., 2016). Furthermore, it has been demonstrated that Asian ZIKV strain SZ01 has shown that ZIKV replicate efficiently in mouse neural precursor cells (NPCs). Next, SZ01 replication was associated with differential expression of genes related to the cell-cycle, immune responses and apoptosis (Li et al., 2016a).
Zika virus association with the neurological abnormalities
ZIKV infections are linked to the GBS and newborn microcephaly (Oehler et al., 2014; Araujo et al., 2016a; Cugola et al., 2016). GBS is a rare neurological syndrome caused by immune system failures wherein the immune system targets myelinating Schwann cells of the peripheral nervous system and leads to a rapid-onset of muscle weakness (Hughes and Cornblath, 2005). Microcephaly is caused by embryonic and fetal developmental failures that lead to reduced cerebral cortex size. The normal development of the cerebral cortex requires an adequate proliferation, differentiation, and migration of neural stem cells. Failure in any of these processes can result in microcephaly (Jackson et al., 2002). Recent reports imply that these neurological abnormalities are also observed in congenital infections (Wakerley and Yuki, 2013). Increase in the number of GBS cases during French Polynesia outbreak and newborn microcephaly cases during Brazil outbreak were observed (Oehler et al., 2014; Araujo et al., 2016a; Cugola et al., 2016). In addition to GBS and microcephaly, infected persons also experienced paralysis of four limbs (tetraparesis) in adults (Broutet et al., 2016). Severe ocular abnormalities were observed in infants with microcephaly (Vasconcelos-Santos et al., 2016). ZIKV positive antibodies have been found in patients with tetraparesis, and the presence of ZIKV RNA has been reported in the brain and tissues of a neonate who died of microcephaly (Araujo et al., 2016a).
The exact mechanism behind ZIKV induced neurological disorders is not clearly known. However, several recent studies have aimed at understanding the link between ZIKV infections and microcephaly (Bayer et al., 2016; Bayless et al., 2016; Brault et al., 2016; Broutet et al., 2016). A recent comprehensive study involving ZIKV (Pf13 strain), DENV-4, and WNV strongly supports the neurotropic nature of ZIKV; wherein, the embryonic brain cells were able to support virus replication, but not closely related DENV-4 replication (Brault et al., 2016) in mice. Cranial neural crest cells (CNCCs) are very crucial in development of facial bones of the skull and play important role in the formation of bones, cartilage, and nerves. ZIKV, but not DENV, efficiently infect CNCCs, promote increased production of neurodevelopmental cytokines such as Vascular endothelial growth factor (VEGF) and leukemia inhibitory factor (LIF) that initiate premature neuronal differentiation (Bayless et al., 2016). At the molecular level, ZIKV NS4A and NS4B inhibit the Akt-mTOR pathway in infected human fetal neural stem cells (fNSCs) leading to dysregulation of autophagy pathways and faulty neurogenesis (Liang et al., 2016). However, the closely related DENV NS4A and NS4B do not demonstrate the same effect on neurogenesis (Liang et al., 2016).
Due to high recombination frequencies, ZIKV has potentially evolved faster and attained the ability to exploit multiple cell surface receptors and cellular factors to facilitate infection in a variety of cells types, including AXL-independent entry into cortical progenitor cells, neuronal progenitor cells, and cerebral organoids in mice and some human ex vivo studies (Hamel et al., 2015; Garcez et al., 2016; Shao et al., 2016; Wells et al., 2016). Also, mice pups delivered from SJL and C57BL/6 mice infected intravenously with a ZIKV Brazil (ZIKVBR) isolate, exhibited delay in whole-body growth and intrauterine restrictions, compared to control mice pups (Cugola et al., 2016). In addition, significant viral RNA and proteins were detected in the brain (Cugola et al., 2016). The extensive microglial activation, massive neuronal death, axonal rarefaction, and blood-brain barrier (BBB) leakage indicate the ability of the virus to cross placenta and cause congenital malformation (Shao et al., 2016). Correspondingly, a quantitative study using three-dimensional forebrain organoids demonstrates that ZIKV infections decrease the neuronal cell-layer volumes by increasing cell-death and decreasing cell proliferation (Qian et al., 2016). This correlates with cortical defects associated with reduced human cortical progenitor cell numbers and cortical thickness due to alteration in the expression of genes involved in immune response, cell-cycle, autophagy and apoptosis (Cugola et al., 2016; Qian et al., 2016; Tang et al., 2016).
Zika virus-host immune system interactions
A contemporary circulating PRVABC59, a ZIKV strain isolated from Puerto Rico in the Western Hemisphere during the 2015 outbreak, was characterized for its cytopathic effects (cell rounding, pyknosis, and caspase 3 activation) in primary human neural progenitors (hNPs), THP-1, Huh7.5, BHK-J, and Vero E6 cells (Hanners et al., 2016). An antibody-based array to detect 102 human inflammatory cytokines in ZIKV-infected hNPs and THP-1 cells showed that multiple cytokines and growth factors were secreted by hNPs cells. However, there was no significant difference in secreted proinflammatory cytokines TNFα, CCL2, and the neuroprotective chemokine CX3CL1 between ZIKV-infected and mock-infected cultures. Thus, confirming that ZIKV can replicate in hNPs without inducing the production of neuroinflammatory or neuroprotective cytokines (Hanners et al., 2016).
Infection of DENV results in a characteristic phenomenon known as a ‘cytokine storm’; wherein, the infected cells produce high levels of circulating pro-inflammatory cytokines, such as IFN-α, IFN-γ, tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-2, IL-6, IL-8, IL-10, CCL2, CCL3, CCL4, CCL5, CXCL-8, CXCL-10, CXCL-11, macrophage migration inhibitory factor, and vascular endothelial growth factor (Mangada and Rothman, 2005; Palmer et al., 2005; Amaral et al., 2011). However, such symptoms are not observed in ZIKV-infected individuals even though there is a high degree of similarities in the viral proteins. The multiplex cytokine serum analyses (MCSA) of acute and convalescent phase of ZIKV infections indicate that the elevation of chemokines was more noticeable than elevation of cytokines (Tappe et al., 2015). In acute phase, substantial increases in concentration of IL-1β, IL-2, IL-4, IL-6, IL-9, IL-10, IL-13, IL-17, interferon-γ-induced protein 10 (IP-10), normal T cell expressed and secreted (RANTES), macrophage inflammatory protein 1 alpha (MIP-1α), and VEGF are observed as compared to normal controls (Tappe et al., 2015). In the convalescent phase, noticeable increase in IL-1β, IL-6, IL-8, IL-10, IL-13, IP-10, RANTES, MIP-1α, MIP-1β, VEGF, fibroblast growth factor (FGF) and granulocyte-macrophage colony stimulating factor (GM-CSF) levels compared to MCSA of uninfected persons (Tappe et al., 2015). A circulating strain, PF-13 was isolated during the French Polynesia outbreak in 2013/2014. It is closely related to strains isolated from Cambodia in 2010 and Yap State in 2007, and it infects permissive cells like human dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells (Musso et al., 2014a; Hamel et al., 2015).
The pathogen recognition receptors (PRR), such as toll-like receptor 3 (TLR-3), retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated gene 5 (MDA5) bind to pathogen associated molecular patterns (PAMP) (Hamel et al., 2015). This leads to restriction of the pathogen through the stimulation of a cascade of signalling events resulting in the activation of the antiviral pathway. The antiviral pathway is comprised of IRF-7-dependent antiviral gene expression that includes: the interferon-stimulated genes OAS2, ISG15, and MX1; type I IFNs; and the inflammatory chemokines CXCL10, CCL5, and CXCL11 (Chen et al., 2013; Hamel et al., 2015). The interferon system is known to play a key role DNEV and WNV infections (Umareddy et al., 2008; Munoz-Jordan, 2009; Nasirudeen et al., 2011; Bayer et al., 2016). Similarly, A129 immunocompromised mice lacking the type I interferon receptor and AG129 mice lacking types I and II IFN receptors infected with the Asian lineage FSS13025 strain exhibited severe neurological symptoms and death. In wild-type immunocompetent mice, this was not observed. In spite of all this, these neurological infections have not been reported in infected rhesus macaques (Dudley et al., 2016). Taken together, this data suggests that the interferon system plays a crucial role in controlling ZIKV pathogenesis (Aliota et al., 2016; Rossi et al., 2016). Interestingly, the antigen presenting system and interferon system is not developed to control these infections and associated diseases in the neonates (Jaspan et al., 2006). Furthermore, pre-treatment of primary skin fibroblasts with human recombinant type I and type II interferon, such as IFN-α, IFN-β, or IFN-γ strongly restrict ZIKV infections in a dose-dependent manner supporting the importance of the interferon system in controlling ZIKV infection and pathogenesis (Navarro-Sanchez et al., 2005; Hamel et al., 2015).
Cross-reactivity of other flavivirus antibodies with ZIKV
It has been shown that DENV and ZIKV are antigenically related with the severity of ZIKV infection significantly increased when antibodies against DENV are present and vice versa (Lanciotti et al., 2008; Dejnirattisai et al., 2016; Priyamvada et al., 2016; Zhu et al., 2016). The DENV has four human serotypes, DENV-1, DENV-2, DENV-3, and DENV-4, which share 65% of genome homology (Holmes, 1998; Mustafa et al., 2015). Each of the serotypes of DENV produce unique humoral immune responses in infected hosts (Holmes, 1998). Infection of humans with a single serotype is typically self-resolving; but when the same individual is infected with a different DENV serotype, it results in higher levels of infectivity. Antibodies generated against the first serotype can bind to other serotypes resulting in enhanced antibody-Fc receptor-mediated entry into the macrophages (Peiris and Porterfield, 1979; Boonnak et al., 2010; Chotiwan et al., 2014; Dejnirattisai et al., 2016). Serum collected from four people who had recovered from DENV showed significant enhancement of ZIKV infection indicating that the DENV neutralizing sera can enhance ZIKV infections in an antibody-Fc receptor dependent pathway. In presence of anti-Fc receptor antibody, the infectivity of ZIKV was significantly reduced (Priyamvada et al., 2016). Similarly, the cross-reactivity of acute and convalescent serum of nine Thai DENV-infected individuals to ZIKV was tested and showed cross-reactivity in the binding as well as in the neutralization of ZIKV (Priyamvada et al., 2016). This is further supported by findings that demonstrated DENV-specific monoclonal antibodies (mAbs) bound to both whole ZIKV virions and ZIKV lysate (Priyamvada et al., 2016). Only a subset of these antibodies could neutralize ZIKV infection, but all the nine sera and 47 mAbs specific to DENV enhanced in vitro ZIKV infection of Fc gamma receptor (FcγR)-bearing cells.
A set of 119 monoclonal antibody-producing B-cells were isolated from the four patients (two had ZIKV-infection and two had serological records of DENV infection) from contemporary epidemics. Due to the high degree of structural similarities between the EDI and EDII loops, 67% (31of 46) of the monoclonal antibodies were cross-reactive with each other and exhibited low neutralizing activity with antibody-mediated enhancement of Fc-receptors (Stettler et al., 2016). However, 90% (27 of 30) of the EDIII-specific monoclonal antibodies isolated from ZIKV or DENV donors were specific for either ZIKV or DENV envelope proteins and exhibited high neutralizing ability (Stettler et al., 2016). Serological examination of German travellers who developed signs of dengue showed ZIKV-specific neutralizing antibodies, as well as anti-ZIKV-IgM and -IgG antibodies (Tappe et al., 2014). However, pre-existing immunity does not confer resistance to other flaviviruses (Murphy and Whitehead, 2011). The ZIKV-immunized rhesus monkeys developed partial resistance to challenge with YFV (Henderson et al., 1970). Neutralizing antibodies developed after ZIKV and YFV infection has shown haemagglutination inhibition that could also partially protect mice from subsequent lethal ZIKV infection (Monath et al., 1973; Musso and Gubler, 2016). It has been shown that 47% (8 of 17 mAbs) of DENV EDE2-specific mAbs and 81% (27 of 33) of DENV EDE1-specific mAbs could cross-react with ZIKV (Dejnirattisai et al., 2016).
Concluding remarks and future perspectives
The global spread of ZIKV infections and pathological mechanisms behind the emergence of Zika virus are not clearly known. The factors influencing the rate of Zika infections include, but are not limited to, viral envelope protein glycosylation and its parallel connection with the evolution in the receptors utilization for cellular entry. Glycosylation plays an important role in both virus entry into host cells and in modulating the host-response (innate and adaptive immune response) towards ZIKV virus. Understanding the unique Zika viral and host factors that determine the tissue tropism and thereby disease severity could delineate the mechanism of Zika viral infections. Therefore, elucidating the ZIKV-host interactions could possibly aid in the development of diagnostic tools and effective therapeutics to prevent Zika viral infections and associated diseases in future outbreaks.
Acknowledgments
This work is supported in part by R01AI113883 and Nebraska Neuroscience Alliance Endowed Fund Award to SNB, and we thank Dr. Norgren for critical reading of the manuscript.
References
- Al-Qahtani AA, Nazir N, Al-Anazi MR, Rubino S, Al-Ahdal MN. Zika virus: a new pandemic threat. J Infect Dev Ctries. 2016;10:201–207. doi: 10.3855/jidc.8350. [DOI] [PubMed] [Google Scholar]
- Alcon-LePoder S, Drouet MT, Roux P, Frenkiel MP, Arborio M, Durand-Schneider AM, Maurice M, Le Blanc I, Gruenberg J, Flamand M. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J Virol. 2005;79:11403–11411. doi: 10.1128/JVI.79.17.11403-11411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DC, Rachid MA, Vilela MC, Campos RD, Ferreira GP, Rodrigues DH, Lacerda-Queiroz N, Miranda AS, Costa VV, Campos MA, Kroon EG, Teixeira MM, Teixeira AL. Intracerebral infection with dengue-3 virus induces meningoencephalitis and behavioral changes that precede lethality in mice. J Neuroinflammation. 2011;8:23. doi: 10.1186/1742-2094-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AQ, Silva MT, Araujo AP. Zika virus-associated neurological disorders: a review. Brain. 2016a;139:2122–2130. doi: 10.1093/brain/aww158. [DOI] [PubMed] [Google Scholar]
- Araujo LM, Ferreira ML, Nascimento OJ. Guillain-Barre syndrome associated with the Zika virus outbreak in Brazil. Arq Neuropsiquiatr. 2016b;74:253–255. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless NL, Greenberg RS, Swigut T, Wysocka J, Blish CA. Zika Virus Infection Induces Cranial Neural Crest Cells to Produce Cytokines at Levels Detrimental for Neurogenesis. Cell Host Microbe. 2016;20:423–428. doi: 10.1016/j.chom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch D, Egloff MP, Mulard L, Guerreiro C, Romette JL, Canard B. A structural basis for the inhibition of the NS5 dengue virus mRNA 2′-O-methyltransferase domain by ribavirin 5′-triphosphate. J Biol Chem. 2004;279:35638–35643. doi: 10.1074/jbc.M400460200. [DOI] [PubMed] [Google Scholar]
- Bollati M, et al. Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral Res. 2009;87:125–148. doi: 10.1016/j.antiviral.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonnak K, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J Virol. 2010;85:1671–1683. doi: 10.1128/JVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault JB, Khou C, Basset J, Coquand L, Fraisier V, Frenkiel MP, Goud B, Manuguerra JC, Pardigon N, Baffet AD. Comparative Analysis Between Flaviviruses Reveals Specific Neural Stem Cell Tropism for Zika Virus in the Mouse Developing Neocortex. EBioMedicine. 2016;10:71–76. doi: 10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkworth RI, Fairlie DP, Leung D, Young PR. Homology model of the dengue 2 virus NS3 protease: putative interactions with both substrate and NS2B cofactor. J Gen Virol. 1999;80(Pt 5):1167–1177. doi: 10.1099/0022-1317-80-5-1167. [DOI] [PubMed] [Google Scholar]
- Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, Espinal M, Low N, Dye C. Zika Virus as a Cause of Neurologic Disorders. N Engl J Med. 2016;374:1506–1509. doi: 10.1056/NEJMp1602708. [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Musso D. Emerging arboviruses in the Pacific. Lancet. 2014;384:1571–1572. doi: 10.1016/S0140-6736(14)61977-2. [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau VM, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TJ, Weir RC, Grakoui A, McCourt DW, Bazan JF, Fletterick RJ, Rice CM. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc Natl Acad Sci U S A. 1990;87:8898–8902. doi: 10.1073/pnas.87.22.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Liao CL, Tsao CH, Chen MC, Liu CI, Chen LK, Lin YL. Membrane permeabilization by small hydrophobic nonstructural proteins of Japanese encephalitis virus. J Virol. 1999;73:6257–6264. doi: 10.1128/jvi.73.8.6257-6264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che P, Tang H, Li Q. The interaction between claudin-1 and dengue viral prM/M protein for its entry. Virology. 2013;446:303–313. doi: 10.1016/j.virol.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Chen HW, King K, Tu J, Sanchez M, Luster AD, Shresta S. The roles of IRF-3 and IRF-7 in innate antiviral immunity against dengue virus. J Immunol. 2013;191:4194–4201. doi: 10.4049/jimmunol.1300799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiwan N, Roehrig JT, Schlesinger JJ, Blair CD, Huang CY. Molecular determinants of dengue virus 2 envelope protein important for virus entry in FcgammaRIIA-mediated antibody-dependent enhancement of infection. Virology. 2014;456–457:238–246. doi: 10.1016/j.virol.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MB, Kinney RM, Miller BR. Deglycosylation of the NS1 protein of dengue 2 virus, strain 16681: construction and characterization of mutant viruses. Arch Virol. 2005;150:771–786. doi: 10.1007/s00705-004-0430-8. [DOI] [PubMed] [Google Scholar]
- Crook KR, Miller-Kittrell M, Morrison CR, Scholle F. Modulation of innate immune signaling by the secreted form of the West Nile virus NS1 glycoprotein. Virology. 2014;458–459:172–182. doi: 10.1016/j.virol.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daep CA, Munoz-Jordan JL, Eugenin EA. Flaviviruses, an expanding threat in public health: focus on dengue, West Nile, and Japanese encephalitis virus. J Neurovirol. 2014;20:539–560. doi: 10.1007/s13365-014-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, Xiao H, Yan J, Shi Y, Qin CF, Qi J, Gao GF. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19:258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Poersch C, Pavoni DP, Queiroz MH, de Borba L, Goldenberg S, dos Santos CN, Krieger MA. Dengue virus infections: comparison of methods for diagnosing the acute disease. J Clin Virol. 2005;32:272–277. doi: 10.1016/j.jcv.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Droll DA, Krishna Murthy HM, Chambers TJ. Yellow fever virus NS2B-NS3 protease: charged-to-alanine mutagenesis and deletion analysis define regions important for protease complex formation and function. Virology. 2000;275:335–347. doi: 10.1006/viro.2000.0488. [DOI] [PubMed] [Google Scholar]
- Dudley DM, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Diamond MS, Fremont DH. Structural basis of Flavivirus NS1 assembly and antibody recognition. Proc Natl Acad Sci U S A. 2014;111:4285–4290. doi: 10.1073/pnas.1322036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgout B, Pethel M, Zhang YM, Lai CJ. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feito MJ, Gomez-Gutierrez J, Ayora S, Alonso JC, Peterson D, Gavilanes F. Insights into the oligomerization state-helicase activity relationship of West Nile virus NS3 NTPase/helicase. Virus Res. 2008;135:166–174. doi: 10.1016/j.virusres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Jr, Tan SL, Katze MG. Translational control of viral gene expression in eukaryotes. Microbiol Mol Biol Rev. 2000;64:239–280. doi: 10.1128/mmbr.64.2.239-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- Gebhard LG, Kaufman SB, Gamarnik AV. Novel ATP-independent RNA annealing activity of the dengue virus NS3 helicase. PLoS One. 2012;7:e36244. doi: 10.1371/journal.pone.0036244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Lim L, Song J. NMR and MD Studies Reveal That the Isolated Dengue NS3 Protease Is an Intrinsically Disordered Chymotrypsin Fold Which Absolutely Requests NS2B for Correct Folding and Functional Dynamics. PLoS One. 2015;10:e0134823. doi: 10.1371/journal.pone.0134823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker K, White L, de Silva AM. N-linked glycans on dengue viruses grown in mammalian and insect cells. J Gen Virol. 2009;90:2097–2106. doi: 10.1099/vir.0.012120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. Biology of Zika Virus Infection in Human Skin Cells. J Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanners NW, Eitson JL, Usui N, Richardson RB, Wexler EM, Konopka G, Schoggins JW. Western Zika Virus in Human Fetal Neural Progenitors Persists Long Term with Partial Cytopathic and Limited Immunogenic Effects. Cell Rep. 2016;15:2315–2322. doi: 10.1016/j.celrep.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BE, Cheshire PP, Kirya GB, Lule M. Immunologic studies with yellow fever and selected African group B arboviruses in rhesus and vervet monkeys. Am J Trop Med Hyg. 1970;19:110–118. doi: 10.4269/ajtmh.1970.19.110. [DOI] [PubMed] [Google Scholar]
- Holmes EC. Molecular epidemiology and evolution of emerging infectious diseases. Br Med Bull. 1998;54:533–543. doi: 10.1093/oxfordjournals.bmb.a011708. [DOI] [PubMed] [Google Scholar]
- Hombach J, Friede M, Moorphy V, Costello A, Kieny MP. Developing a vaccine against Zika. BMJ. 2016;355:i5923. doi: 10.1136/bmj.i5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Jackson AP, Eastwood H, Bell SM, Adu J, Toomes C, Carr IM, Roberts E, Hampshire DJ, Crow YJ, Mighell AJ, Karbani G, Jafri H, Rashid Y, Mueller RF, Markham AF, Woods CG. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am J Hum Genet. 2002;71:136–142. doi: 10.1086/341283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MG, Robinson PJ, Bletchly C, Mackenzie JM, Young PR. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 2000;14:1603–1610. doi: 10.1096/fj.14.11.1603. [DOI] [PubMed] [Google Scholar]
- Jamil Z, Waheed Y, Durrani TZ. Zika virus, a pathway to new challenges. Asian Pac J Trop Med. 2016;9:626–629. doi: 10.1016/j.apjtm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Jaspan HB, Lawn SD, Safrit JT, Bekker LG. The maturing immune system: implications for development and testing HIV-1 vaccines for children and adolescents. AIDS. 2006;20:483–494. doi: 10.1097/01.aids.0000210602.40267.60. [DOI] [PubMed] [Google Scholar]
- Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94:675–686C. doi: 10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klema VJ, Ye M, Hindupur A, Teramoto T, Gottipati K, Padmanabhan R, Choi KH. Dengue Virus Nonstructural Protein 5 (NS5) Assembles into a Dimer with a Unique Methyltransferase and Polymerase Interface. PLoS Pathog. 2016;12:e1005451. doi: 10.1371/journal.ppat.1005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. Structure of the thermally stable Zika virus. Nature. 2016;533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- Ladner JT, Wiley MR, Prieto K, Yasuda CY, Nagle E, Kasper MR, Reyes D, Vasilakis N, Heang V, Weaver SC, Haddow A, Tesh RB, Sovann L, Palacios G. Complete Genome Sequences of Five Zika Virus Isolates. Genome Announc. 2016;4 doi: 10.1128/genomeA.00377-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA. Role of nonstructural protein NS2A in flavivirus assembly. J Virol. 2008;82:4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016a;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, Gleeson JG. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 2016b;19:593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Shan C, Deng CL, Ye HQ, Shi PY, Yuan ZM, Gong P, Zhang B. The interface between methyltransferase and polymerase of NS5 is essential for flavivirus replication. PLoS Negl Trop Dis. 2014;8:e2891. doi: 10.1371/journal.pntd.0002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, Ge J, Wang S, Goldman SA, Zlokovic BV, Zhao Z, Jung JU. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell. 2016;19:663–671. doi: 10.1016/j.stem.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J Virol. 1999;73:4611–4621. doi: 10.1128/jvi.73.6.4611-4621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Adv Virus Res. 2003;59:23–61. doi: 10.1016/s0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Sedlak PL, Kondratieva N, Khromykh AA. Complementation analysis of the flavivirus Kunjin NS3 and NS5 proteins defines the minimal regions essential for formation of a replication complex and shows a requirement of NS3 in cis for virus assembly. J Virol. 2002;76:10766–10775. doi: 10.1128/JVI.76.21.10766-10775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Gong P. Crystal Structure of the full-length Japanese encephalitis virus NS5 reveals a conserved methyltransferase-polymerase interface. PLoS Pathog. 2013;9:e1003549. doi: 10.1371/journal.ppat.1003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Xu T, Hunke C, Gruber G, Vasudevan SG, Lescar J. Crystal structure of the NS3 protease-helicase from dengue virus. J Virol. 2008;82:173–183. doi: 10.1128/JVI.01788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton K. Zika virus disease: a public health emergency of international concern. Br J Nurs. 2016;25:198, 200–192. doi: 10.12968/bjon.2016.25.4.198. [DOI] [PubMed] [Google Scholar]
- Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Malet H, Egloff MP, Selisko B, Butcher RE, Wright PJ, Roberts M, Gruez A, Sulzenbacher G, Vonrhein C, Bricogne G, Mackenzie JM, Khromykh AA, Davidson AD, Canard B. Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5. J Biol Chem. 2007;282:10678–10689. doi: 10.1074/jbc.M607273200. [DOI] [PubMed] [Google Scholar]
- Malone RW, Homan J, Callahan MV, Glasspool-Malone J, Damodaran L, Schneider Ade B, Zimler R, Talton J, Cobb RR, Ruzic I, Smith-Gagen J, Janies D, Wilson J. Zika Virus: Medical Countermeasure Development Challenges. PLoS Negl Trop Dis. 2016;10:e0004530. doi: 10.1371/journal.pntd.0004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–2683. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- Markoff L, Falgout B, Chang A. A conserved internal hydrophobic domain mediates the stable membrane integration of the dengue virus capsid protein. Virology. 1997;233:105–117. doi: 10.1006/viro.1997.8608. [DOI] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D, Shieh WJ, Luz KG, Ramos AM, Davi HP, Kleber de Oliveria W, Lanciotti R, Lambert A, Zaki S. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses–Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–160. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- Miller S, Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6:363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Kastner S, Krijnse-Locker J, Buhler S, Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J Biol Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79:1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, Wilson DC, Casals J. The 1970 yellow fever epidemic in Okwoga District, Benue Plateau State, Nigeria. 3. Serological responses in persons with and without pre-existing heterologous group B immunity. Bull World Health Organ. 1973;49:235–244. [PMC free article] [PubMed] [Google Scholar]
- Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013;98:192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan JL. Subversion of interferon by dengue virus. Curr Top Microbiol Immunol. 2009;338:35–44. doi: 10.1007/978-3-642-02215-9_3. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- Musso D. Zika Virus Transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21:1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014a;20:O595–596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014b;19 doi: 10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med J Armed Forces India. 2015;71:67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirudeen AM, Wong HH, Thien P, Xu S, Lam KP, Liu DX. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLoS Negl Trop Dis. 2011;5:e926. doi: 10.1371/journal.pntd.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Despres P, Cedillo-Barron L. Innate immune responses to dengue virus. Arch Med Res. 2005;36:425–435. doi: 10.1016/j.arcmed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Nishiura H, Kinoshita R, Mizumoto K, Yasuda Y, Nah K. Transmission potential of Zika virus infection in the South Pacific. Int J Infect Dis. 2016;45:95–97. doi: 10.1016/j.ijid.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016;111:287–293. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome–case report, French Polynesia, December 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- Olagnier D, Amatore D, Castiello L, Ferrari M, Palermo E, Diamond MS, Palamara AT, Hiscott J. Dengue Virus Immunopathogenesis: Lessons Applicable to the Emergence of Zika Virus. J Mol Biol. 2016;428:3429–3448. doi: 10.1016/j.jmb.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Palmer DR, Sun P, Celluzzi C, Bisbing J, Pang S, Sun W, Marovich MA, Burgess T. Differential effects of dengue virus on infected and bystander dendritic cells. J Virol. 2005;79:2432–2439. doi: 10.1128/JVI.79.4.2432-2439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Porterfield JS. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature. 1979;282:509–511. doi: 10.1038/282509a0. [DOI] [PubMed] [Google Scholar]
- Perera-Lecoin M, Meertens L, Carnec X, Amara A. Flavivirus entry receptors: an update. Viruses. 2014;6:69–88. doi: 10.3390/v6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016;113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor MJ, Gualano RC, Lin B, Davidson AD, Wright PJ. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J Gen Virol. 1998;79(Pt 11):2631–2639. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- Qian X, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016;94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, Santos LA, Nery N, Jr, Vasilakis N, Ko AI, de Almeida AR. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10:e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Herrlinger S, Yang SL, Lai F, Moore JM, Brindley MA, Chen JF. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143:4127–4136. doi: 10.1242/dev.143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 A resolution cryo-EM structure of Zika virus. Science. 2016;352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithburn KC. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J Immunol. 1952;69:223–234. [PubMed] [Google Scholar]
- Stettler K, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM, Didier RA, Jin P, Song H, Ming GL. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe D, Rissland J, Gabriel M, Emmerich P, Gunther S, Held G, Smola S, Schmidt-Chanasit J. First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.4.20685. [DOI] [PubMed] [Google Scholar]
- Tappe D, Perez-Giron JV, Zammarchi L, Rissland J, Ferreira DF, Jaenisch T, Gomez-Medina S, Gunther S, Bartoloni A, Munoz-Fontela C, Schmidt-Chanasit J. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2015;205:269–273. doi: 10.1007/s00430-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz N, Kaiser A, Thiel HJ. NS3 serine protease of bovine viral diarrhea virus: characterization of active site residues, NS4A cofactor domain, and protease-cofactor interactions. Virology. 2000;273:351–363. doi: 10.1006/viro.2000.0425. [DOI] [PubMed] [Google Scholar]
- Umareddy I, Tang KF, Vasudevan SG, Devi S, Hibberd ML, Gu F. Dengue virus regulates type I interferon signalling in a strain-dependent manner in human cell lines. J Gen Virol. 2008;89:3052–3062. doi: 10.1099/vir.0.2008/001594-0. [DOI] [PubMed] [Google Scholar]
- Vasconcelos-Santos DV, Andrade GM, Caiaffa WT. Zika Virus, Microcephaly, and Ocular Findings. JAMA Ophthalmol. 2016;134:946. doi: 10.1001/jamaophthalmol.2016.1313. [DOI] [PubMed] [Google Scholar]
- Vasudevan SG, Johansson M, Brooks AJ, Llewellyn LE, Jans DA. Characterisation of inter- and intra-molecular interactions of the dengue virus RNA dependent RNA polymerase as potential drug targets. Farmaco. 2001;56:33–36. doi: 10.1016/s0014-827x(01)01014-x. [DOI] [PubMed] [Google Scholar]
- Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain-Barre syndrome. Expert Rev Clin Immunol. 2013;9:627–639. doi: 10.1586/1744666X.2013.811119. [DOI] [PubMed] [Google Scholar]
- Wang P, Hu K, Luo S, Zhang M, Deng X, Li C, Jin W, Hu B, He S, Li M, Du T, Xiao G, Zhang B, Liu Y, Hu Q. DC-SIGN as an attachment factor mediates Japanese encephalitis virus infection of human dendritic cells via interaction with a single high-mannose residue of viral E glycoprotein. Virology. 2015;488:108–119. doi: 10.1016/j.virol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Watrin L, Ghawche F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barre Syndrome (42 Cases) Occurring During a Zika Virus Outbreak in French Polynesia. Medicine (Baltimore) 2016;95:e3257. doi: 10.1097/MD.0000000000003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, Kommineni S, Bilican B, Klim JR, Hill EJ, Kane LT, Ye C, Kaykas A, Eggan K. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell. 2016;19:703–708. doi: 10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- WHO Bot. Promising new tools to fight Aedes mosquitoes. Bull World Health Organ. 2016;94:562–563. doi: 10.2471/BLT.16.020816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26:645–654. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Gayen S, Kang C, Yuan Z, Shi PY. Membrane topology and function of dengue virus NS2A protein. J Virol. 2013;87:4609–4622. doi: 10.1128/JVI.02424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Takeda K, Markoff L. Protein-protein interactions among West Nile non-structural proteins and transmembrane complex formation in mammalian cells. Virology. 2013;446:365–377. doi: 10.1016/j.virol.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Yusof R, Clum S, Wetzel M, Murthy HM, Padmanabhan R. Purified NS2B/NS3 serine protease of dengue virus type 2 exhibits cofactor NS2B dependence for cleavage of substrates with dibasic amino acids in vitro. J Biol Chem. 2000;275:9963–9969. doi: 10.1074/jbc.275.14.9963. [DOI] [PubMed] [Google Scholar]
- Zhang B, Dong H, Zhou Y, Shi PY. Genetic interactions among the West Nile virus methyltransferase, the RNA-dependent RNA polymerase, and the 5′ stem-loop of genomic RNA. J Virol. 2008;82:7047–7058. doi: 10.1128/JVI.00654-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat Struct Mol Biol. 2012;20:105–110. doi: 10.1038/nsmb.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chan JF, Tee KM, Choi GK, Lau SK, Woo PC, Tse H, Yuen KY. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect. 2016;5:e22. doi: 10.1038/emi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]


