Abstract
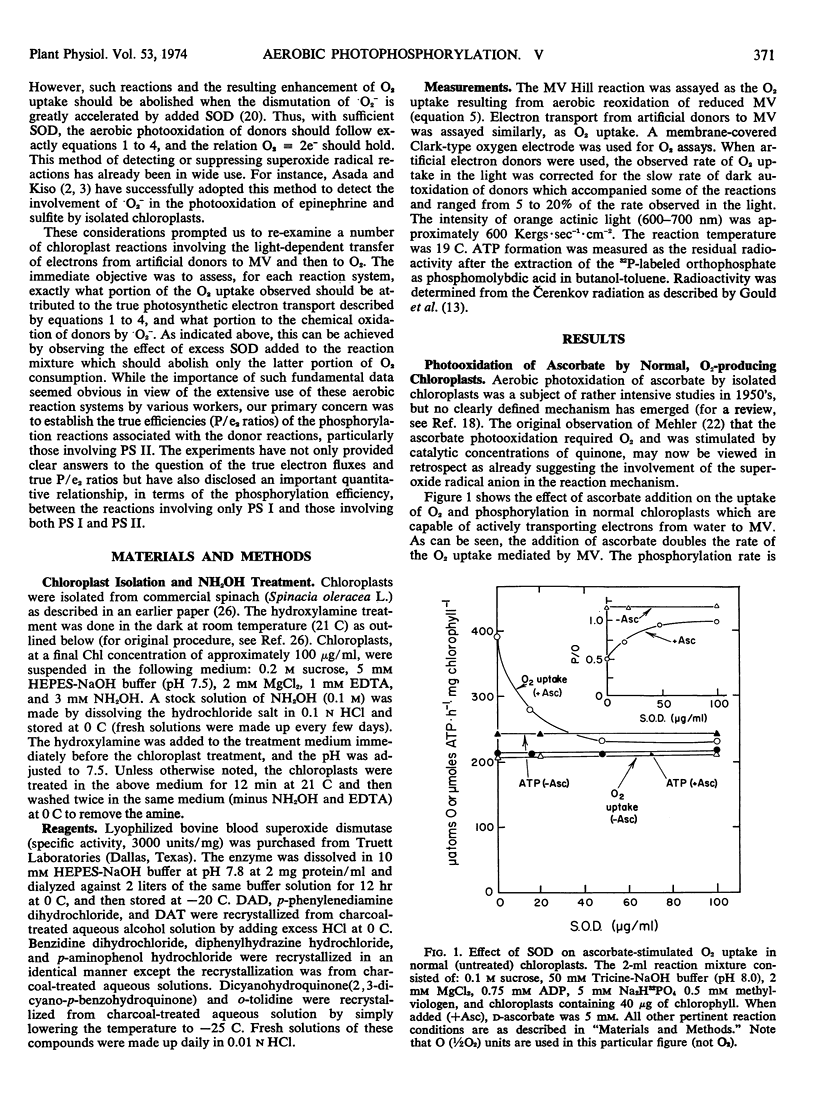
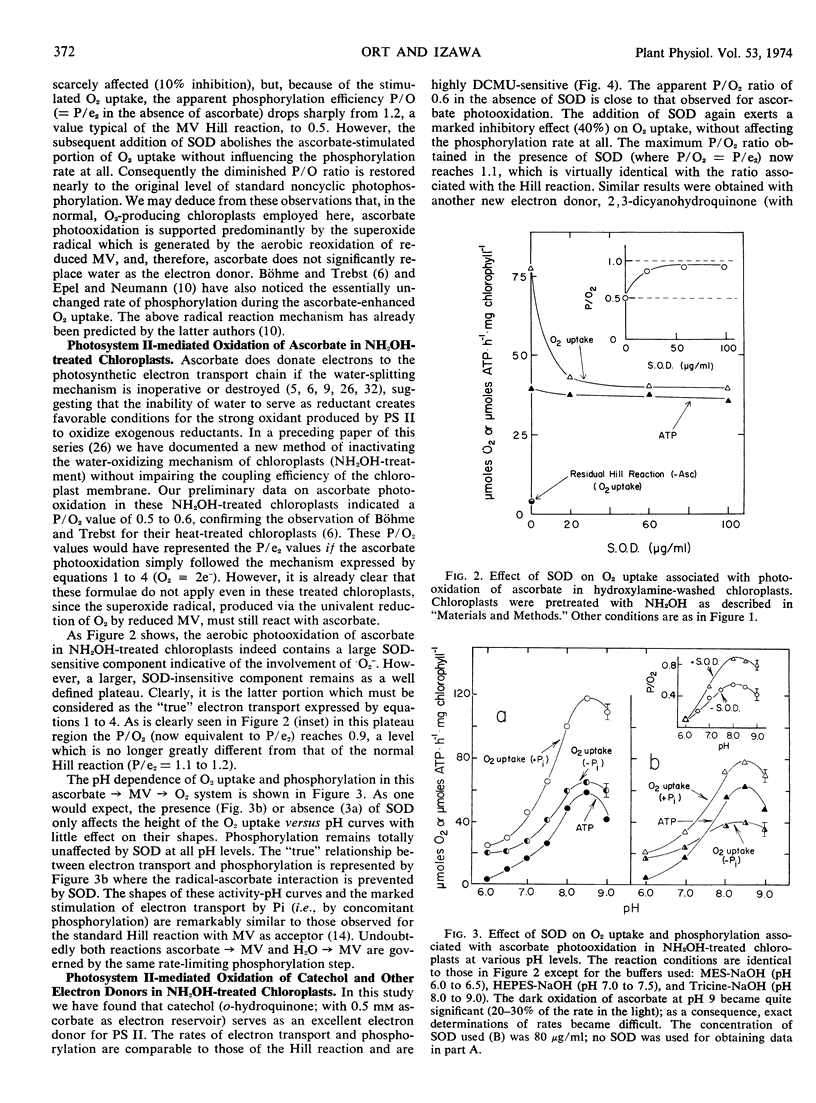
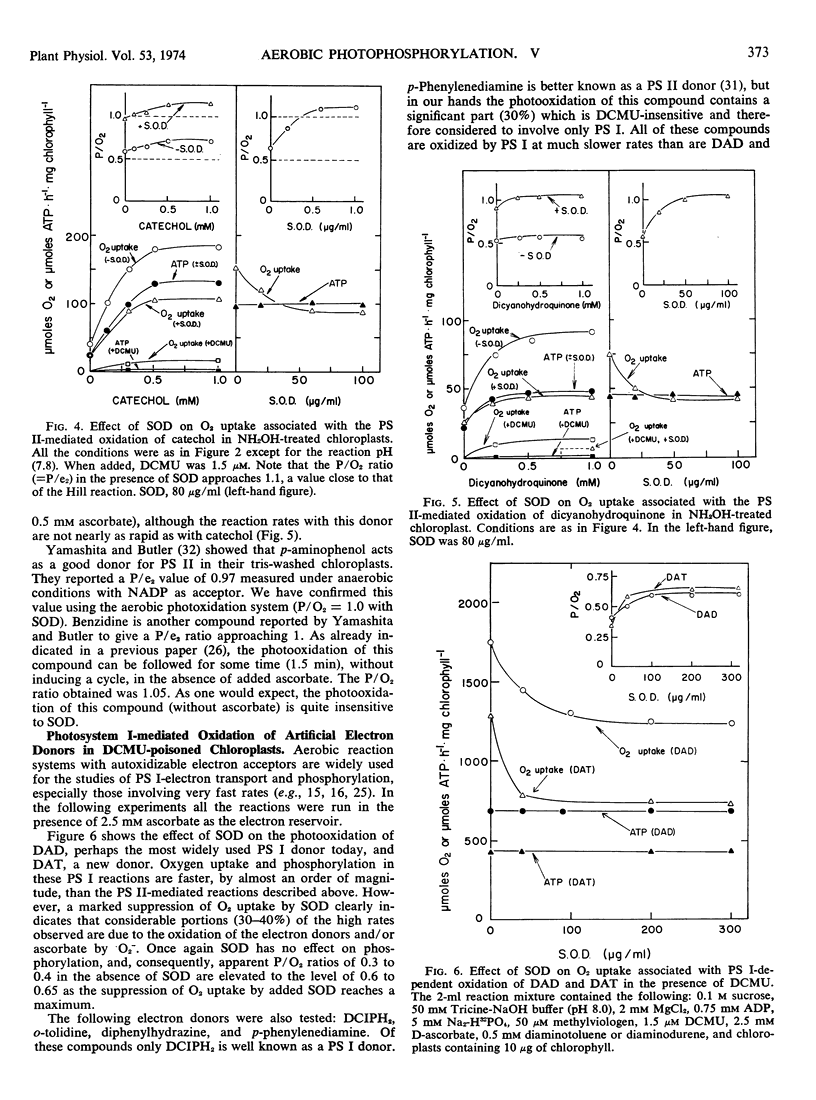
The rate of Hill reaction can be measured accurately as O2 uptake (the Mehler reaction) if a rapidly autoxidizable electron acceptor (e.g., methylviologen) is used. However, when an artificial electron donor-ascorbate couple (or ascorbate alone) replaces the natural donor, water, the rate of O2 consumption is no longer a reliable measure of the electron flux, because superoxide radical reactions contribute to O2 uptake. Such radical reactions, however, can be suppressed by adding enough superoxide dismutase to the reaction mixture. Indeed in all of the photosystem I- and photosystem II-donor reactions tested (except with benzidine which was tested without ascorbate added), the O2 uptake was inhibited by 30 to 50% by the addition of superoxide dismutase. The rate of phosphorylation was totally unaffected by the enzyme. The reasessment of the phosphorylation efficiencies thus made by the use of superoxide dismutase led us to the following conclusions. The phosphorylation efficiency associated with the transfer of electrons from a donor to methlylviologen (than to O2) through both photosystems II and I is practically independent of the donor used—catechol, benzidine, p-aminophenol, dicyanohydroquinone, or water. The P/e2 ratio is 1.0 ± 0.1. Only ascorbate gives a slightly lower value (P/e2 = 0.9). (NH2OH-treated, non-water-splitting chloroplasts were used for reactions with these artificial donors.) The phosphorylation efficiency associated with DCMU-insensitive, photosystem I-mediated transfer of electrons from a donor to methylviologen (then to O2) is again largely independent of the donor used, such as diaminodurene, diaminotoluene, and reduced 2,6-dichlorphenol-indophenol. The P/e2 ratio is 0.6 ± 0.08.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F., Hall D. O. Superoxide reduction as a mechanism of ascorbate-stimulated oxygen uptake by isolated chloroplasts. Biochem Biophys Res Commun. 1973 Jun 8;52(3):856–862. doi: 10.1016/0006-291x(73)91016-4. [DOI] [PubMed] [Google Scholar]
- Asada K., Kiso K. Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur J Biochem. 1973 Mar 1;33(2):253–257. doi: 10.1111/j.1432-1033.1973.tb02677.x. [DOI] [PubMed] [Google Scholar]
- Avron M., Chance B. Relation of phosphorylation to electron transport in isolated chloroplasts. Brookhaven Symp Biol. 1966;19:149–160. [PubMed] [Google Scholar]
- BROWN A. H., GOOD N. Photochemical reduction of oxygen in chloroplast preparations and in green plant cells. I. The study of oxygen exchanges in vitro and in vivo. Arch Biochem Biophys. 1955 Aug;57(2):340–354. doi: 10.1016/0003-9861(55)90297-6. [DOI] [PubMed] [Google Scholar]
- Ben-Hayyim G., Avron M. Involvement of photosystem two in non-oxygen evolving non-cyclic, and in cyclic electron flow processes in chloroplasts. Eur J Biochem. 1970 Jul;15(1):155–160. doi: 10.1111/j.1432-1033.1970.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Böhme H., Cramer W. A. Localization of a site of energy coupling between plastoquinone and cytochrome f in the electron-transport chain of spinach chloroplasts. Biochemistry. 1972 Mar 28;11(7):1155–1160. doi: 10.1021/bi00757a007. [DOI] [PubMed] [Google Scholar]
- Böhme H., Trebst A. On the properties of ascorbate photooxidation in isolated chloroplasts. Evidence for two ATP sites in noncyclic photophosphorylation. Biochim Biophys Acta. 1969 May;180(1):137–148. doi: 10.1016/0005-2728(69)90201-1. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Sites of function of manganese within photosystem II. Roles in O2 evolution and system II. Biochim Biophys Acta. 1970 Mar 3;197(2):219–239. doi: 10.1016/0005-2728(70)90033-2. [DOI] [PubMed] [Google Scholar]
- GOOD N., HILL R. Photochemical reduction of oxygen in chloroplast preparations. II. Mechanisms of the reaction with oxygen. Arch Biochem Biophys. 1955 Aug;57(2):355–366. doi: 10.1016/0003-9861(55)90298-8. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Izawa S. Studies on the energy coupling sites of photophosphorylation. I. Separation of site I and site II by partial reactions of the chloroplast electron transport chain. Biochim Biophys Acta. 1973 Aug 31;314(2):211–223. doi: 10.1016/0005-2728(73)90136-9. [DOI] [PubMed] [Google Scholar]
- Hauska G. A., McCarty R. E., Racker E. The site of phosphorylation associated with photosystem. Biochim Biophys Acta. 1970 Mar 3;197(2):206–218. doi: 10.1016/0005-2728(70)90032-0. [DOI] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys. 1951 Dec;34(2):339–351. doi: 10.1016/0003-9861(51)90012-4. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miller R. W. Reactions of superoxide anion, catechols, and cytochrome c. Can J Biochem. 1970 Aug;48(8):935–939. doi: 10.1139/o70-145. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Neumann J., Arntzen C. J., Dilley R. A. Two sites for adenosine triphosphate formation in photosynthetic electron transport mediated by photosystem I. Evidence from digitonin subchloroplast particles. Biochemistry. 1971 Mar 2;10(5):866–873. doi: 10.1021/bi00781a021. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: II. Treatment of Chloroplasts with NH(2)OH Plus Ethylenediaminetetraacetate to Inhibit Water Oxidation while Maintaining Energy-coupling Efficiencies. Plant Physiol. 1973 Dec;52(6):595–600. doi: 10.1104/pp.52.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouitrakul R., Izawa S. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. II. Acceptor-specific inhibition by KCN. Biochim Biophys Acta. 1973 Apr 27;305(1):105–118. doi: 10.1016/0005-2728(73)90236-3. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Hayon E. Experimental determination of the redox potential of the superoxide radical O 2 . Biochem Biophys Res Commun. 1973 Mar 17;51(2):468–473. doi: 10.1016/0006-291x(73)91280-1. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of the Hill Reaction by Tris and Restoration by Electron Donation to Photosystem II. Plant Physiol. 1969 Mar;44(3):435–438. doi: 10.1104/pp.44.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


