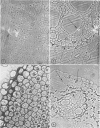
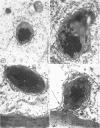
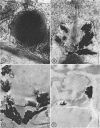
Abstract
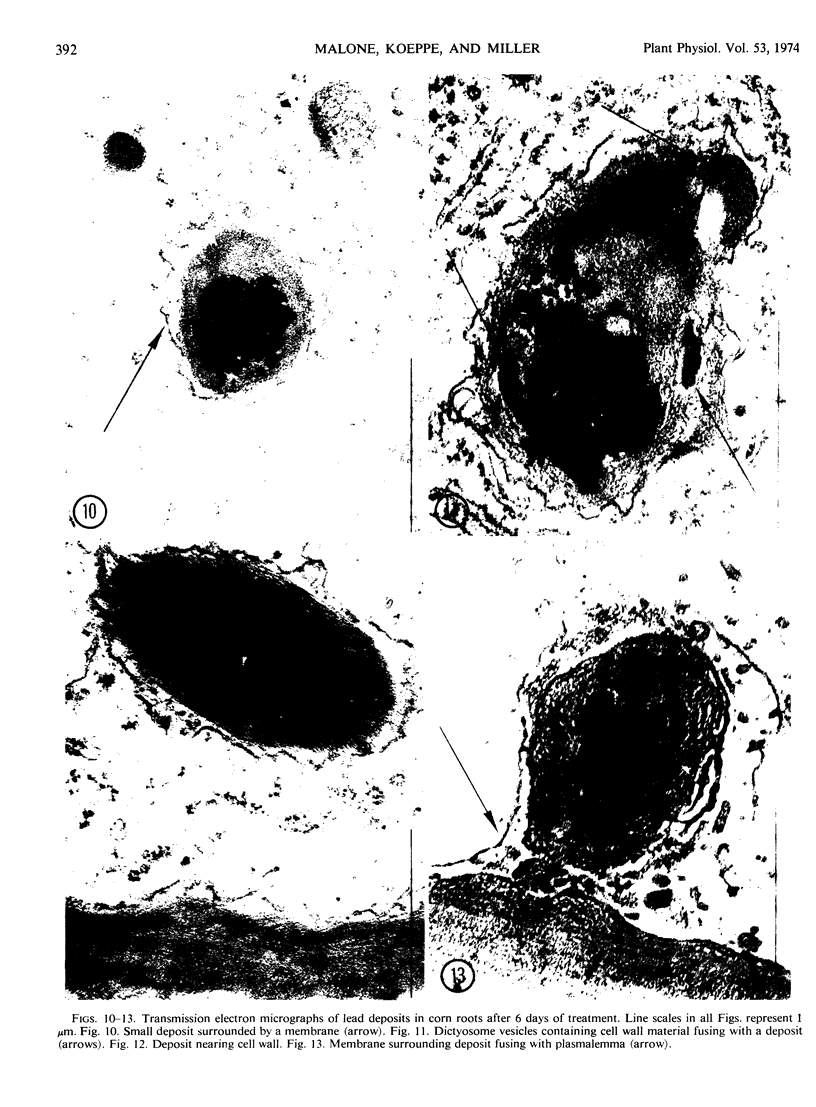
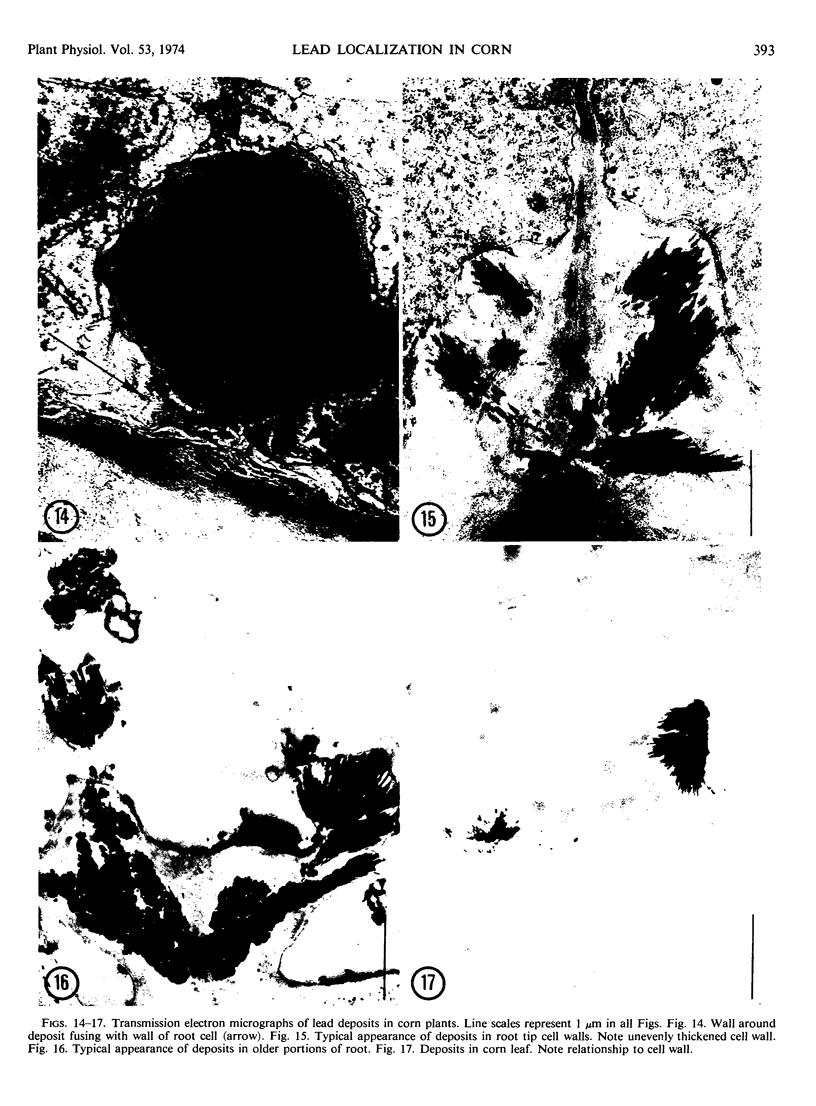
Light and electron microscopic studies of corn plants (Zea mays L.) exposed to Pb in hydroponic solution showed that the roots generally accumulated a surface Pb precipitate and slowly accumulated Pb crystals in the cell walls. The root surface precipitate formed without the apparent influence of any cell organelles. In contrast, Pb taken up by roots was concentrated in dictyosome vesicles. Dictyosome vesicles containing cell wall material fused with one another to encase the Pb deposit. This encased deposit which was surrounded by a membrane migrated toward the outside of the cell where the membrane surrounding the deposit fused with the plasmalemma. The material surrounding the deposit then fused with the cell wall. The result of this process was a concentration of Pb deposits in the cell wall outside the plasmalemma. Similar deposits were observed in stems and leaves suggesting that Pb was transported and deposited in a similar manner.
Full text
PDF
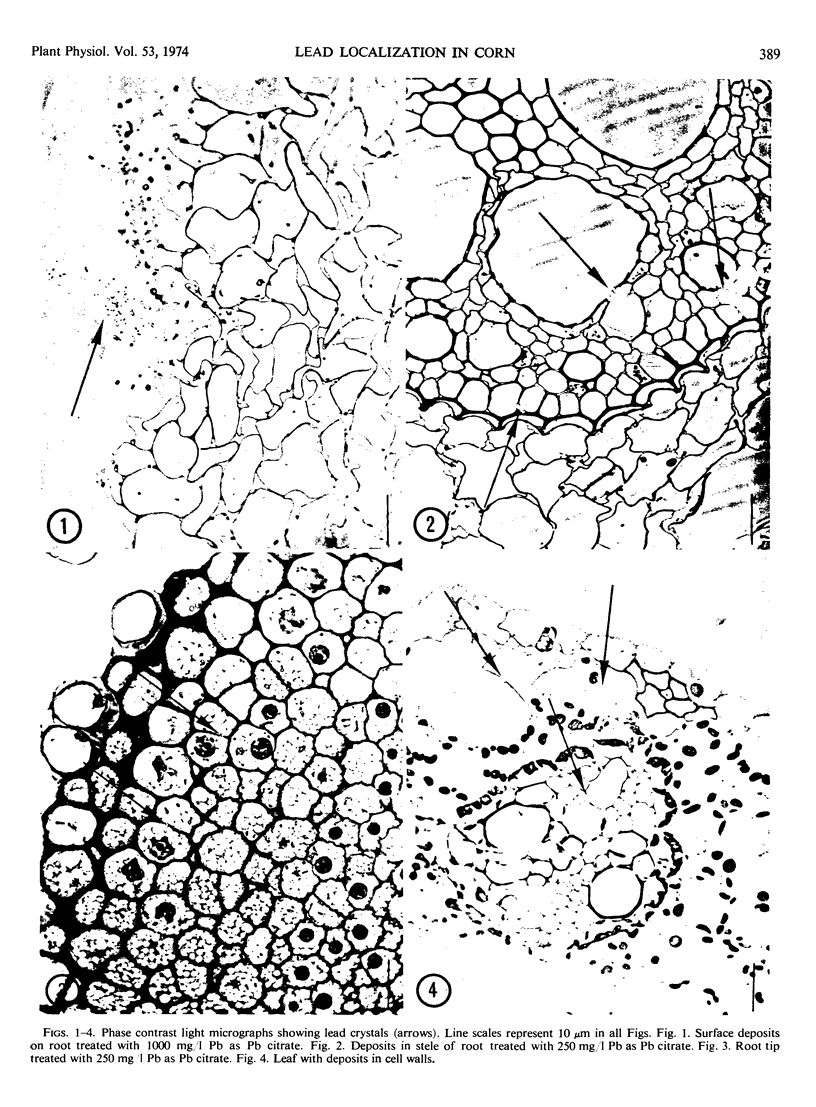



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon H. L., Bowles J. M. Contamination of Vegetation by Tetraethyl Lead. Science. 1962 Sep 7;137(3532):765–766. doi: 10.1126/science.137.3532.765. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote D. H., Pickett-Heaps J. D. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J. 1966 Jan;98(1):159–167. doi: 10.1042/bj0980159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps J. D. Further observations on the Golgi apparatus and its functions in cells of the wheat seedling. J Ultrastruct Res. 1967 May;18(3):287–303. doi: 10.1016/s0022-5320(67)80119-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis D. D., Gordon M., Galston A. W. A site with an affinity for heavy metals on the thylakoid membranes of chloroplasts. Plant Physiol. 1969 Sep;44(9):1355–1363. doi: 10.1104/pp.44.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton R., Cronshaw J., Hall J. L. A study of the biochemistry and cytochemical localization of -glycerophosphatase activity in root tips of maize and pea. Protoplasma. 1971;73(3):417–441. doi: 10.1007/BF01273944. [DOI] [PubMed] [Google Scholar]
- Squier C. A., Waterhouse J. P., Linder J. E. A comparison between osmiophilic reagents and the Gomori lead method for the electron cytochemical demonstration of two lysosomal enzymes in oral epithelium. Histochem J. 1970 Mar;2(2):91–107. doi: 10.1007/BF01003537. [DOI] [PubMed] [Google Scholar]
- Walton J. R. Granules containing lead in isolated mitochondria. Nature. 1973 May 11;243(5402):100–101. doi: 10.1038/243100a0. [DOI] [PubMed] [Google Scholar]