Abstract
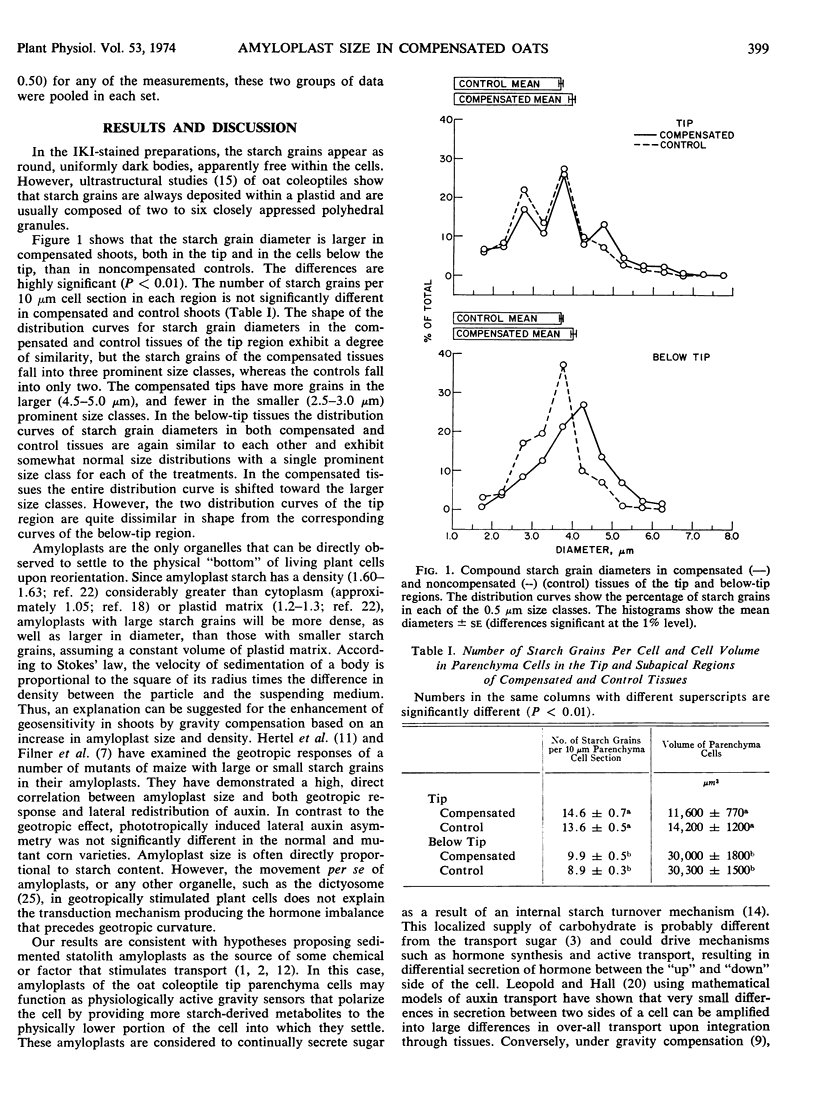
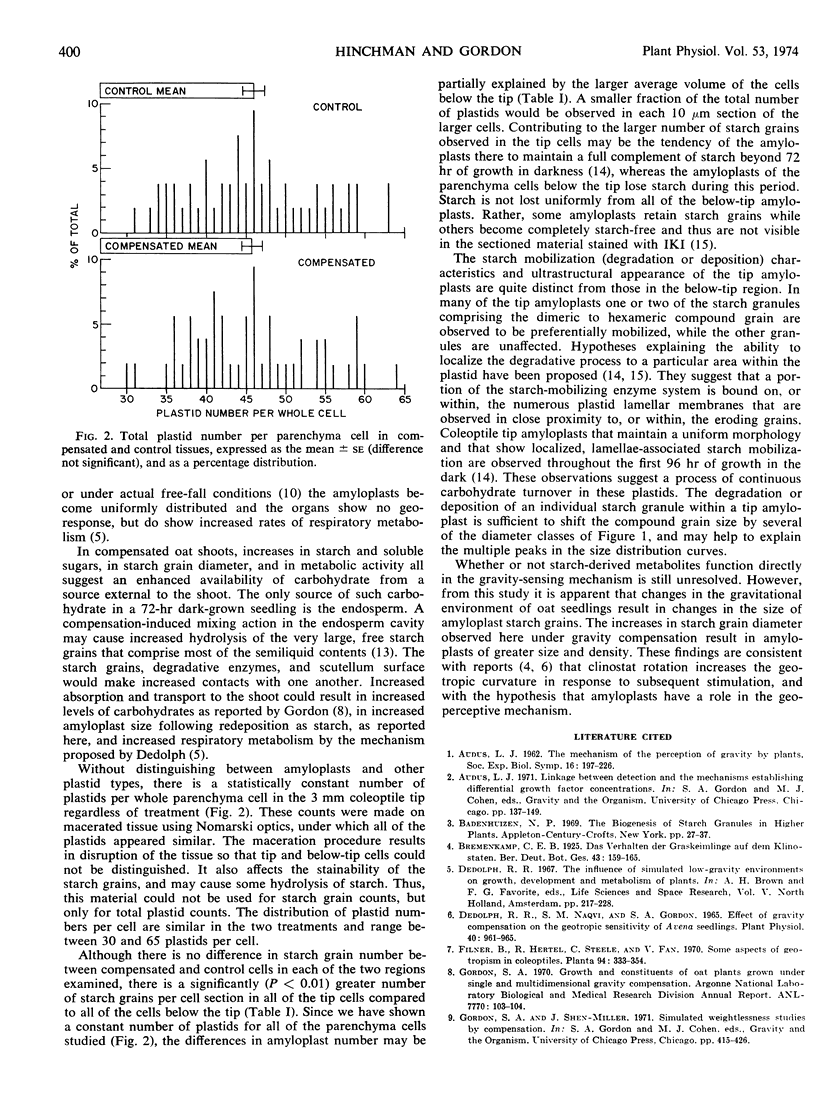
Gravity compensation by the horizontal clinostat increases the diameter of amyloplast starch grains of oat (Avena sativa cv. Victory) coleoptile parenchyma cells, as compared to vertically rotated and stationary controls. In dark-grown coleoptile tip parenchyma cells, measured starch grain sizes exhibit a wide distribution of diameters, from approximately 1.5 to approximately 8.0 μm, but fall into three prominent diameter classes. The compensated tissues from both the tip and the subapical region have more starch grains in the larger, and fewer in the smaller size classes, compared to controls. The total number of starch grains per cell, the total plastid number per cell, and cell volume are unaffected by gravity compensation. Amyloplasts with large starch grains are denser, as well as larger in diameter, than those with smaller starch grains. The amyloplast is considered as a geosensor with an active metabolic role in the geotropic transduction mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dedolph R. R., Naqvi S. M., Gordon S. A. Effect of Gravity Compensation on the Geotropic Sensitivity of Avena Seedlings. Plant Physiol. 1965 Sep;40(5):961–965. doi: 10.1104/pp.40.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedolph R. R. The influence of simulated low-gravity environments on growth, development and metabolism of plants. Life Sci Space Res. 1967;5:217–228. [PubMed] [Google Scholar]
- Hinchman R. R. A permanent iodine stain-mountant combination for starch in plant tissues. Stain Technol. 1973 Nov;48(6):344–346. doi: 10.3109/10520297309116653. [DOI] [PubMed] [Google Scholar]
- Hinchman R. Cytology of the young oat seedling. ANL-7635. ANL Rep. 1969 Dec;:323–327. [PubMed] [Google Scholar]
- Leopold A. C., Hall O. F. Mathematical model of polar auxin transport. Plant Physiol. 1966 Nov;41(9):1476–1480. doi: 10.1104/pp.41.9.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard B. G., Thimann K. V. Geotropic response of wheat coleoptiles in absence of amyloplast starch. J Gen Physiol. 1966 May;49(5):1065–1086. doi: 10.1085/jgp.49.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Gordon S. A. Gravitational compensation and the phototropic response of oat coleoptiles. Plant Physiol. 1967 Mar;42(3):352–360. doi: 10.1104/pp.42.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Gordon S. A. Hormonal Relations in the Phototropic Response: III. The Movement of C-labeled and Endogenous Indoleacetic Acid in Phototropically Stimulated Zea Coleoptiles. Plant Physiol. 1966 Jan;41(1):59–65. doi: 10.1104/pp.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Miller C. Distribution and activation of the Golgi apparatus in geotropism. Plant Physiol. 1972 Apr;49(4):634–639. doi: 10.1104/pp.49.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]


