Abstract
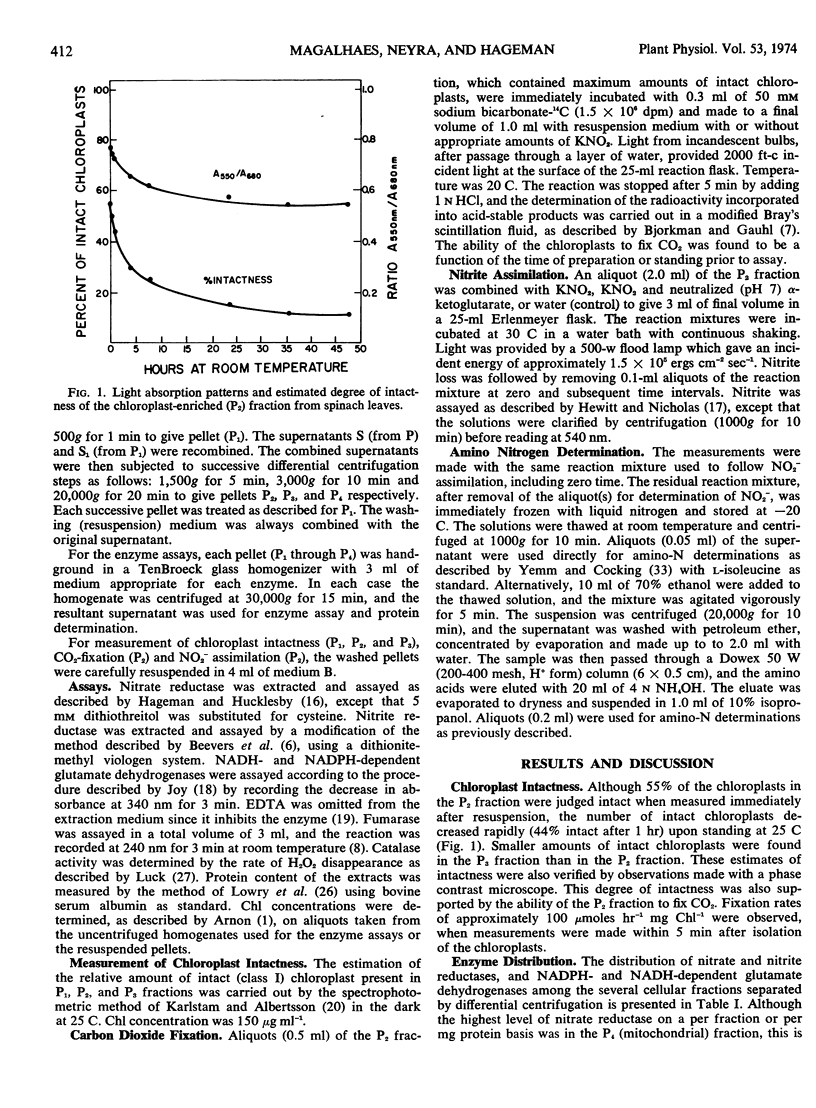
The assimilation of nitrite leading to de novo synthesis of amino nitrogen in a chloroplast-enriched fraction isolated from freshly harvested young spinach (Spinacia oleracea L.) leaves was demonstrated. The preparations showed approximately 55% intact chloroplasts as determined by light scattering properties and fixed CO2 at rates of approximately 100 μmoles hr−1 mg chlorophyll−1.
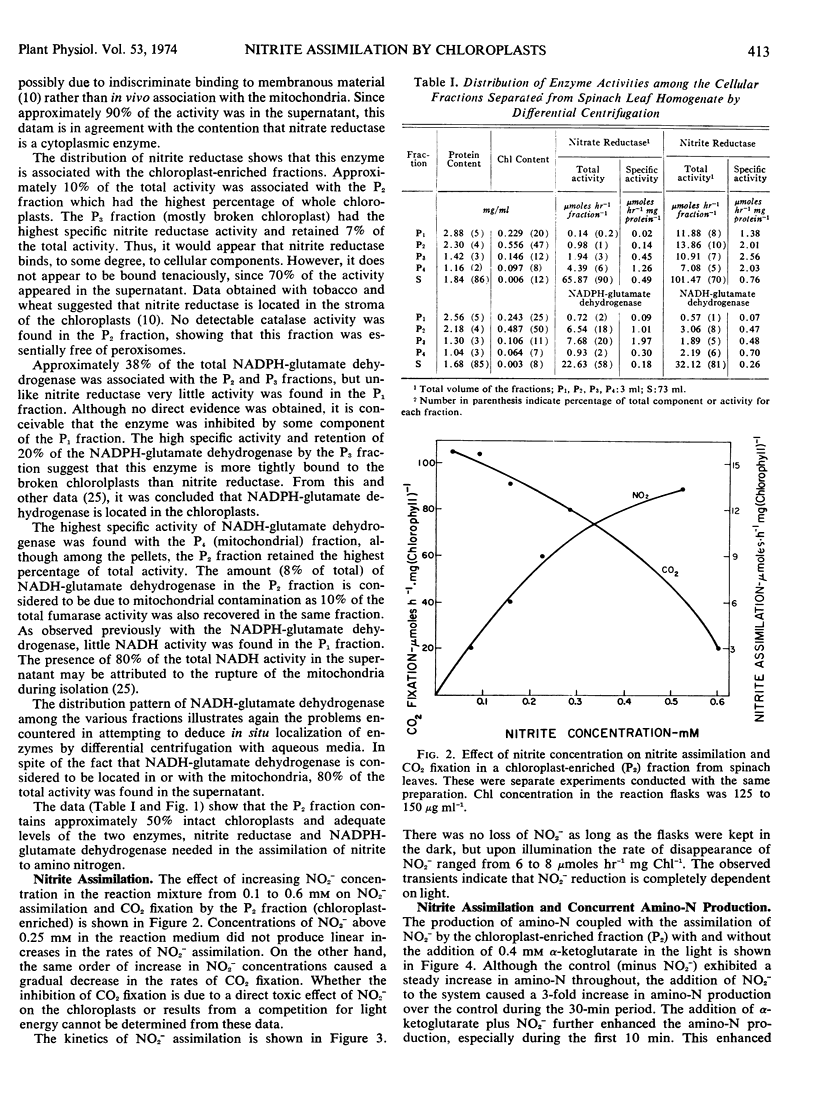
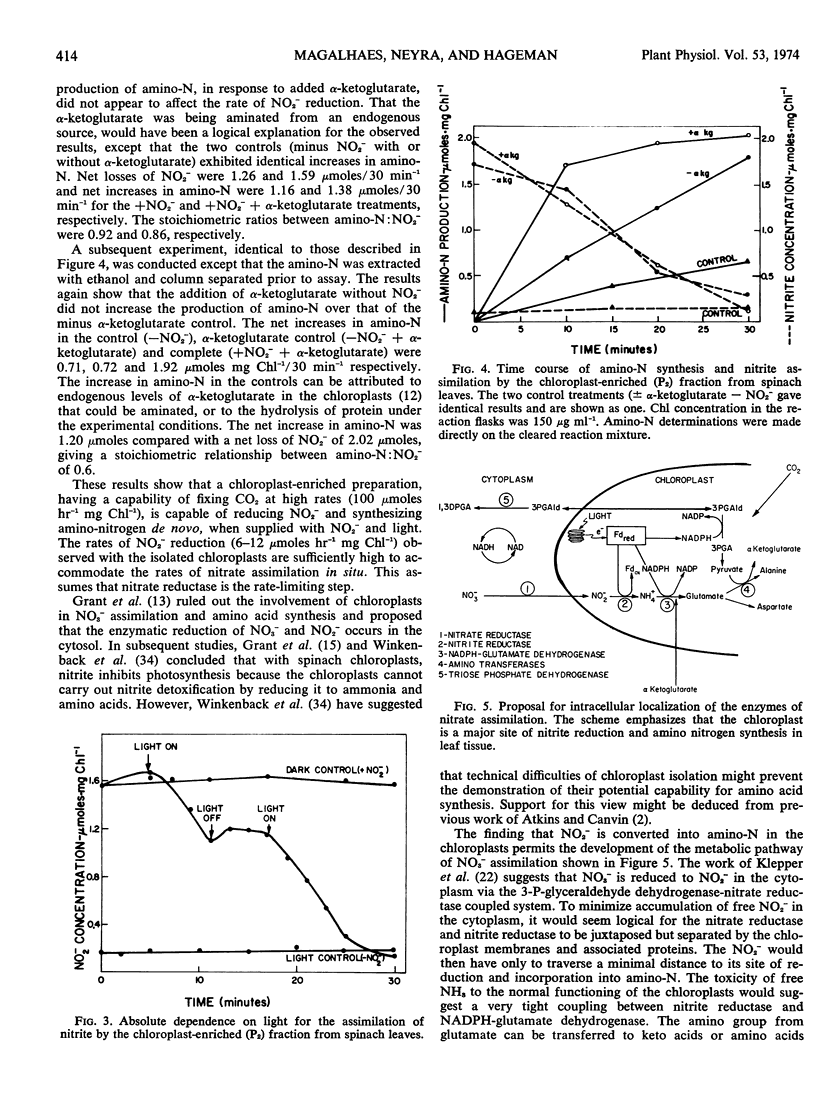
The chloroplast-enriched fraction contained the enzymes, nitrite reductase and NADPH-glutamate dehydrogenase, needed for the reduction of nitrite and incorporation of ammonia into glutamate. Kinetic studies showed that the reduction of nitrite by the chloroplast-enriched fraction is light-dependent, and the process proceeds at rates of 6 to 12 μmoles hr−1 mg chlorophyll−1. The addition of nitrite to the chloroplast preparation caused a 3-fold increase in the production of α-amino nitrogen when compared with the control without nitrite. There was a stoichiometric relation between amino-nitrogen synthesis and nitrite disappearance from the medium. The ratio of amino-nitrogen: NO2− ranged from 0.6 to 0.9. The initial rate of amino-nitrogen production was faster when α-ketoglutarate was added to the nitrite reducing chloroplast medium than when it was omitted. However, these high rates were not sustained and the total amino-nitrogen production at the end of a 30-minute period was only slightly higher. These data show that chloroplasts are functionally able and contain the enzyme complement necessary to utilize light energy for the reduction of nitrite to amino nitrogen. Thus, chloroplasts should be considered as a major site for in vivo amino-nitrogen synthesis in green plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSHAM J. A., KIRK M. PHOTOSYNTHESIS OF AMINO ACIDS. Biochim Biophys Acta. 1964 Sep 4;90:553–562. doi: 10.1016/0304-4165(64)90234-x. [DOI] [PubMed] [Google Scholar]
- BASSHAM J. A., MORAWIECKA B., KIRK M. PROTEIN SYNTHESIS DURING PHOTOSYNTHESIS. Biochim Biophys Acta. 1964 Sep 4;90:542–552. doi: 10.1016/0304-4165(64)90233-8. [DOI] [PubMed] [Google Scholar]
- BEEVERS L., FLESHER D., HAGEMAN R. H. STUDIES ON THE PYRIDINE NUCLEOTIDE SPECIFICITY OF NITRATE REDUCTASE IN HIGHER PLANTS AND ITS RELATIONSHIP TO SULFHYDRYL LEVEL. Biochim Biophys Acta. 1964 Sep 18;89:453–464. doi: 10.1016/0926-6569(64)90071-9. [DOI] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. I. Spinach and tobacco leaves. Biochim Biophys Acta. 1972 Dec 14;283(3):505–512. doi: 10.1016/0005-2728(72)90266-6. [DOI] [PubMed] [Google Scholar]
- Del Campo F. F., Ramírez J. M., Paneque A., Losada M. Ferredoxin and the dark and light reduction of dinitrophenol. Biochem Biophys Res Commun. 1966 Mar 8;22(5):547–553. doi: 10.1016/0006-291x(66)90309-3. [DOI] [PubMed] [Google Scholar]
- Givan C. V., Givan A. L., Leech R. M. Photoreduction of alpha-Ketoglutarate to Glutamate by Vicia faba Chloroplasts. Plant Physiol. 1970 May;45(5):624–630. doi: 10.1104/pp.45.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W. Glutamate dehydrogenase changes in lemna not due to enzyme induction. Plant Physiol. 1971 Mar;47(3):445–446. doi: 10.1104/pp.47.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W. Nitrogen metabolis of Lemna minor. II. Enzymes of nitrate assimilation and some aspects of their regulation. Plant Physiol. 1969 Jun;44(6):849–853. doi: 10.1104/pp.44.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstam B., Albertsson P. A. A spectrophotometric method for the quantitative estimation of intact (Class I) chloroplasts. Biochim Biophys Acta. 1970 Aug 4;216(1):220–222. doi: 10.1016/0005-2728(70)90173-8. [DOI] [PubMed] [Google Scholar]
- Kirk P. R., Leech R. M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972 Aug;50(2):228–234. doi: 10.1104/pp.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leech R. M., Kirk P. R. An NADP-dependent L-glutamate dehydrogenase from chloroplasts of Vicia faba L. Biochem Biophys Res Commun. 1968 Aug 21;32(4):685–690. doi: 10.1016/0006-291x(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Puritch G. S., Barker A. V. Structure and function of tomato leaf chloroplasts during ammonium toxicity. Plant Physiol. 1967 Sep;42(9):1229–1238. doi: 10.1104/pp.42.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Stocking C. R. Intracellular localization of enzymes in leaves and chloroplast membrane permeability to compounds involved in amino acid syntheses. Z Naturforsch B. 1969 Sep;24(9):1170–1179. doi: 10.1515/znb-1969-0915. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Phosphoglycerate as a hill oxidant in a reconstituted chloroplast system. Plant Physiol. 1971 Aug;48(2):163–165. doi: 10.1104/pp.48.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]


