Abstract
Current animal models of sepsis often incorporate antibiotics to be consistent with clinical standards for treatment of patients in the ICU. However, such experimental intervention is commonly initiated very early after infectious insult, which likely blunts the progression of systemic inflammation and downstream pathology. The objective of this study was to establish an animal model of sepsis with delayed therapeutic intervention, allowing a longer disease course and downstream pathology, but still resulting in a high survival rate. Severe lethal abdominal infection was initiated in young adult (17-18 week-old) C57BL/6 mice by cecal slurry (CS) injection. When initiated early (1- or 6-hours post-CS injection), antibiotic treatment (imipenem, 1.5mg/mouse i.p., twice/day for 5 days) rescued the majority of mice; however, few of these mice showed evidence of bacteremia, cytokinemia, or organ injury. When antibiotic treatment was delayed until late time-points (12- or 24-hours post-CS injection) the majority of animals did not survive beyond 48 hours. When fluid resuscitation (physiological saline, s.c.) was performed in combination with antibiotic treatment (twice daily) beginning at these late time-points, the majority of mice survived (75%) and showed bacteremia, cytokinemia, organ dysfunction, and prolonged body weight loss (<90% for 4 weeks). We recommend that this new repeated combination treatment with antibiotics and fluids resuscitation be initiated at a late time point after bacteremia becomes evident because this model more closely mimics the downstream pathological characteristics of severe clinical sepsis yet maintains a high survival rate. This model would be advantageous for studies on severe sepsis and post intensive care illness.
Keywords: bacteremia, cecal slurry, imipenem, mortality, mouse, peritonitis, post intensive care syndrome (PICS), survival
INTRODUCTION
Sepsis is a condition characterized by severe infection, profound inflammation, and organ dysfunction which can progress to septic shock with a significantly higher risk for mortality, as is true for approximately 60% of sepsis cases (1-3). Over recent decades, expedited identification of Systemic Inflammatory Response Syndrome (SIRS) in the ICU as well as advancements in critical care medicine have resulted in a decrease in sepsis mortality rate (4). However, sepsis remains an increasingly common illness, being the 6th most common principal reason for hospitalization in the US in 2009, surmounting to nearly 1.7 million hospital stays when sepsis as a secondary diagnosis is taken into account (5-7).
As the sepsis incidence rate continues to grow and the mortality rate in patients with sepsis and septic shock remains high, the need for a highly translational animal model has become of upmost importance. In recent years, the applicability of animal models for sepsis studies has come under question, spurred by the Seok et al. report that genomic responses to inflammatory diseases show little to no correlation among mice and humans (8). This study was highly publicized and sparked unfortunate criticism of biomedical research despite having many study limitations such as disregard of sex, age, strain, or disease severity in their data analysis (9). Takao et al. recently reanalyzed the same data sets taking into account disease conditions and utilizing more conventional statistical methods which resulted in a reversal of the conclusion: gene expression patterns are highly similar among mice and humans (10). Murine models of sepsis are in fact powerful tools for biomedical research, but come with an urgent need to understand the various strengths and weaknesses of different models which must be weighed in the context of the research question being posed (11). The choice of model is a critical decision that heavily influences the relevance of the experimental outcomes in respect to clinical translation ability (12).
To more closely mimic the clinical situation, antibiotic therapy is often included in infectious models (13-21); however the timing of therapeutic intervention is largely inconsistent due to many unresolved questions in the field regarding how animal models relate to clinical sepsis. Most of these studies administer antibiotics immediately or within a few hours after infectious insult. The time-course of sepsis is accelerated in animals compared to patients, making early intervention with antibiotic therapy a plausible therapeutic strategy, but patients are seldom treated in this narrow window (14).
We hypothesized that therapeutic intervention initiated prior to the development of bacteremia would halt the progression from local to systemic infection, thus preventing the development of severe sepsis and downstream pathology. To test this hypothesis, we first assessed the kinetics of bacteremia following infectious insult in adult mice using the cecal slurry model of abdominal sepsis. Further, we methodically determined the most appropriate timing and combination of therapies which when initiated at late time-points allows the progression of local to systemic infection, maintains sepsis pathophysiology, and still results in high survival. Here we present a new repeated combination treatment procedure initiated at a late time point which would be useful for studies on severe sepsis and post sepsis dysfunctions.
MATERIALS AND METHODS
Animals
Young adult (16 week-old) male C57BL/6 mice were obtained from The Jackson Laboratory, and acclimated for at least 7 days prior to experimentation to eliminate influence of transportation stress. Animals were maintained in the Division of Laboratory Animal Resources at the University of Kentucky in pressurized intraventilated (PIV) cages housed 5 animals per cage under controlled temperature (21-23°C), humidity (30-70%), and lighting (14/10 light/dark cycle) with free access to drinking water and chow (Teklad Global No. 2918, 18% Protein Rodent Diet, Madison WI). All experimental procedures were approved by the Institutional Animal Care and Use Committee. All animal handling techniques in these studies were performed as described and approved in our Animal Use Protocol #2009-0541 and were in accordance with the National Institutes of Health guidelines for ethical treatment.
Preparation of Cecal Slurry (CS) stock
A large-volume stock of cecal slurry (CS) was prepared and stored long-term at −80°C according to our recently reported protocol (22) with some modifications. Briefly, the ceca from donor mice were dissected, the cecal contents were removed and slurry was made by suspending them in 10% glycerol-PBS at a ratio of 1-mL for every 100mg wet cecal content weight. The slurry was passed through a series of sterile mesh strainers (860, 380, 190, 74μm; Bellco Glass Inc., Vineland NJ). For maximal recovery, a sterile pestle was used to press the slurry through each strainer. Then, under continuous stirring using a magnetic stir bar, the slurry was dispensed into cryovials (1-2mL each) and stored at −80°C (protocol flowchart in Figure 1).
Figure 1. Protocol flowchart for preparing cecal slurry (CS) stock.
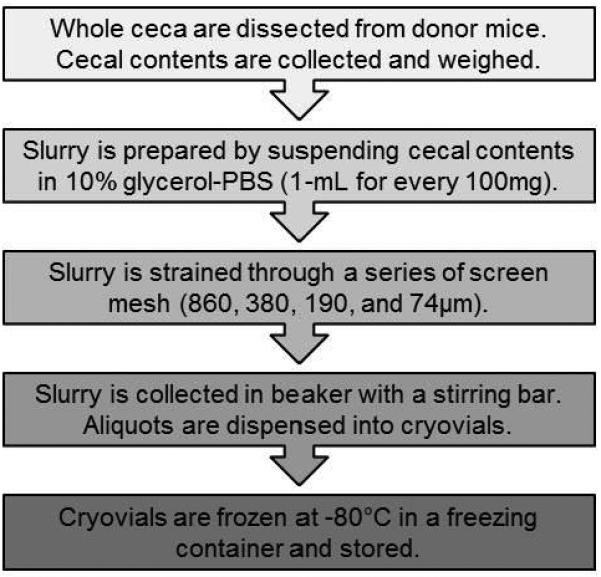
Cecal contents are collected and prepared for long-term cryopreservation according to the diagram.
This CS preparation protocol has two major modifications from our previous protocol (22). First, the concentration of glycerol in PBS was reduced from 15% to 10% to lessen possible physiological stress upon injection but still maintain bacteria viability during storage. The second modification was increasing the number of straining steps from two (860 and 190μm) to four (addition of 380 and 74μm) which removes debris from the slurry more efficiently and prevents clogging of the needle during injection.
Cecal Slurry-induced polymicrobial sepsis
Frozen CS stocks were thawed rapidly by agitating the cryovials in a 37°C water bath immediately before injection. The CS was mixed thoroughly and injected to mice intraperitoneally (i.p.) using a 25-gauge needle. In this study, the minimum lethal dose of CS was determined to be 500μL in young adult mice. This injection volume resulted in 100% mortality within 48 hours whereas 400μL only resulted in 66% mortality. Vehicle-injected controls were administered 10% glycerol-PBS (i.p.). Survival was monitored daily for at least 10 days. Health was assessed by body weight measurements and body temperature using a rectal temperature probe (P/N 4600-1.2.6 YSI, Dayton OH) daily for 5 days and continued up to 14 days. Plasma blood glucose levels were also monitored in selected experiments using the Accu-Check SmartView system which requires <1μL of blood obtained by tail prick.
Therapeutic intervention with antibiotics and fluid resuscitation
For antibiotic treatment, we used imipenem as it is a broad-spectrum antibiotic with potent capabilities for treating infection and is widely used in animal models of sepsis (15, 17, 19). Imipenem (IPM; Primaxin® I.V., imipenem 500mg stabilized in cilastatin, NDC 0006-3516-59, Merck & Co., Inc., Whitehouse Station NJ) was reconstituted in sterile physiological saline (0.9%, Ref. # 04930-04-10, Abbott Laboratories, North Chicago IL) for a final concentration of 0.005mg/mL. The dose of IPM equivalent to the maximum dose administered to patients in hospitals was determined to be 1.5 mg. Assuming young lean mice have 2-mL of blood, the final concentration of IPM in the blood (0.75 mg/mL) was used for in vitro bacteria culture and confirmed that CS bacteria (up to 500 CFU/mL) were unable to grow in the presence of IPM, even at 100-times diluted concentrations (Supplemental Digital Content 1A). IPM was aliquoted and frozen at −20°C for up to 1 week (under these storage conditions IPM maintained 95% efficacy). For each use, an aliquot was thawed in a 37°C water bath and 1.5mg IPM (300μL) was administered i.p. to mice beginning 1, 6, 12, or 24 hours after CS injection. The 1 and 6 hour groups received another treatment 12 hours post-CS injection, and all groups then received treatments every 12 hours for 5 days or until death.
In some experiments, fluid resuscitation (700μL, physiological saline, s.c.) was administered alone or in addition to the 300μL of antibiotics beginning at 12 or 24 hours after CS injection. Antibiotics and fluids were administered twice daily. Antibiotic therapy was continued for 5 days, and fluid resuscitation was continued until body temperature recovered to at least 35.0°C.
Assessment of bacteremia
Small blood samples were collected to assess bacteria load. The tail vein was wiped with alcohol, nicked with a sterile razor blade, and 10μL of blood was collected, immediately diluted in 90μL of sterile saline, and spread onto agar plates containing 3.7% w/v brain-heart infusion broth and 1.5 % w/v agar (Product Number 211059 and 214530, respectively, Becton, Dickenson and Company, Sparks, MD). In selected experiments, half of the blood sample was spread onto agar plates containing antibiotics (2 mg/mL IPM), and the remaining half of the blood sample was spread on plates without antibiotics. Plates were incubated at 37°C for 24 ± 2 hours, colonies were counted, and CFU was calculated.
Assessment of cytokinemia and organ injury
To assess cytokinemia, animals were injected with either CS or vehicle (10% glycerol-PBS). The animals which received CS injection were subdivided into four groups: nonresuscitated, 1h antibiotics, 6h antibiotics, and 12h antibiotics with fluid resuscitation. All animals were euthanized 24 hours after CS or vehicle injection. Mice were anesthetized by isoflurane (NDC 66794-017, Piramal Critical Care Inc., Bethlehem PA) inhalation (5% in air for induction, 2.5% to maintain) and blood was collected from the inferior vena cava with 10% volume of 0.1M sodium citrate to prevent clotting. Plasma samples were obtained by immediately centrifuging blood at 4°C which were then stored at −80°C and subsequently analyzed for interleukin-6 (IL-6) levels by ELISA (Thermo Scientific Ref # EM2IL6, Frederick MD), and IL-10, IL-1β, and TNF-α by multiplex assay (Meso Scale Discovery Catalog #K15048G, Rockville, MD).
For histological analysis of lung injury, lungs were slowly infused with 10% buffered formalin phosphate (SF100, Fisher Scientific, Fair Lawn NJ) from the trachea immediately after euthanasia as we previously described (23, 24). After fixation in 10% buffered formalin phosphate for 24 hours, the tissues were transferred to PBS (Fisher BioReagents BP6651-1, Fair Lawn NJ) and embedded in paraffin. Tissue sections (5μm thick) were cut, mounted on glass slides, and stained with hematoxylin and eosin (H&E). Photomicrographs were taken (Nikon Eclipse E200 microscope, Nikon digital Sight DS-U3/DSFil digital software integrated with NIS Elements F3.2 Imaging Software), and histology was scored in a blinded semi-quantitative fashion adapted from the established method by Hirano et al. (25) with slight modifications. Scoring was conducted for the degrees of (A) alveolar thickening, (B) cellular infiltration, (C) hemorrhage, (D) presence of debris, and (E) cellular hyperplasia with each assigned a score: 0 (normal; absence of markers), 1 (mild; sporadic pathological markers), 2 (moderate; frequent pathological markers) or 3 (most severe; ubiquitous pathological markers) which were totaled for a final score from 0 to 15.
For biochemical analysis of liver damage, plasma alanine aminotransferase (ALT) levels were quantified using a commercially available assay kit (Alanine Aminotransferase (ALT or SGPT) Activity Colorimetric/Fluorometric Assay Kit, Biovision Catalog #K752-100).
Statistical analysis
Survival curves were analyzed by Kaplan Meier LogRank test. Data for two-group comparisons were analyzed by Student's t-test. When multiple comparisons were made, the Shapiro-Wilk normally test was run. If the data passed the normality test, one-way ANOVA and Holm-Sidak post-hoc test were used to analyze the data. Alternatively, when the data was not normally distributed, the Kruskal-Wallis test and Dunn's post-hoc test were used. In instances where one group was assessed multiple times (i.e. bacteria load), repeated-measures one-way ANOVA was used, and in times when multiple groups were assessed multiple times (i.e. body temperature data), repeated-measures two-way ANOVA was used and the Holm-Sidak post-hoc test was run. All data are expressed as means and standard deviations and p<0.05 was considered statistically significant.
RESULTS
The majority of animals cannot be rescued by delayed antibiotic treatment initiated after all animals have detectable bacteremia
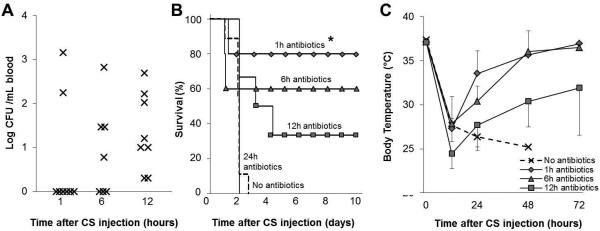
First, we determined the kinetics of bacteremia in adult mice after CS-mediated severe infection. Severe infection was initiated by administration of a minimum lethal dose of CS (500μL, see Materials and Methods) and no therapeutic intervention was performed. Circulating bacteria was assessed 1, 6, and 12 hours after CS injection. Of the 8 animals assessed, only 2 (25%) had circulating bacteria by 1 hour, which increased to 4 (50%) by 6 hours, whereas all 8 (100%) animals had bacteremia by the 12-hour time-point (Figure 2A). Based on these data, we defined “early intervention” time-points as ≤6 hours after CS injection at which time bacteremia is not always confirmed, and alternatively “late intervention” time-points as ≥12 hours after CS injection when 100% of animals have circulating bacteria.
Figure 2. Survival rate and disease severity correlate with time of intervention after CS injection.
All mice were given a minimum lethal dose of cecal slurry (CS; 500μL). (A) Blood bacteria load was assessed 1, 6, and 12 hours after CS injection in animals (n=8) in animals which did not receive therapeutic intervention. (B-C) In another experiment, animals received antibiotic treatment (imipenem, IPM; 1.5mg i.p.) beginning 1, 6, 12, and 24 hours after CS injection. Antibiotics were continued twice daily for five days (n=5-6 per group). (B) Survival (star notates p<0.05 compared to no-antibiotics group) and (C) body temperature were monitored. Data represent mean ± standard deviation.
Next, we investigated whether mice can be rescued from lethal sepsis if antibiotic therapy is initiated after blood bacteria became detectable in all animals. The potent broad-spectrum antibiotic imipenem (1.5mg/mouse i.p.) was administered to mice beginning at late time-points (12 and 24 hours after CS injection) and the treatment was repeated twice daily for 5 days. As a comparison, some mice also received antibiotic treatment beginning at early time-points (1 and 6 hours after CS injection). A significant improvement in survival (80%, p<0.05 compared with no antibiotics group) was observed when antibiotic treatment was begun 1 hour after CS injection, and the majority of animals (60%) were also rescued when antibiotics were initiated at the 6-hour time-point. However, only 33% (2 of 6) of animals were rescued when initiation of antibiotic treatment was delayed to 12 hours, and no animals survived when delayed further to 24 hours (n=5; Figure 2B). Important to note, the blood bacteria of moribund mice showed no resistance to the antibiotic (Supplementary Digital Content 1B). Early intervention (starting at 1 and 6 hours) resulted in rapid recovery of body temperature (35.7± 2.3, 36.0 ± 1.9°C respectively) within 48 hours, whereas mice with late intervention developed sustained hypothermia (30.4±2.7°C at 48 hours) indicating that late intervention allows for a prolonged disease time-course (Figure 2C). Taken together, bacteremia does not develop in all mice until 12 hours after CS injection and therefore late therapeutic intervention should be initiated at this time or later. However, antibiotic treatment alone cannot rescue the majority of mice if delayed until these late time-points.
Late therapeutic intervention with a combination of antibiotics and fluid resuscitation rescues the majority of animals
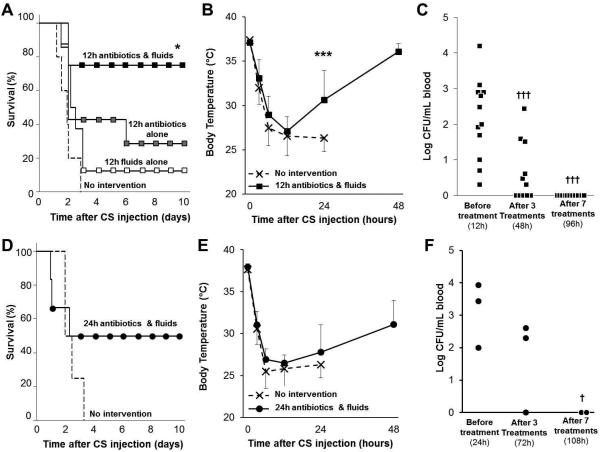
We aimed to elucidate a therapeutic protocol which could be initiated after development of bacteremia and still achieve a high survival rate. Antibiotic treatment alone (i.p.), fluid resuscitation alone (s.c.), or a combination of both were administered to septic mice beginning 12 hours after CS injection (500 μL) and continued twice daily for 5 days. Without therapeutic intervention, 100% (5 out of 5) mortality was observed by 48 hours confirming our earlier results shown in Figure 2. Neither antibiotic therapy nor fluid resuscitation alone could rescue a significant number of animals (2 out of 7 and 1 out of 8, respectively). Conversely, combination treatment with antibiotics and fluid resuscitation when initiated 12 hours after CS injection resulted in 75% (6 out of 8) survival rate (Figure 3A). Late intervention with this combination therapeutic strategy resulted in significantly increased body temperature 12 hours after the first treatment (i.e. 24 hours after CS injection; p<0.01 compared to no-intervention group) although body temperature did not fully recover until 48 hours after CS injection (36 hours after therapeutic intervention; 36.1±1.3°C; Figure 3B). Assessment of blood bacteria load 12 hours after CS injection confirmed that bacteremia had developed in all mice before therapeutic treatment was initiated. After 3 treatments with antibiotics and fluid resuscitation (administered every 12 hours), bacteria load was significantly reduced and was completely resolved in all animals after 7 treatments (96 hours after CS injection; Figure 3C). Further, because mice with sepsis tend to develop hypoglycemia, we tested the effects of glucose control by administering 1-2mg of glucose in the resuscitation fluid to animals with glucose levels below 75mg/dL. Although the plasma glucose level was transiently increased, hypoglycemia persisted and survival rate was not improved by inclusion of this treatment (data not shown).
Figure 3. Late intervention with antibiotics and fluid resuscitation rescues the majority of mice from otherwise lethal sepsis.
All animals were given a lethal dose of cecal slurry (CS). Mice received antibiotic treatment alone (imipenem, IPM; 1.5mg i.p.), fluid resuscitation alone (1mL physiological saline, s.c.), or a combination treatment of antibiotics and fluid resuscitation (n=5-9 per group) beginning 12 or 24 hours after CS injection. Therapeutic treatment was continued twice daily for five days. (A, D) Survival and (B, E) body temperature were monitored for multiple days. Data is represented as mean ± standard deviation. (C, F) Circulating bacteria load was assessed in the combination treatment groups by culturing blood obtained by micropuncture of the tail vein immediately before the first therapeutic treatment (12, 24h), after 3 treatments (48, 72h) and after 7 treatments (96, 108h). Symbols * and *** represent p<0.05 and p<0.001 respectively compared to no intervention group; † and †† represent p<0.05 and p<0.01 respectively compared to bacteria load before intervention (i.e. 12 and 24h).
To determine whether high survival is still achievable if therapeutic intervention was postponed even further, combination treatment with antibiotic and fluid resuscitation was delayed until 24 hours after CS injection. This therapeutic timeline resulted in 50% 10-day survival rate (Figure 3D). The survivors of this delayed intervention exhibited prolonged hypothermia for 48 hours post-CS injection (Figure 3E). Animals recovered body temperature (36.5±0.8°C) 96 hours after CS injection (72 hours after therapeutic intervention). Bacteremia was confirmed at 24 hours after CS injection and bacteria load was reduced after 3 treatments with therapeutics (72 hours after CS injection) and was resolved after 7 treatments (108 hours after CS injection, Figure 3F), similar to the trend observed in the animals that were resuscitated beginning at 12 hours post-CS injection.
Delayed, but not early, therapeutic intervention allows for development of cytokinemia, organ dysfunction, and sustained body weight loss
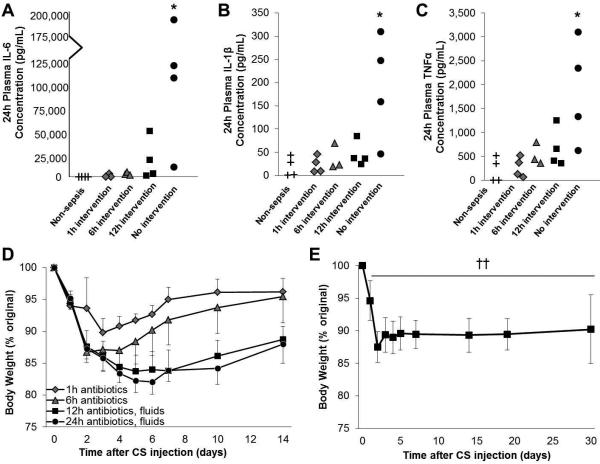
Next we aimed to determine if delayed therapeutic intervention resulted in clinically-relevant characteristics of severe sepsis. Severe infection was initiated by CS injection and animals received either early (≤6 hours) intervention with antibiotics or late (≥12 hours) intervention with combination treatment of antibiotics and fluid resuscitation. Plasma samples were prepared at time of euthanasia (24 hours after CS injection) and interleukin-6 (IL-6), interleukin 1β (IL-1β), and tumor necrosis factor α (TNFα) levels were assayed. As shown in Figure 4, non-resuscitated animals had significantly higher levels of all three proinflammatory cytokines 24 hours after CS injection (the time at which half of animals could still be rescued using repeated antibiotic and fluid treatment) compared to non-sepsis controls (p<0.05). Although significance was not achieved, on average the 12h resuscitated group had elevated cytokine levels compared to controls (18,028 pg/mL vs. nondetectable IL-6 levels, 46.1 vs 18.4 pg/mL IL-1β levels, and 67.1 vs. 5.0 pg/mL TNFα levels). Similarly, the 12h resuscitated group had higher cytokine levels on average compared to that of the early (≤6 hours) resuscitated groups. A similar trend was observed without statistical significance when the anti-inflammatory cytokine interleukin 10 (IL-10) levels were compared among the groups (Supplemental Digital Content 2).
Figure 4. Late, but not early, therapeutic intervention results in cytokinemia and prolonged reduction in body weight.
Severe infection was induced by cecal slurry (CS) injection and mice were divided into groups (n=4-8). Mice received antibiotic treatment (imipenem, 1.5 mg/mouse, i.p.) beginning 1 or 6 hours after CS injection, or combination treatment with antibiotics and fluid resuscitation (physiological saline, s.c.) beginning 12 hours later. A group of animals (n=4) received vehicle injection (10% glycerol-PBS) for comparison. (A-C) Plasma samples obtained 24 hours after CS injection were subjected to IL-6, IL-1β, and TNFα quantification. (D) Body weight was monitored for 14 days after CS injection (n=5-9). (E) In a separate experiment, the body weight of animals resuscitated with antibiotics and fluid resuscitation beginning 12 hours after CS injection was monitored up to 30 days post-CS injection (n=7). * indicates p<0.05 by one-way ANOVA and †† indicates p<0.01 at all time-points compared to baseline (paired student's t-test).
In order to assess potential long-term effects of delaying therapeutic intervention, the body weight of each mouse was monitored for 14 days after CS injection. Mice which received early intervention (starting at 1h or 6h) experienced rapid recovery of body weight (> 95% or >90% of original body weight by 7 days, respectively), whereas delayed intervention (starting at 12h or 24h) resulted in long term body weight depression (<85% original body weight by day 7, and <90% by day 14, p<0.05 early time-points vs late time-points; Figure 4D). In another experiment under the same sepsis and therapeutic conditions, we confirmed that the sepsis-induced reduction in body weight (<90% original) persisted for at least 4 weeks (Figure 4E). Delayed therapeutic intervention also resulted in abscess formation which was identified in ~50% of animals two weeks after sepsis induction (Supplementary Digital Content 3).
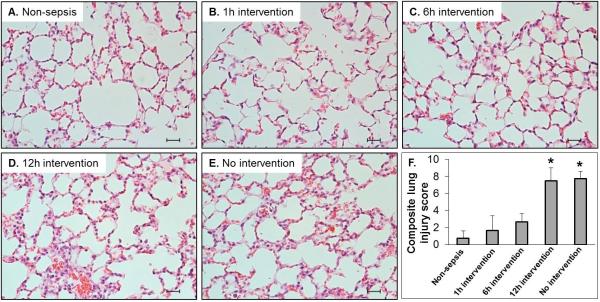
To evaluate the effect of timing of therapeutic intervention on sepsis-induced organ injury, analysis of lung injury of animals euthanized 24 hours after CS injection were histologically examined. As shown in Figure 5, H&E staining of tissue sections revealed that late resuscitation, started ≥12 hours after CS injection, resulted in pathological abnormalities including infiltration of inflammatory cells, marked edema in the interstitial space, and thickened alveolar walls. The average composite lung injury score of 12-hour intervention group (7.5) was equivalent to the non-intervention group's average score (7.8), both being much higher than the control group (0.8). Conversely, animals which received early intervention (1 or 6 hours after CS injection) showed few markers of inflammation (average composite score of 2.2). All composite lung injury scores in these early intervention groups were less than 4 without statistical significance compared to non-sepsis control group, whereas the inflammatory scores of the 12 hour and non-resuscitated groups were significantly higher compared to the early resuscitated groups (1 and 6 hours) (p<0.001).
Figure 5. Early, but not late, therapeutic intervention prevents sepsis-induced lung injury.
Mice received either cecal slurry (CS; n=14) or vehicle (10% glycerol; n=4) injection (i.p.). CS-injected mice were divided among the following groups: nonresuscitated, 1h antibiotics, 6h antibiotics, or 12h combination treatment with antibiotics and fluid resuscitation (n=3-4 per group). All animals were euthanized 24 hours after CS or vehicle injection. (A-E) Lung tissue sections stained with H&E. Representative images (400X, scale bar represents 50 μm) are shown for each group. (F) Hisopathological scoring (n=3-4 per group). *** represents p<0.001.
To further assess the effect of delaying therapeutic intervention on organ injury, plasma alanine aminotransferase (ALT), a commonly used marker of liver damage, was quantified in the samples obtained 24 hours after CS or vehicle injection. As displayed in Figure 6, the ALT levels of animals which received delayed intervention (12 hours after CS injection) was comparable to those which did not receive therapeutics (63.2 and 69.9 ng/μL, respectively) (p<0.05) which were both statistically higher than the vehicle injected non-sepsis controls (14.2 ng/μL). ALT levels in animals which received early therapeutic intervention (1 and 6 hours after CS injection) showed moderate but non-significant ALT increases compared to controls. Together, these data suggest that delaying intervention to late time-points allows pathological characteristics of severe sepsis to develop, which were not observed in early intervention groups.
Figure 6. Delayed therapeutic intervention results in heightened plasma ALT levels.
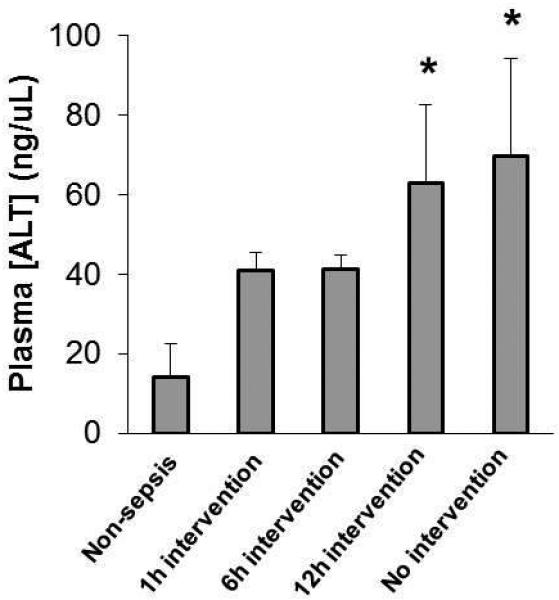
Animals were divided among treatment groups as described in Figure 5. Plasma samples obtained 24 hours after CS injection were subjected to ALT quantification by colorimetric assay (n=3-4 per group). * indicates p<0.05.
DISCUSSION
To mimic the clinical situation, antibiotics are often included in animal models of sepsis. However, most of the studies using these animal models intervene either immediately or within a few hours after infectious insult. Since previous studies have not evaluated the effect of the timing of therapeutic intervention in relation to timing of bacteremia after induction of severe infection in animal models, there has been a question on whether therapeutic intervention is conducted in a manner to maximize survival without allowing severe sepsis to develop. We hypothesized that intervention, if initiated before the development of bacteremia, would halt the progression from local to systemic infection and block downstream pathology otherwise characteristic of severe sepsis. On the other hand, we believed that delaying intervention until after bacteria is detectable in circulation would allow propagation of the inflammatory cascade and organ damage, but still result in a high survival rate if the therapeutic strategy is aggressive.
For this study, the cecal slurry (CS) injection model was ideal due to being highly time efficient, allowing the induction of sepsis in a large number of animals within a short period of time (26-28). Additionally, it is highly reproducible using our new CS preparation protocol (22, 29). Using this model, we showed that bacteria are not always detectable in the circulation of animals until 12 hours after injection with a lethal dose of CS. Only 25% of animals were positive for circulating bacteria 1 hour after CS injection, and even 6 hours after CS injection, 50% of animals remained negative for circulating bacteria.
Using these data, we attempted to establish an intervention model which is initiated after all animals are positive for bacteria in the circulation (i.e. ≥12h after sepsis induction). Initiating antibiotic therapy prior to this time-point resulted in relatively high survival rates: i.e. intervention at 1 and 6 hours resulted in 80 and 60% survival, respectively, which is in agreement of the study by Gonnert et al. in which antibiotic therapy initiated 2 hours after CS injection resulted in a 50% survival rate (30). However, our data indicate that many of these animals did not develop sepsis at such early time points. When we delayed intervention until late time-points (≥12 hours when bacteremia is apparent in all mice), antibiotic treatment alone could not rescue the majority of animals and >67% died within 4 days. Importantly, the blood bacteria of moribund animals (determined by body temperature less than 32°C for over 24 hours following CS injection) was unable to grow in the presence of IPM when cultured on agar plates containing the antibiotic. This evidence verifies that mortality in this model is not a result of antibiotic resistance of the circulating bacteria.
We then attempted to increase survival rates of animals under late intervention by including fluid resuscitation to reduce hypotension, another lethal component of sepsis. In attempt to mimic the continuous fluid resuscitation state without the use of anesthesia, we conducted fluid resuscitation via subcutaneous injection two times a day. We observed a significant improvement in survival rate (≥75% of animals with otherwise completely lethal sepsis) when antibiotic treatment and fluid resuscitation were given in combination starting even 12 hours after sepsis induction. This 75% survival rate by the combined treatment is a striking improvement compared to 29% by antibiotics alone and 13% by fluid resuscitation alone. Our recent study confirmed that this resuscitation protocol can effectively rescue approximately 70% of older mice as well (data not shown). Importantly, when the combined therapeutic intervention was further delayed for a full 24 hours after CS injection, half of the animals could still be rescued using this combination treatment. In these experiments, some of the animals with the most severe bacteremia could be rescued using the combination treatment. This demonstrates the effectiveness of this resuscitation protocol, especially since our group previously showed that without therapeutic intervention, bacteremia following CS injection did correlate with 15 day survival (22).
In this study, we also provided data demonstrating that animals with late intervention, but not early intervention, develop pathophysiological conditions that are characteristics of sepsis. We found that intervening after bacteria was confirmed in circulation (≥12 hours after CS injection) resulted in the elevation of plasma IL-6, IL-1β, and TNFα levels 24 hours after CS injection when compared to animals in which therapeutic intervention was initiated at early (1 and 6 hours) time-points. Further, late intervention resulted in marked lung injury, characterized by inflammatory cells, thickened alveolar walls, and edema, as well as liver damage assessed by heightened plasma ALT levels. We also found that surviving animals of the late intervention groups exhibited an inability to recover body weight for four weeks after sepsis. Being that the majority of animals showed no signs of body weight recovery beyond the 1 week time-point, it is highly likely that these mice will have reduced body weight beyond this 4-week time-point. Therefore, we conclude that experimental sepsis model with late intervention allows good survival rates with development of severe sepsis, organ injury, and long-term body weight reduction, as opposed to sepsis models with early intervention which tend to blunt pathophysiological processes, and sepsis models without intervention which result in high early mortality (Figure 7).
Figure 7. Early vs. Late therapeutic intervention: A comparison of sepsis outcomes after severe infection in murine models.
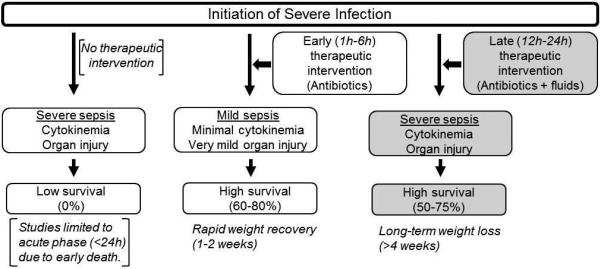
Late therapeutic intervention starting 12 hours after severe infection allows development of severe sepsis with organ injury and long-term weight reduction.
We predict that this late therapeutic strategy would be highly applicable to other animal models of sepsis, including the widely used CLP model (21, 31). The CS and CLP models are similar in that they induce severe intra-abdominal infections via exposure to endogenously-derived polymicrobial bacteria. Indeed, many have found that antibiotic administration after CLP significantly improves survival (15, 17, 18, 21), especially in the young (13). When administration of antibiotics was delayed until 12 hours after CLP and given every 12 hours for 5 days, animals with IL-6 levels >14,000 pg/mL at 6 hours were unable to be rescued (14). In our model, the average IL-6 level was 106,445 pg/mL 24 hours after CS injection (non-resuscitated group), which is presumably lower than levels at the 6 hours. When antibiotics alone were administered beginning at this time point, we too found that animals were not able to be rescued. However, when we used combination treatment of antibiotics and fluid resuscitation, half of the animals were able to be rescued, despite having 7-times greater IL-6 levels than the cutoff previously established. Thus, we have reason to believe that if this resuscitation strategy were applied to the CLP model, similar survival rate would be achieved. Important to note, the development of bacteremia in the CLP model is presumably slower than what we observed in the CS model because the cecal contents may more slowly leak into the peritoneum in the CLP model as opposed to the bolus injection of cecal contents in the CS model. Therefore, the more clinically relevant appropriate time of intervention may need to be determined and therapeutic treatments may need to be continued for more than 5 days. In our current study, we chose to use CS model instead of CLP model partly because of the concern that CLP-induced sepsis outcome could be affected by repeated intraperitoneal injection of antibiotics. It was previously observed that daily intraperitoneal injection with saline after CLP significantly increased mortality rates in mice (13), which is possibly explained by a disruption of abscess formation (i.e. wound healing) by the fluid at the site of cecal puncture. In our model, abscesses were present in the peritoneum in at least half of the animals which received CS injection despite numerous intraperitoneal injections of the antibiotic.
To apply this late-intervention model to other sepsis models or even different experimental conditions (i.e. different age, strains, or gender), we recommend that the kinetics of bacteremia following infectious insult should first be assessed before deciding the time point for therapeutic intervention. For example, bacteremia was confirmed in all mice just 2 hours following CS injection to 5-7 day-old neonate mice (32), indicating that intervention at 12 hours may be too late and thus not effective in this case. It is our opinion that this protocol, combination treatment with antibiotics and fluid resuscitation performed twice daily for multiple days, should be initiated only after bacteremia is confirmed to ensure the development of sepsis and a longer disease course.
Although this late-intervention protocol is multifactorial, we acknowledge that this is not a comprehensive therapeutic strategy. The infection and hypotension aspects of sepsis are targeted therapeutically in this protocol, as they are primary concerns clinically. Although we did not characterize which bacteria grew in the blood cultures or the peritoneum, we confirmed that the bacteria in both the CS stocks and blood bacteria were not resistant to IPM. We also examined the efficacy of glycemic control in our late-intervention model with antibiotic treatment and fluid resuscitation; however it showed no further benefit to survival outcome. In this study we did not include ventilatory support, but predict that it would be beneficial not only for clinical relevance but also for survival. However, we aimed to develop a therapeutic strategy which could be adopted by many laboratory groups, and prolonged murine ventilation is a technically challenging technique which requires specialized equipment and also adds variables (such as prolonged use of anesthesia (33, 34)) to the experiment which is often undesirable, but would be worthwhile to include in future experiments.
In conclusion, to the best of our knowledge, this is the first study which examined the effect of therapeutic intervention in relation to the kinetics of the development of bacteremia following infectious insult in an animal model of sepsis. Here we elucidate a late-intervention combination treatment protocol in which antibiotic therapy and fluid resuscitation are repeatedly administered and results in significantly improved survival after bacteremia, cytokinemia, and organ damage. This study highlights the importance of (1) inducing a severe infection that would otherwise be completely lethal without therapeutics, and (2) delaying intervention until later time-points to ensure that progression from local to systemic infection is achieved, as is profound cytokinemia and organ damage (characteristics of severe clinical sepsis). As summarized in Figure 7, late intervention with combination therapeutics after infectious insult has several advantages over existing sepsis models in which intervention is either not performed or initiated at early time points. We recommend use of this new procedure as it will allow for more clinically relevant studies on severe sepsis and on investigating post intensive care illness.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Health (R01 AG039732 and F31 GM117868). This study was presented at a poster session of the 39th Annual Conference on Shock, where Allison M. Steele received a Travel Award. We thank Ms. Dana Napier and the Biospecimen Procurement and Translational Pathology Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558) for lung tissue processing and the Center for Clinical and Translational Science for performing the multiplex assay (supported by Grant UL1TR001998). We also thank Dr. Arnold Stromberg at the Department of Statistics of the University of Kentucky for his helpful advice and certification of our statistical analyses. Additionally, the authors would like to recognize Mr. Damon Wallace for biochemical analysis assistance and Ms. Beverly Balasuriya for her help with animal monitoring.
Footnotes
Conflict of interest: none
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–61. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The N Engl J of Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–74. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 6.Elixhauser A, Friedman B, Stranges E. HCUP Statistical Brief #122 Online. Agency for Healthcare Research and Quality; 2011. Septicemia in U.S. Hospitals, 2009. [PubMed] [Google Scholar]
- 7.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(suppl 7):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 8.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, et al. Abandon the mouse research ship? Not just yet! Shock. 2014;41(6):463–75. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2015;112(4):1167–72. doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deitch EA. Rodent models of intra-abdominal infection. Shock. 2005;24(suppl 1):19–23. doi: 10.1097/01.shk.0000191386.18818.0a. [DOI] [PubMed] [Google Scholar]
- 12.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9(1):1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19(4):310–3. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull IR, Javadi P, Buchman TG, Hotchkiss RS, Karl IE, Coopersmith CM. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock. 2004;21(2):121–5. doi: 10.1097/01.shk.0000108399.56565.e7. [DOI] [PubMed] [Google Scholar]
- 15.Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10(2):110–7. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Xiao H, Remick DG. Correction of perioperative hypothermia decreases experimental sepsis mortality by modulating the inflammatory response. Crit Care Med. 2005;33(1):161–167. doi: 10.1097/01.ccm.0000151049.19253.54. [DOI] [PubMed] [Google Scholar]
- 17.Coopersmith CM, Amiot DM, 2nd, Stromberg PE, Dunne WM, Davis CG, Osborne DF, Husain KD, Turnbull IR, Karl IE, Hotchkiss RS, Buchman TG. Antibiotics improve survival and alter the inflammatory profile in a murine model of sepsis from Pseudomonas aeruginosa pneumonia. Shock. 2003;19(5):408–14. doi: 10.1097/01.shk.0000054370.24363.ee. [DOI] [PubMed] [Google Scholar]
- 18.Brown I, Bellevue O, Shawo A, Woldesemayat H, Lyo V, Rayikanti B, Lee M, Uzosike ED, Kasravi S, Harris HW. Low-dose cyclophosphamide improves survival in a murine treatment model of sepsis. Shock. 2015;43(1):92–8. doi: 10.1097/SHK.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marques M, Perre S, Aertgeerts A, Derde S, Guiza F, Casaer MP, Hermans G, Van den Berghe G, Langouche L. Critical illness induces nutrient-independent adipogenesis and accumulation of alternatively activated tissue macrophages. Crit Care. 2013;17(5):R193. doi: 10.1186/cc12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanotti-Cavazzoni SL, Guglielmi M, Parrillo JE, Walker T, Dellinger RP, Hollenberg SM. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med. 2009;35(4):748–54. doi: 10.1007/s00134-008-1360-9. [DOI] [PubMed] [Google Scholar]
- 21.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94(2):331–5. [PubMed] [Google Scholar]
- 22.Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE. 2014;9(12):e115705. doi: 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic Biol Med. 2011;50(2):371–80. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamura D, Starr ME, Lee EY, Stromberg AJ, Evers BM, Saito H. Age-dependent vulnerability to experimental acute pancreatitis is associated with increased systemic inflammation and thrombosis. Aging Cell. 2012;11(5):760–9. doi: 10.1111/j.1474-9726.2012.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M, Khader A, Wang P. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care. 2015;19:53. doi: 10.1186/s13054-015-0782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Szpila BE, Cuenca AG, Joseph A, Moore FA, Leeuwenburgh C, et al. Host responses to sepsis vary in different low-lethality murine models. PLoS ONE. 2014;9(5):e94404. doi: 10.1371/journal.pone.0094404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, Mele T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Research Notes. 2014;7:233. doi: 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sam AD, 2nd, Sharma AC, Law WR, Ferguson JL. Splanchnic vascular control during sepsis and endotoxemia. Front Biosci. 1997;2:e72–92. doi: 10.2741/a229. [DOI] [PubMed] [Google Scholar]
- 29.Starr ME, Steele AM, Cohen DA, Saito H. Short-Term Dietary Restriction Rescues Mice From Lethal Abdominal Sepsis and Endotoxemia and Reduces the Inflammatory/Coagulant Potential of Adipose Tissue. Crit Care Med. 2016;44(7):e509–19. doi: 10.1097/CCM.0000000000001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonnert FA, Recknagel P, Seidel M, Jbeily N, Dahlke K, Bockmeyer CL, Winning J, Losche W, Claus RA, Bauer M. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J Surg Res. 2011;170(1):e123–34. doi: 10.1016/j.jss.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(suppl 1):52–7. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 32.Wynn JL, Scumpia PO, Delano MJ, O'Malley KA, Ungaro R, Abouhamze A, Moldawer LL. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28(6):675–83. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 33.Muller-Redetzky HC, Will D, Hellwig K, Kummer W, Tschernig T, Pfeil U, Paddenberg R, Menger MD, Kershaw O, Gruber AD, et al. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Crit Care. 2014;18(2):R73. doi: 10.1186/cc13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schellekens WJ, van Hees HW, Linkels M, Dekhuijzen PN, Scheffer GJ, van der Hoeven JG, Heunks LM. Levosimendan affects oxidative and inflammatory pathways in the diaphragm of ventilated endotoxemic mice. Crit Care. 2015;19:69. doi: 10.1186/s13054-015-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.