Abstract
Background/Objectives
Behavioral and psychological symptoms of dementia (BPSD) are nearly universal in dementia and associated with multiple negative outcomes. Current real-world management is largely pharmacologic, despite poor risk/benefit. The WeCareAdvisor™ was designed to enable family caregivers to assess, manage and track BPSD using nonpharmacologic strategies.
Design
In-depth qualitative data were collected from family caregivers of people with dementia in order to inform: 1) style of approach and “look and feel” of the tool, and 2) the types of psycho-education most needed by caregivers.
Results
We conducted four focus groups and a technology survey (n=26) as well as additional individual semi-structured interviews (n=12) with family caregivers. Main themes of the qualitative work included: 1) need to minimize difficulty and training time; 2) importance of “one-stop shopping” for information; and 3) necessity for information to be tailored to the caregiver and person with dementia. This information was then combined with effective existing evidence-based behavioral strategies to create a web-based tailored caregiver-support tool.
Conclusions
The WeCareAdvisor™ was designed with input on functionality and content by end-users, family caregivers. The randomized controlled trial of WeCareAdvisor™ will test whether the tool improves outcomes including caregiver upset and burden and frequency and severity of BPSD.
Keywords: neuropsychiatric symptoms of dementia, non-pharmacologic approaches, informal caregivers
Introduction
Dementia is a devastating syndrome affecting over 5 million people in the US, and potentially affecting16 million people by 2050 1. Although cognitive impairment is the clinical hallmark of dementia, non-cognitive behavioral and psychological symptoms (BPSD) are exceedingly common (affecting 98% of individuals at some point in the illness course) and often dominate disease presentation 2. BPSD including depression, psychosis, psychomotor agitation, aggression, apathy, sleep disturbances, and inappropriate behaviors occur in dementia of all types 3. BPSD appear to be a consequence of multiple, but sometimes modifiable, interacting factors internal and external to the person with dementia including patient (e.g. undiagnosed medical conditions and untreated pain), caregiver (e.g. ineffective communication style) and environmental (e.g. overstimulation or lack of activity/structure) factors4.
BPSD, as opposed to core cognitive symptoms, create the most difficulties for patients, caregivers, and providers, and commonly lead to earlier nursing home placement4. Other negative outcomes include excess morbidity, mortality, and hospital stays, caregiver stress and depression, and reduced caregiver employment income4. Thirty percent of the cost of caring for community-dwelling patients with dementia is directly attributable to BPSD management 5. Managing BPSD is one of the most challenging aspects of caring for a person with dementia, causing intense caregiver burden and upset6. Caregivers of individuals with BPSD are more distressed and depressed than those not managing behaviors 7.
In real-world settings, few treatment options are currently available to family caregivers for BPSD. Typically, if a caregiver expresses concern about a BPSD to a physician, a psychiatric medication is prescribed to try to control these symptoms. However, the FDA has not approved any medications for BPSD, and the medication class with the strongest evidence for effect for BPSD, antipsychotics, demonstrates limited efficacy at best 8. Further, antipsychotic use in dementia has been shown to have significant risks including mortality9 resulting in FDA black box warnings. Additionally, BPSD can be a “moving target” with different symptoms appearing over time, and caregivers frequently managing multiple BPSD simultaneously, and thus, unpredictability and complexity makes a simple “magic bullet” medication solution impossible.
In contrast, nonpharmacologic behavioral management strategies are increasingly recognized as a critical part of comprehensive, state of the art dementia care 10. Nonpharmacologic strategies are recommended by multiple medical organizations and expert groups as the preferred first-line treatment approach to BPSD, except in emergency situations when behaviors could lead to imminent danger 10–13. An emerging evidence base supports the use of a range of nonpharmacologic approaches to manage BPSD. The common goal of nonpharmacologic approaches is prevention, symptom relief and reduction of caregiver distress.
To date, however, only “hands-on” staff- and training-intensive interventions have been developed and tested to help families manage BPSD. These approaches have demonstrated effectiveness, but are time-consuming, labor-intensive and intervene for a limited period of time in the trajectory of a family’s care provision. Additionally, unfortunately few of these proven interventions have been translated into a deliverable and sustainable service, and thus, most families continue to be underserved or receive services that are not evidence-based. While a number of prior studies have incorporated technology components 14–17, there have been no web-based, easy to use, comprehensive interactive tools to help families manage BPSD and track their modifiable underlying causes such as pain, sleep disturbances or poor nutrition and which can be used over the course of the disease.
As part of an NIH-funded project (R01NR014200), we sought to overcome this research-practice gap by creating a tool that would guide caregivers through a clinical reasoning process to identify, monitor and manage behaviors while simultaneously addressing motivation, self-efficacy and problem-solving skills. This type of approach has been offered to consumers for management of other complex health conditions such as cancer, smoking cessation, weight management, and asthma 18,19.
The keystone of the proposed tool was the “DICE” approach to screen, identify and manage BPSD that was developed from a multidisciplinary expert consensus panel20. DICE™ is an algorithmic evidence-based approach comprised of four steps: DESCRIBE (describe the behavior to derive an accurate characterization and the context in which it occurs; INVESTIGATE (examine, exclude and identify possible underlying causes of the behavior); CREATE (create and implement a treatment plan for the behavior); and EVALUATE (assess what parts of the treatment plan were attempted and effective). Within the DICE™ approach, caregiver (expectations, caregiver stress/depression, etc); person with dementia (medical conditions, functional status, etc); and environmental (overstimulation, lack of routines, etc) considerations are evaluated. We proposed to involve end-users (e.g. family caregivers) in guiding us in the design of a tool incorporating the DICE™ approach that would: 1) be easy to use; 2) be tailored to the person with dementia’s and caregiver’s specific behavioral concerns, environment and personal characteristics; 3) teach new transferrable skills to the end-users; 4) provide an alternative to the risks and limited efficacy associated with medication treatment; and 5) could be used throughout the disease course.
To assure our approach met the needs of end users, we conducted a study consisting of a series of focus groups and in-depth interviews with family caregivers. The purpose of this paper is to report the outcomes of these interviews and the implications for tool development for dementia caregivers.
Methods
Four focus groups were held with a total of 26 family caregivers. Study participants for the focus groups were recruited via two methods: 1) by the caregiver responding to fliers placed at local senior resource centers and contacting research staff by phone or email; and 2) by referral from senior resource center staff. Eligible persons were primary caregivers of a person diagnosed with dementia. Directly prior to the beginning of the group meeting, participants provided informed consent and also completed a brief survey that contained questions regarding: 1) demographic information; 2) caregiving characteristics; and 3) technology familiarity. An additional twelve semi-structured interviews for further detail on tool “look and feel” were purposively sampled from the focus group sample.
All focus groups were conducted by an experienced facilitator. Using an outline developed by the investigative team to ensure systematic coverage of participants' concerns, ideas, and experiences, study participants were asked about: 1) the perceived need for psychoeducation about BPSD and their management; 2) the language used to describe BPSD; 3) opinions regarding medication vs. behavioral management for symptoms; and 4) previous experiences and communication with providers and family members related to BPSD. Further prompts investigated the caregivers’ understanding of BPSD’s relationship to the underlying dementing illness (vs. willful behaviors) and past experiences with symptom treatment. Focus groups provided an opportunity to generate ideas about treatment preferences, the language to be used in the tool, and identifying desired tool components which are the data presented here. Focus group sessions lasted approximately 90 minutes and were audio-recorded. Sessions were then transcribed and transcriptions verified for accuracy by research staff.
A qualitative data research expert (DW) led the study team (BS, MT and HCK) in data analysis using a spreadsheet or “all-inclusive data table” technique to organize, manage and analyze the data as a group21,22 . The spreadsheet consisted of seven row headings: transcript number, outline section, question asked, participant response, notes, code and theme. The team then used a data reduction technique called “rigorous and accelerated data reduction” (RADaR) the purpose of which is to generate results quickly and meticulously for translation and dissemination22,23. Working together in Microsoft Excel, the team placed segments of raw text from the transcripts into the spreadsheet. Next, a two-level process was used to code the data in order to analyze it for embedded meaning. First, open coding was used to identify categories, concepts and themes germane to the project goals (e.g. what would be most useful for caregivers to see in a tool created to assess and manage behavioral and psychological symptoms of dementia). Second, the team worked together to identify the frequency of codes in order to determine which concepts were most cited throughout the data. Data from each focus group were analyzed separately (e.g. individual group data not initially combined). While the basic focus group script questions did not change from the first group to the other three groups, material was added to include additional probes (the purpose of which is to get more detail for given questions) if suggested from analysis of prior groups.
Results
Table 1 shows the characteristics of the family caregivers (n=26) who were involved in the four focus groups. The majority of caregivers were adult children of the person with dementia, followed by spouse as the next most common category and then by other relative. Mean age of caregiver was 52 years old and the person with dementia was 82 years old. Most caregivers were women, had a college degree or higher, were married and white. Most caregivers had been providing care for 2 years or more and were providing more than 10 hours of care per week to the person with dementia.
Table 1.
Characteristics of Family Caregivers (n=26)
| Characteristic | N (%) | |
|---|---|---|
| Relationship to care recipient |
Adult child | 15 (57.7%) |
| Spouse | 6 (23.1%) | |
| Other relative | 5 (19.2%) | |
| Mean age | 52 (24–76) | |
| Gender | Female | 21 (80.8%) |
| Male | 5 (19.2%) | |
| Race | White | 25 (96.2%) |
| African American | 1 (3.8%) | |
| Marital Status | Married | 18 (69.2%) |
| Divorced | 5 (19.2%) | |
| Never married | 2 (7.7%) | |
| Widowed | 1 (3.8%) | |
| Education | High school or less | 1 (3.8%) |
| Some college/associate’s degree | 5 (19.2.%) | |
| Bachelor’s degree | 11 (42.3%) | |
| Graduate degree | 9 (34.6%) | |
| Time providing care (years) |
1 year or less | 3 (11.5%) |
| 2–3 years | 9 (34.6%) | |
| 4–5 years | 7 (26.9%) | |
| >5 years | 7 (26.9%) | |
| Care hours per week |
<5 | 1 (3.8%) |
| 5–10 | 5 (19.2%) | |
| 11–15 | 8 (30.8%) | |
| 16–20 | 1 (3.8%) | |
| >20 | 11 (42.3%) | |
| Mean age of care recipient (years) |
82 (61–96) | |
Table 2 depicts caregiver technology familiarity. All had access to a computer, the internet and email, and most used the computer several times per day. The majority used multiple devices (e.g. smart phone, desktop, laptop). Top sources from which participants obtained dementia information were: online internet searches; Alzheimer’s Association; physician; magazines; and social workers. All but two participants expressed interest in using a web-based program to help with BPSD management; the two who were not interested cited concerns with “privacy” and “discomfort using computer except for email”.
Table 2.
Technology Familiarity Questions
| Characteristic | N (%) | |
|---|---|---|
| Ever used computer | Yes | 26 (100%) |
| Access to computer | Yes | 26 (100%) |
| Computer use frequency | Few times a month or less | 1 (3.8%) |
| Once a week | 0 (0%) | |
| Every day or two | 6 (23.1%) | |
| Several times per day | 19 (73.1%) | |
| Place of access of
computers (subjects selected all that applied) |
Home | 25 (96.2%) |
| Work | 5 (19.2%) | |
| School | 0 (0%) | |
| Friends/family | 6 (23.1%) | |
| Library | 7 (26.9%) | |
| Public places with access | 5 (19.2%) | |
| Email address | Yes | 26 (100%) |
| Email use frequency | Few times a month or less | 1 (3.8%) |
| Once a week | 0 (0%) | |
| Every day or two | 8 (30.1%) | |
| Several times per day | 17 (65.4%) | |
| Internet access | Yes | 26 (100%) |
| Device used for internet
access (subjects selected all that applied) |
Desktop | 16 (61.5%) |
| Laptop | 18 (69.2%) | |
| Tablet | 8 (30.1%) | |
| Smart phone | 14 (53.8%) | |
| Multiple devices | 18 (69.2%) | |
| Internet access frequency | Few times a month or less | 0 (0%) |
| Once a week | 0 (0%) | |
| Every day or two | 8 (30.1%) | |
| Several times per day | 18 (69.2%) | |
| Dementia caregiving information
source (subjects selected all that applied) |
Alzheimer’s association | 19 (73.1%) |
| Physician | 15 (57.7%) | |
| Social Worker | 11 (42.3%) | |
| Internet | 21 (80.1%) | |
| Magazines | 15 (57.7%) | |
| Radio/TV | 6 (23.1%) | |
| Newspapers | 5 (19.2%) | |
| Other | 13 (50.0%) | |
| Willingness to use a web-based
program to manage BPSD |
Yes | 23 (88.5%) |
| No | 2* (7.7%) | |
| Maybe | 1 (3.8%) | |
“no” response reflected concerns for privacy (1 subject) and comfort in only using the computer for email (1 subject).
The primary themes related to tool development from the focus groups centered on three main areas:
-
Need to minimize difficulty and training time:
“If the tool needs training, it’s too complicated.”
“Make it intuitive or you will scare people away. We are stressed already.”
-
“One-stop shopping” for information:
“Information is all piece meal (on the internet), nothing is brought together.”
“I had a specific question and there was no systematic way to find an answer. I had to look through topic threads and hope that someone responded.”
-
The need for tailored output:
“I’m going to strangle somebody if I see a frequently asked questions, I can’t be put into one category.”
“One of my frustrations about support groups is the disparity in the kind of issues people are dealing with.”
Caregivers also gave feedback about specific features they would like to see included in the proposed tool:
“It would have arms and legs” (e.g. human touches)
“Contain strategies on how to speak with someone who is confused and upset”
“Provide an understanding of how medical problems like urinary tract infections cause behaviors.”
“It would be great to get an email each day with some words of encouragement…to ground you, give you strength and energy to keep doing what you are doing.”
“It would be nice to have a little search engine to put in whatever you are dealing with and it pops up some strategies.”
“Build in feedback so the user can say ‘this didn’t work’”.
“I would like things [ideas] for taking care of me, the caregiver. When I am feeling thus and so to know that this is part of the game and ideas of how to get out of that mode.”
In the semi-structured interviews (n=12), we received additional feedback on the tool in several areas: 1) naming of tool sections (e.g. Caregiver Survival Guide preferred over Caregiver College and Caregiver Corner for section on education); 2) suggestions for types of education included in the tool (e.g. material on medication side effects); 3) types of graphics that would be appealing (e.g. caregivers preferred a horizontal graded pain scale over a face scale: “these things are childish”or a vertical “thermometer” scale: “reminds me of heat and raging”); and 4) wording of the strategies contained in the tool.
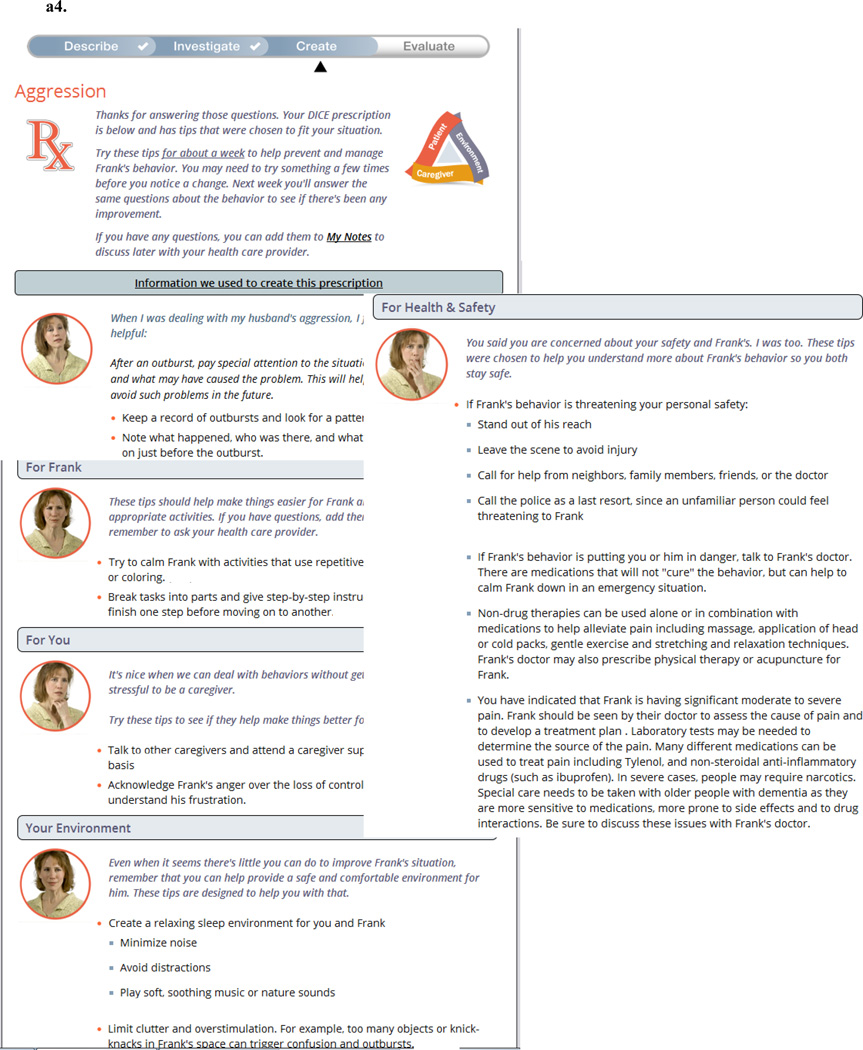
Screenshots from the completed WeCareAdvisor™ tool are contained in Figure 1. WeCareAdvisor™ has two main sections:
A guided DICE™ approach (Figure 1a) where the caregiver inputs contextual factors associated with a given BPSD, including consideration of possible medical illnesses and pain. The contextual factors (examples of context screens shown in1a.2 and 1a.3) allow the algorithm to select from over 900 strategies that were included in the tool and help to create the BPSD “prescription” (1a.4) that can be printed or emailed to others. After using the prescription for one week, caregivers are prompted to evaluate how the tips worked for them. The strategies included in the tool are based upon effective evidence-based interventions for family caregivers. During the DICE™ process within the tool, depending upon the type of behavior selected and contextual variables (e.g. is sleep impacted? Is there underlying pain or a possible medical cause? Is the onset acute? Is the environment over or understimulating?), the program selects the strategies that are most likely to be helpful to the person with dementia and caregiver. Selection rules for the algorithm within the tool were developed by one member of the team (HCK), and then reviewed and checked by another team member (LNG). Inconsistencies were then discussed and the approach tested independently on multiple occasions by 4 team members (HCK, LNG, BS, KM).
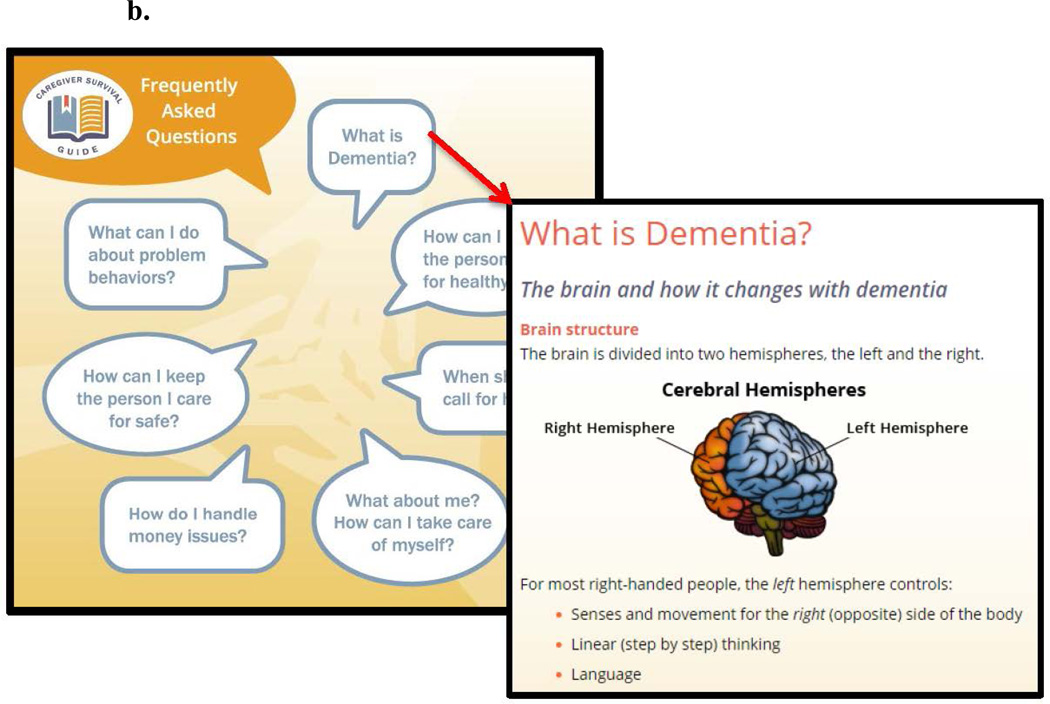
The Caregiver Survival Guide™ (Figure 1b) which is a compendium of information for dementia caregivers (e.g.“one-stop shopping”) that they can read when time allows with chapters on "what is dementia”; “behaviors in dementia”; “keeping a person with dementia healthy”; “communicating with the healthcare team”; “medications for BPSD: uses and common side effects”; “keeping a person with dementia safe”; “taking care of yourself”; “financial matters”; glossary of commonly used terms in aging and dementia; and other links and resources. Strategies for managing BPSD that get tailored in the DICE™ approach also appear in the Caregiver Survival Guide™ where they are listed under behavior type (e.g agitation, aggression, depression, etc).
Figure 1.
- DICE Steps explained by “Peer Navigator”
- Selecton of contextual variables for a given BPSD (here Aggression)
- Pain scale with training of how to assess pain in dementia incorporated
- Example of a WeCareAdvisor™ Prescription; tips in the Prescription are tailored (selected from almost 1000 strategies in the tool) based upon caregiver input
b. Caregiver Survival Guide™ Contents and Sample Content
Caregiver feedback was used in creating many of the features of the tool, including:
A daily messaging feature that provides an encouraging daily communication to caregivers for support and motivation.
A peer navigator (1a.1; tailored to the age, race and gender of the caregiver user) to describe the features of the tool to the caregiver to “humanize” the experience.
Incorporating teaching into the tool’s approach (e.g. DICE™ pain screen shown in Figure 1a.3. teaches how to assess for pain in people with dementia) to “minimize time/training”.
A notes section where caregivers can write notes to themselves about care issues that they wish to discuss with other members of the health care team.
Because the consideration of urgent situations from a safety and risk standpoint is a significant concern in assessing and managing BPSD (an area lacking with many of the currently used psychosocial approaches), assessment of safety and potential risk is purposefully built into the WeCareAdvisor™ at multiple levels. First, caregiver and person with dementia (PWD) safety and risk is assessed in every DICE™ session. If the caregiver indicates ANY risk to either self or PWD, one of the strategies that will appear in the DICE™ Prescription will be a clear recommendation for the caregiver to seek help from the PWD’s physician. For serious risk, something like the following would be included in the DICE™ Prescription:
If Frank’s behavior is putting you or him in danger, talk to his doctor. There are medications that will not “cure” the behavior, but can help to calm him down in an emergency situation.
Secondly, caregivers are asked about potential medical contributors to BPSD in multiple ways during DICE™ sessions including: 1) changes in chronic medical conditions they have listed; 2) the appearance of new symptoms suggesting delirium (e.g. behaviors starting suddenly or worsening at night); and 3) consideration of pain as a contributor. If any of these items are noted by the caregiver, something like the following would be included in the prescription.
You have indicated that the behavior may have had a sudden onset or other characteristics that could suggest a medical problem underlying the behavior. Please check with Elizabeth's doctor for a review of possible medical causes of the behavior you are seeing.
You have indicated that Howard has recently taken a new prescribed medication or there has been a recent change in medication or its dosing. Medication additions or changes can trigger behaviors. Please check with his doctor to see if the behavior could be a medication side effect.
You have indicated that Dorothy has a new physical symptom that started around the same time as the behavior. Please check with her doctor to see if the behavior could have been triggered by this new medical problem. Treating the medical problem may alleviate the behavior.
Mild pain can often be managed at home after you talk to your doctor. First, try to localize figure the source of the pain (for example, is it a headache? Was Walter sitting too long in one position? Could he be constipated?). The treatment depends on the source of the pain. If the pain is due to Walter sitting too long in one position, try to introduce an active time into his daily routine. If the pain is from a headache, Walter’s doctor might recommend Tylenol. For constipation, the doctor may recommend increasing fluids and fiber in the diet and stool softeners.
Discussion
In real-world settings, few treatment options are currently available to family caregivers for BPSD despite a growing evidence base for the effectiveness of nonpharmacological strategies. We sought to overcome this research-practice gap by creating a tool that would guide caregivers through a clinical reasoning process to identify, monitor and manage behaviors while simultaneously addressing motivation, self-efficacy and problem-solving skills.
The tool created, the WeCareAdvisor™, builds upon the DICE™ approach for BPSD assessment and management that was created by a multidisciplinary expert panel20. In addition to DICE™, the tool was “built” with direct input from the intended end-users, family caregivers. We included the features that caregivers told us that they wanted including comprehensive information on dementia and related issues; daily messaging for encouragement; and “human” touches.
In the final part of the project, we are testing the WeCareAdvisor™ tool in a randomized controlled trial of 60 family caregiver/person with dementia dyads. We will evaluate the effects of WeCareAdvisor™ on the primary outcomes of caregiver confidence and upset in the treatment group (30 dyads) compared to the waitlist control group (30 dyads) after one month of tool use. In addition, we will be collecting data on other caregiver (stress level, change in negative communications, perceived change of well-being) and patient outcomes (behavioral symptom frequency and severity). Dyads randomized to the waitlist condition will receive the tool after a 1-month delay, and will be retested one month later to evaluate whether they benefit on same indicators as the initial WeCareAdvisor™ group. This design will yield a total of 60 dyads to evaluate WeCareAdvisor™ use parameters, including: ease and frequency of use, time required to learn and length of time engaged in using the tool, overall usability, and perceived benefit.
As the WeCareAdvisor™ is currently being testing in the NIH-sponsored randomized controlled trial, it is not yet available to the public. When the trial is completed and results are reported (estimated as December 2016), we anticipate making the tool available to family caregivers at a low cost (under $20) which would enable them to have on-going access to the on-line platform. The fee would be intended to offset our costs for maintaining and updating the on-line program. Costs could be reduced further in partnership with state, foundation, or private donor funding that will be sought during development of the commercial product.
Our approach to tool development highlights the value of including end-users in the process. We obtained critical and invaluable insight which directly informed tool construction. On-line programs for dementia must be responsive to the needs of caregivers and reflect their values including ease of use, on-demand/easily understood materials, one-stop-shopping, relevance across disease trajectory, and tailoring to their particular context.
Limitations of the research described include the limited number as well as the relative homogeneity of the caregivers (in terms of geographical area, race, gender and education level in particular) interviewed. In the trial as well as in future research, we will be determining what iterative changes may be necessary for more diverse populations (such as in our current Department of Defense grant with families of military veterans with dementia, and in a planned larger RCT within a competing renewal of the current family caregiver grant). Additionally, the sample was also fairly technologically savvy in terms of the ubiquity of using computers, smart phones and the internet. In our current trial, we are limiting recruitment to caregivers who have some experience with such technology as we did not wish to test caregivers’ ability to use technology in general, but rather the use of this particular web-based program. Moving forward, we will examine how to provide less technology savvy caregivers access to the information and support they need.
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NR014200-01). The funding organization had no role in any of the following: design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Data from this study were presented in part at the Alzheimer’s Association International Conference, Toronto Canada, July 25, 2016.
References
- 1.Thies W, Bleiler L. Alzheimer’s Association report: 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 2011;7(5):532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kales HC, Gitlin LN, Lyketsos CG. State of the Art Review: Assessment and management of behavioral and psychological symptoms of dementia. BMJ: British Medical Journal. 2015;350 doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer's disease patients. International journal of geriatric psychiatry. 2002;17(5):403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 6.Van Den Wijngaart MA, Vernooij-Dassen MJ, Felling AJ. The influence of stressors, appraisal and personal conditions on the burden of spousal caregivers of persons with dementia. Aging Ment Health. 2007;11(6):626–636. doi: 10.1080/13607860701368463. [DOI] [PubMed] [Google Scholar]
- 7.de Vugt ME, Stevens F, Aalten P, et al. Do caregiver management strategies influence patient behaviour in dementia? International journal of geriatric psychiatry. 2004;19(1):85–92. doi: 10.1002/gps.1044. [DOI] [PubMed] [Google Scholar]
- 8.Schneider L, Tariot P, Dagerman K, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. New England Journal of Medicine, The. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 9.Maust DT, Kim HM, Seyfried LS, et al. Number Needed to Harm for Antipsychotics and Antidepressants in Dementia. The American Journal of Geriatric Psychiatry. 2014;22(3):S119–S120. [Google Scholar]
- 10.Improvement PCfP. Dementia: Performance Measure Set. American Medical Association; 2010. [Google Scholar]
- 11.Workgroup C. In: Guideline for Alzheimer's Disease Managment--Final Report. State of California DoPH, editor. 2008. [Google Scholar]
- 12.NICE-SCIE Supporting Caregivers and patients. London: British Psychological Society; 2011. [Google Scholar]
- 13.Detection, diagnosis, and managment of dementia. St. Paul: American Academy of Neurology; 2011. [Google Scholar]
- 14.Beauchamp N, Irvine AB, Seeley J, Johnson B. Worksite-based internet multimedia program for family caregivers of persons with dementia. The Gerontologist. 2005;45(6):793–801. doi: 10.1093/geront/45.6.793. [DOI] [PubMed] [Google Scholar]
- 15.Czaja SJ, Rubert MP. Telecommunications technology as an aid to family caregivers of persons with dementia. Psychosom Med. 2002;64(3):469–476. doi: 10.1097/00006842-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Dang S, Nedd N, Nair S, Roos B, et al. Utilization of TLC technology by dementia family caregivers. The Gerontologist. 2004;44(1):604. [Google Scholar]
- 17.Eisdorfer C, Czaja SJ, Loewenstein DA, et al. The effect of a family therapy and technology-based intervention on caregiver depression. Gerontologist. 2003;43(4):521–531. doi: 10.1093/geront/43.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strecher VJ, McClure J, Alexander G, et al. The role of engagement in a tailored web-based smoking cessation program: randomized controlled trial. J Med Internet Res. 2008;10(5):e36. doi: 10.2196/jmir.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph CL, Havstad SL, Johnson D, et al. Factors associated with nonresponse to a computer-tailored asthma management program for urban adolescents with asthma. J Asthma. 2010;47(6):667–673. doi: 10.3109/02770900903518827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kales HC, Gitlin LN, Lyketsos CG the Detroit Expert Panel on the A, Management of the Neuropsychiatric Symptoms of D. Management of Neuropsychiatric Symptoms of Dementia in Clinical Settings: Recommendations from a Multidisciplinary Expert Panel. Journal of the American Geriatrics Society. 2014;62(4):762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guest G, MacQueen KM, editors. Handbook for team-based qualitative research. Lanham, MD: AltaMira Press; 2008. Reevaluating guidelines for qualitative research. [Google Scholar]
- 22.Watkins DC. Qualitative research: The importance of conducting research that doesn’t ‘count.’. Health Promot Pract. 2012;13(13):153–158. doi: 10.1177/1524839912437370. [DOI] [PubMed] [Google Scholar]
- 23.Watkins DC, Gioia D. Mixed methods research. Pocket guides to social work research series. New York, New York: Oxford University Press; 2015. [Google Scholar]