Abstract
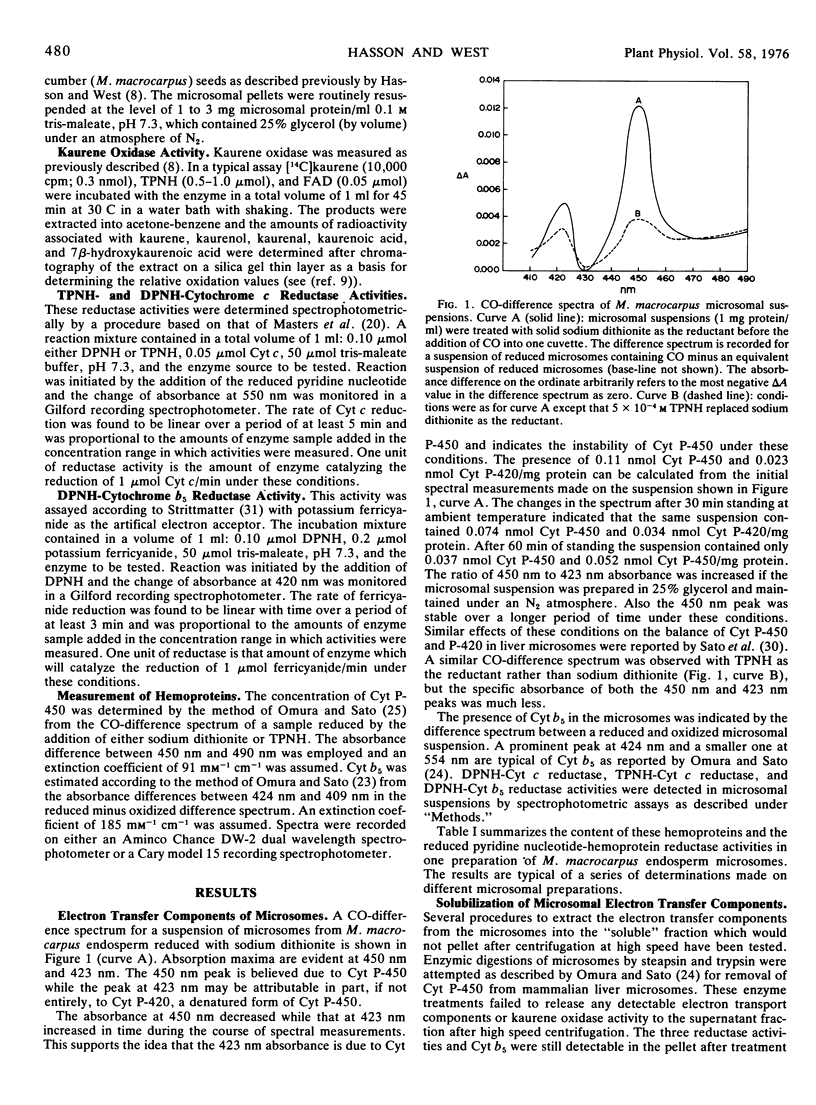
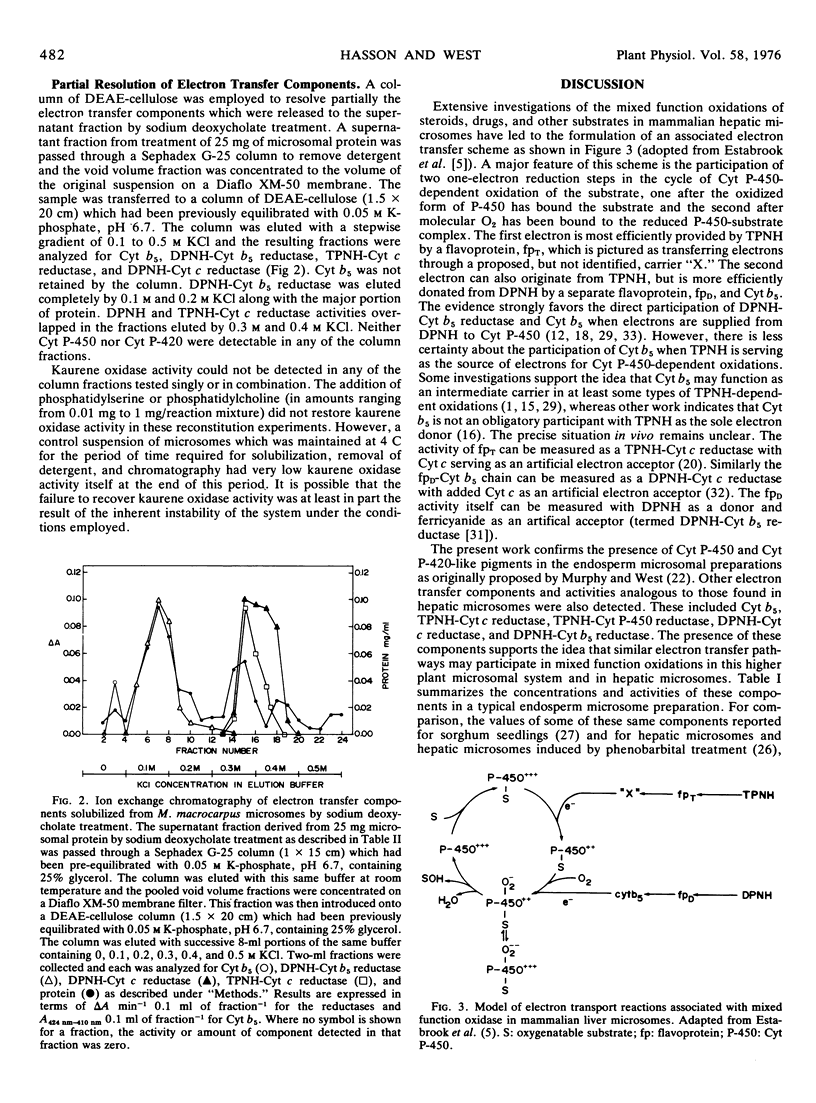
Cytochrome P-450 and cytochrome b5 at levels of approximately 0.10 and 0.60 nanomole per milligram of microsomal protein were detected by spectral measurements in microsomes prepared from endosperm tissue of immature Marah macrocarpus seeds. TPNH-cytochrome c reductase, DPNH-cytochrome c reductase, andDPNH-cytochrome b5 reductase activities were also present in these microsomes at levels of approximately 0.060, 0.22, and 0.52 unit per milligram of microsomal protein, respectively. (One unit of reductase is the amount of enzyme catalyzing the reduction of 1 micromole of electron acceptor per minute.) Treatments of microsomes with steapsin or trypsin were not effective in solubilizing any of these electron transport components in detectable form. However, treatment of a microsomal suspension in 25% glycerol with 1% sodium deoxycholate led to the release of about 60% of the protein and each of the above hemoproteins and electron transfer activities to the fraction which was not pelleted after centrifugation for 2 hours at 105,000g. Some ent-kaur-16-ene oxidase activity could be detected in the solubilized fraction after removal of the detergent. Cytochrome b5 and DPNH-cytochrome b5 reductase activity were largely separated from one another and from an overlapping mixture of TPNH-cytochrome c reductase and DPNH-cytochrome c reductase when the sodium deoxycholate-solubilized fraction was chromatographed on a DEAE-cellulose column. No cytochrome P-450 or cytochrome P-420 was detected in the column fractions and no ent-kaur-16-ene oxidase activity was detected when the column fractions were tested singly or in combination.
The possible participation of these components in the mixed function oxidation of ent-kaur-16-ene and a number of its oxidized derivatives catalyzed by these microsomes is discussed in relation to the model which has been developed to explain the function of analogous components in mixed function oxidase reactions in mammalian liver microsomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J., Hildebrandt A. G., Peterson J. A., Estabrook R. W. The role of oxygenated cytochrome P-450 and of cytochrome b5 in hepatic microsomal drug oxidations. Drug Metab Dispos. 1973 Jan-Feb;1(1):129–138. [PubMed] [Google Scholar]
- Cohen B. S., Estabrook R. W. Microsomal electron transport reactions. 3. Cooperative interactions between reduced diphosphopyridine nucleotide and reduced triphosphopyridine nucleotide linked reactions. Arch Biochem Biophys. 1971 Mar;143(1):54–65. doi: 10.1016/0003-9861(71)90185-8. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Mason J. I., Baron J., Lambeth D., Waterman M. Drugs, alcohol and sex hormones: a molecular perspective of the receptivity of cytochrome P-450. Ann N Y Acad Sci. 1973;212:27–49. doi: 10.1111/j.1749-6632.1973.tb47584.x. [DOI] [PubMed] [Google Scholar]
- GILLETTE J. R., BRODIE B. B., LA DU B. N. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957 Apr;119(4):532–540. [PubMed] [Google Scholar]
- Gunsalus I. C., Pederson T. C., Sligar S. G. Oxygenase-catalyzed biological hydroxylations. Annu Rev Biochem. 1975;44:377–407. doi: 10.1146/annurev.bi.44.070175.002113. [DOI] [PubMed] [Google Scholar]
- Hasson E. P., West C. A. A microsomal ATP-activated pyridine nucleotide transhydrogenase. Arch Biochem Biophys. 1973 Apr;155(2):258–269. doi: 10.1016/0003-9861(73)90114-8. [DOI] [PubMed] [Google Scholar]
- Hasson E. P., West C. A. Properties of the System for the Mixed Function Oxidation of Kaurene and Kaurene Derivatives in Microsomes of the Immature Seed of Marah macrocarpus: Cofactor Requirements. Plant Physiol. 1976 Oct;58(4):473–478. doi: 10.1104/pp.58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A., Estabrook R. W. Evidence for the participation of cytochrome b 5 in hepatic microsomal mixed-function oxidation reactions. Arch Biochem Biophys. 1971 Mar;143(1):66–79. doi: 10.1016/0003-9861(71)90186-x. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., Prough R. A. Reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase and cytochrome b5 as electron carriers in NADH-supported cytochrome P-450 -dependent enzyme activities in liver microsomes. Arch Biochem Biophys. 1974 Nov;165(1):331–339. doi: 10.1016/0003-9861(74)90171-4. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Levin W., Selander H., Jerina D. M. Liver microsomal electron transport systems. III. The involvement of cytochrome b5 in the NADPH-supported cytochrome P-450-dependent hydroxylation of chlorobenzene. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1348–1355. doi: 10.1016/s0006-291x(74)80432-8. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Levin W. The resolution and reconstitution of the liver microsomal hydroxylation system. Biochim Biophys Acta. 1974 Sep 16;344(2):205–240. doi: 10.1016/0304-4157(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., West S. B., Vore M., Ryan D., Levin W. Role of cytochrome b5 in hydroxylation by a reconstituted cytochrome P-450-containing system. J Biol Chem. 1974 Nov 10;249(21):6701–6709. [PubMed] [Google Scholar]
- Mannering G. J., Kuwahara S., Omura T. Immunochemical evidence for the participation of cytochrome b5 in the NADH synergism of the NADPH-dependent mono-oxidase system of hepatic microsomes. Biochem Biophys Res Commun. 1974 Mar 25;57(2):476–481. doi: 10.1016/0006-291x(74)90956-5. [DOI] [PubMed] [Google Scholar]
- Meehan T. D., Coscia C. J. Hydroxylation of geraniol and nerol by a monooxygenase from Vinca rosea. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1043–1048. doi: 10.1016/0006-291x(73)90570-6. [DOI] [PubMed] [Google Scholar]
- Murphy P. J., West C. A. The role of mixed function oxidases in kaurene metabolism in Echinocystis macrocarpa Greene endosperm. Arch Biochem Biophys. 1969 Sep;133(2):395–407. doi: 10.1016/0003-9861(69)90468-8. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Russell D. W. The metabolism of aromatic compounds in higer plants. X. Properties of the cinnamic acid 4-hydroxylase of pea seedlings and some aspects of its metabolic and developmental control. J Biol Chem. 1971 Jun 25;246(12):3870–3878. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. A microsomal cytochrome reductase specific for diphosphopyridine nucleotide. J Biol Chem. 1956 Jul;221(1):277–286. [PubMed] [Google Scholar]
- Sasame H. A., Thorgeirsson S. S., Mitchell J. R., Gillette J. R. The possible involvement of cytochrome b5 in the oxidation of lauric acid by microsomes from kidney cortex and liver of rats. Life Sci. 1974 Jan 1;14(1):35–46. doi: 10.1016/0024-3205(74)90243-4. [DOI] [PubMed] [Google Scholar]
- Sato R., Satake H., Imai Y. Partial purification and some spectral properties of hepatic microsomal cytochrome P-450. Drug Metab Dispos. 1973 Jan-Feb;1(1):6–13. [PubMed] [Google Scholar]
- West S. B., Levin W., Ryan D., Vore M., Lu A. Y. Liver microsomal electron transport systems. II. The involvement of cytochrome b5 in the NADH-dependent hydroxylation of 3,4-benzpyrene by a reconstituted cytochrome P-448-containing system. Biochem Biophys Res Commun. 1974 May 20;58(2):516–522. doi: 10.1016/0006-291x(74)90395-7. [DOI] [PubMed] [Google Scholar]


