Abstract
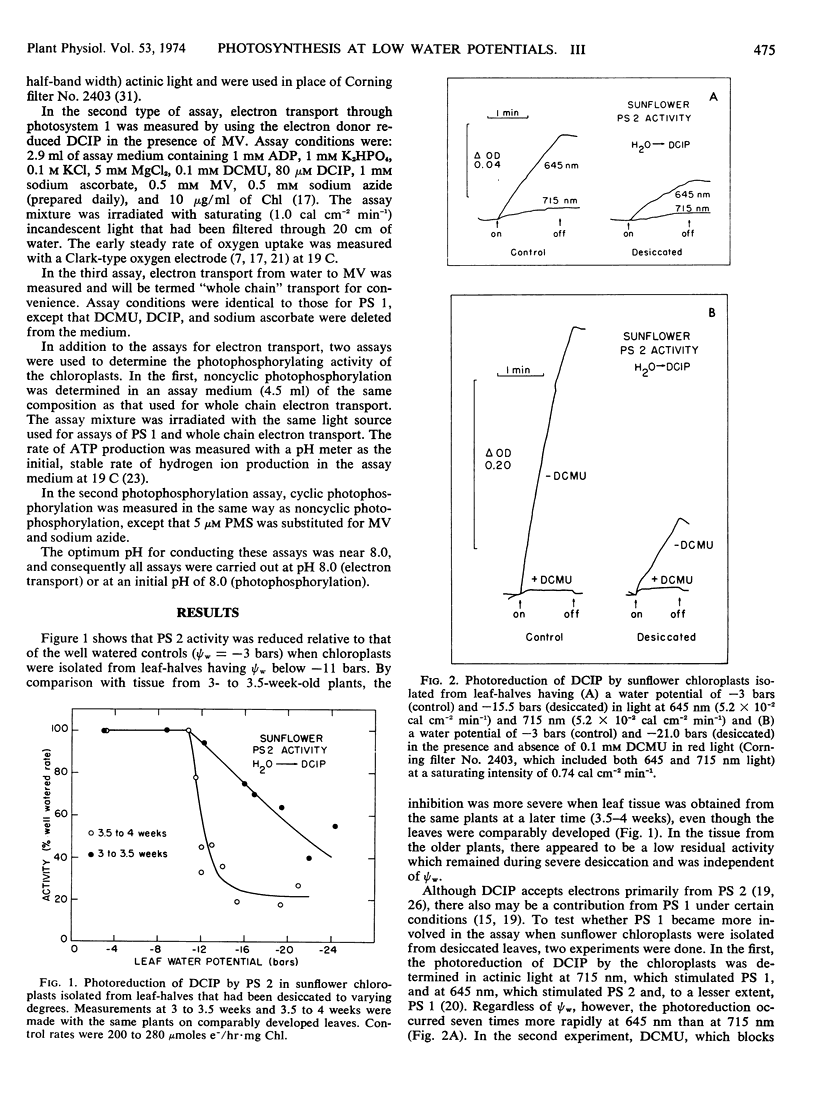
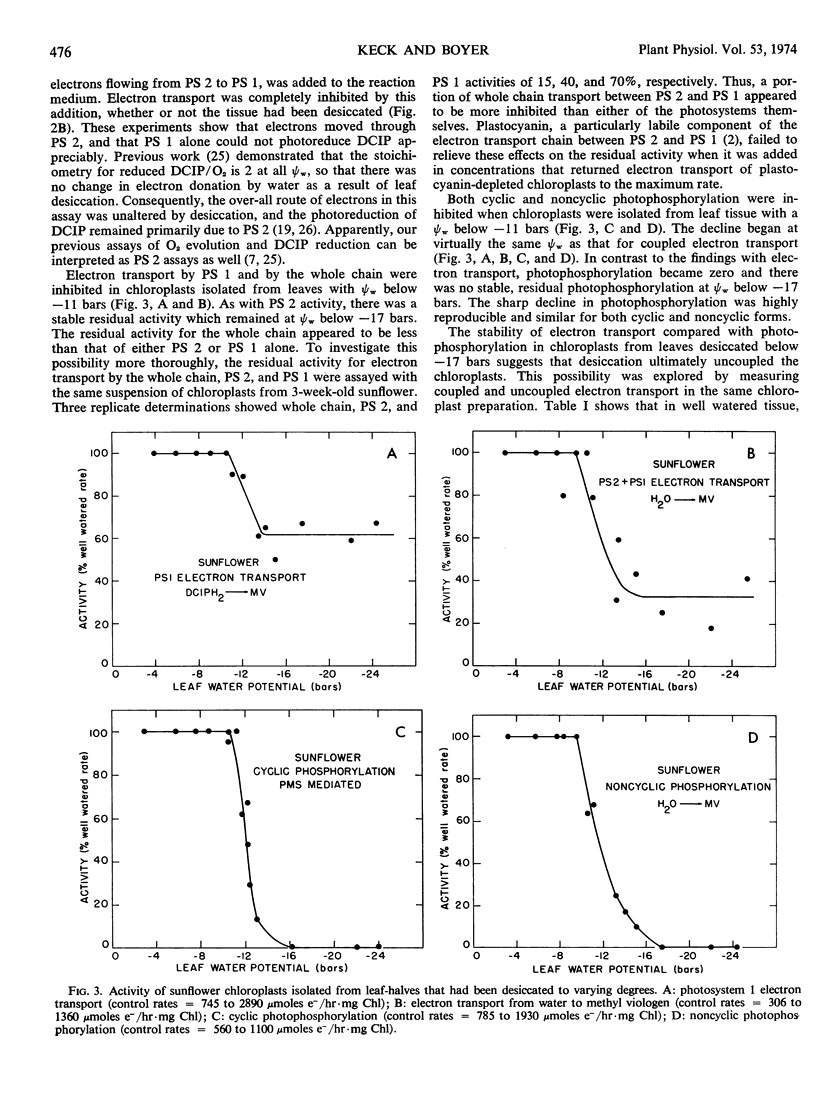
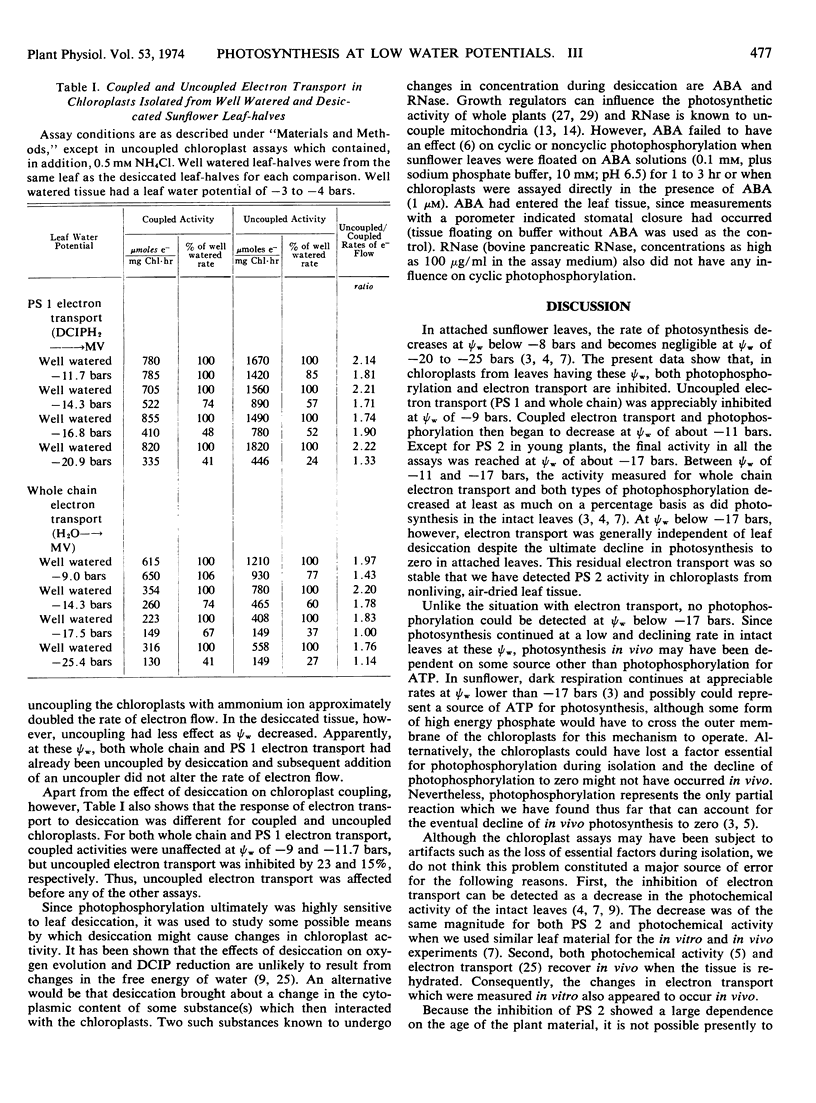
Cyclic and noncyclic photophosphorylation and electron transport by photosystem 1, photosystem 2, and from water to methyl viologen (“whole chain”) were studied in chloroplasts isolated from sunflower (Helianthus annus L. var Russian Mammoth) leaves that had been desiccated to varying degrees. Electron transport showed considerable inhibition at leaf water potentials of −9 bars when the chloroplasts were exposed to an uncoupler in vitro, and it continued to decline in activity as leaf water potentials decreased. Electron transport by photosystem 2 and coupled electron transport by photosystem 1 and the whole chain were unaffected at leaf water potentials of −10 to −11 bars but became progressively inhibited between leaf water potentials of −11 and −17 bars. A low, stable activity remained at leaf water potentials below −17 bars. In contrast, both types of photophosphorylation were unaffected by leaf water potentials of −10 to −11 bars, but then ultimately became zero at leaf water potentials of −17 bars. Although the chloroplasts isolated from the desiccated leaves were coupled at leaf water potentials of −11 to −12 bars, they became progressively uncoupled as leaf water potentials decreased to −17 bars. Abscisic acid and ribonuclease had no effect on chloroplast photophosphorylation. The results are generally consistent with the idea that chloroplast activity begins to decrease at the same leaf water potentials that cause stomatal closure in sunflower leaves and that chloroplast electron transport begins to limit photosynthesis at leaf water potentials below about −11 bars. However, it suggests that, during severe desiccation, the limitation may shift from electron transport to photophosphorylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Shneyour A. On the siteof action of plastocyanin in isolated chloroplasts. Biochim Biophys Acta. 1971 Mar 2;226(2):498–500. doi: 10.1016/0005-2728(71)90120-4. [DOI] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970 Aug;46(2):233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Nonstomatal inhibition of photosynthesis in sunflower at low leaf water potentials and high light intensities. Plant Physiol. 1971 Nov;48(5):532–536. doi: 10.1104/pp.48.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Potter J. R. Chloroplast response to low leaf water potentials: I. Role of turgor. Plant Physiol. 1973 Jun;51(6):989–992. doi: 10.1104/pp.51.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Recovery of photosynthesis in sunflower after a period of low leaf water potential. Plant Physiol. 1971 Jun;47(6):816–820. doi: 10.1104/pp.47.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo P., Sacher J. A. Control of ribonuclease and acid phosphatase by auxin and abscisic acid during senescence of Rhoeo leaf sections. Plant Physiol. 1970 Dec;46(6):806–811. doi: 10.1104/pp.46.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley R. A. Effect of poly-L-lysine on energy-linked chloroplast reactions. Biochemistry. 1968 Jan;7(1):338–346. doi: 10.1021/bi00841a043. [DOI] [PubMed] [Google Scholar]
- Fry K. E. Some factors affecting the Hill reaction activity in cotton chloroplasts. Plant Physiol. 1970 Apr;45(4):465–469. doi: 10.1104/pp.45.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON J. B. The effect of ribonuclease on oxidative phosphorylation by mitochondria. J Biol Chem. 1959 May;234(5):1303–1306. [PubMed] [Google Scholar]
- Hanson J. B. Ion transport induced by polycations and its relationship to loose coupling of corn mitochondria. Plant Physiol. 1972 May;49(5):707–715. doi: 10.1104/pp.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haveman J., Duysens L. N., Geest T. C., van Gorkom H. J. Hydrazobenzene oxidation by 2,6-dichlorophenol-indophenol in a photoreaction catalyzed by system I of photosynthesis. Hydrazine compounds as donors for photosystem II. Biochim Biophys Acta. 1972 Nov 17;283(2):316–327. doi: 10.1016/0005-2728(72)90247-2. [DOI] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Joliot P., Joliot A., Kok B. Analysis of the interactions between the two photosystems in isolated chloroplasts. Biochim Biophys Acta. 1968 Apr 2;153(3):635–652. doi: 10.1016/0005-2728(68)90191-6. [DOI] [PubMed] [Google Scholar]
- Keck R. W., Dilley R. A., Ke B. Photochemical characteristics in a soybean mutant. Plant Physiol. 1970 Nov;46(5):699–704. doi: 10.1104/pp.46.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMURA M., ITO T., CHANCE B. Studies on bacterial photophosphorylation. III. A sensitive and rapid method of determination of photophosphorylation. Biochim Biophys Acta. 1962 May 7;59:177–182. [PubMed] [Google Scholar]
- Plaut Z. Inhibition of photosynthetic carbon dioxide fixation in isolated spinach chloroplasts exposed to reduced osmotic potentials. Plant Physiol. 1971 Nov;48(5):591–595. doi: 10.1104/pp.48.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. R., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: II. Role of Osmotic Potential. Plant Physiol. 1973 Jun;51(6):993–997. doi: 10.1104/pp.51.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. S., Sauer K. Pigment systems and electron transport in chloroplasts. I. Quantum requirements for the two light reactions in spinach chloroplasts. Biochim Biophys Acta. 1971 Jun 15;234(3):399–414. doi: 10.1016/0005-2728(71)90207-6. [DOI] [PubMed] [Google Scholar]
- Treharne K. J., Stoddart J. L. Effects of gibberellin on photosynthesis in red clover (Trifolium pratense L.). Nature. 1968 Nov 2;220(5166):457–458. doi: 10.1038/220457a0. [DOI] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Wareing P. F., Khalifa M. M., Treharne K. J. Rate-limiting processes in photosynthesis at saturating light intensities. Nature. 1968 Nov 2;220(5166):453–457. doi: 10.1038/220453a0. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


