Abstract
HIV entry into target cells is a highly sequential and time-sensitive process. In recent years, potent HIV Env-targeting antibodies, such as VRC01, have been identified. However, antibodies bind only to a single epitope, and mutations that confer resistance to antibody-mediated inhibition of HIV entry have been detected. In contrast, HIV cannot escape from binding to soluble CD4 (sCD4) without a fitness disadvantage. sCD4 has the unique ability to induce conformational changes within the HIV envelope glycoproteins (Env) that allow fusion inhibitors to bind. We have previously linked sCD4 to the fusion inhibitor FIT45 (sCD4-FIT45) and examined delivery of the bifunctional entry inhibitor via gene therapy. Here, we extend our studies and analyze the ability of sCD4-FIT45 to inhibit HIV Env-mediated cell fusion and HIV entry of several primary isolates. sCD4-FIT45 inhibited both cell fusion and HIV entry with remarkable antiviral activity. The mean 50% inhibitory concentrations (IC50) for sCD4-FIT45 were <0.2 μg/mL in both assays. Importantly, inhibition by sCD4-FIT45 was more potent than by VRC01, sCD4, or the previously described bifunctional protein sCD4-scFv17b. In contrast to sCD4, sCD4-FIT45 as well as VRC01 and sCD4-scFv17b did not mediate cell fusion between HIV Env+ and CD4−CCR5+ cells. The results presented here provide further evidence for the testing of sCD4-FIT45 and development of bifunctional proteins based on the sCD4-fusion inhibitor architecture.
Keywords: bifunctional antiviral proteins, entry inhibitor, fusion inhibitor, sCD4, VRC01, sCD4-FIT45, gene therapy, HIV, entry, cell fusion
Introduction
HIV entry is a highly sequential and time-sensitive process that can be divided into receptor binding, co-receptor binding, and membrane fusion. CD4 serves as the primary receptor, while CCR5 and CXCR4 function as co-receptors. Entry into target cells is mediated by HIV Env, which is comprised of a gp120/gp41 heterotrimer. Interaction of the gp120 subunit with CD4 enables gp120 binding to CCR5 or CXCR4. Co-receptor binding triggers gp41 to assume a hairpin formation that mediates the fusion of viral and cellular membranes.
Targeting HIV Env remains challenging due to the ability of the virus to shield conserved immunogenic epitopes with extensive glycan structures.1, 2, 3, 4 The CD4-binding site on gp120 constitutes one of the few sites that are not shielded in the absence of target cell binding.1, 5 The antibody VRC01 targets the CD4-binding site by partially mimicking the interaction of gp120 with the CD4 receptor.6 While VRC01 is one of the best broadly neutralizing antibodies identified to date, HIV isolates with naturally occurring resistance to VRC01 have evolved in patients.7 Further studies have shown that a single mutation in gp120 is sufficient to negatively impact the affinity of VRC01 to gp120 without affecting the capability of gp120 to bind to CD4.8
In contrast to VRC01, soluble CD4 (sCD4) is identical to the extracellular gp120-binding domains of CD4.9, 10 Since HIV needs to retain its ability to interact with CD4 on target cells, mutations that affect binding to sCD4 inevitably reduce viral fitness.11 However, some HIV isolates require relatively high concentrations of sCD4 for inhibition without an evident loss of fitness.12 Further analyses of HIV Env have revealed no consistent correlation between higher resistance to sCD4-mediated inhibition and sCD4-binding affinity.13, 14 Interaction of sCD4 with gp120 prematurely triggers the entry process in the absence of target cells and induces extensive conformational changes within HIV Env. This sCD4-induced conformational state is transient and followed by the permanent inactivation of HIV Env.15 It has been shown that the sCD4-induced conformational state of resistant isolates is more stable than that of sensitive isolates.15 Thus, sensitive isolates rapidly progress to the inactivated state upon sCD4-binding, while resistant isolates remain infectious for a prolonged period of time despite binding of sCD4.15
Importantly, otherwise inaccessible epitopes are exposed on HIV Env in the sCD4-induced conformational state. Therefore, combining sCD4 with inhibitors that target these epitopes could represent a method to counteract the viral escape from sCD4-mediated inhibition. The gp120 unit of HIV Env is comprised of five highly variable regions (V1–V5) and five conserved regions16 that form an inner and outer domain that is connected by a bridging sheet.17 sCD4-induced changes in gp120 are well documented. Binding of sCD4 to gp120 results in the movement of V1/V2, V3, and the bridging sheet, thereby exposing the co-receptor binding site as well as several conserved discontinuous epitopes.17, 18 The single-chain variable fragment of the human mAb 17b (scFv17b) targets a conserved sCD4-induced epitope on gp120 that partially overlaps with the co-receptor-binding site.17, 19 Covalently linking sCD4 to scFv17b (sCD4-scFv17b) has previously been shown to drastically increase the antiviral activity of sCD4,20, 21 thereby providing evidence for the benefit of exploiting the sCD4-induced conformational state.
In contrast to gp120, exposure of gp41 epitopes upon binding of sCD4 to HIV Env is less studied. The ectodomain of gp41 contains a fusion peptide and two heptad repeats (HR1 and HR2) that are connected by a loop region. It has previously been shown that binding of sCD4 to HIV Env increases binding of gp41-targeting antibodies.22 Several studies have shown that sCD4-induced changes in HIV Env result in the exposure of the HR1 of gp41 and allow fusion inhibitors to bind in the absence of co-receptor engagement.15, 23, 24, 25 Fusion inhibitors are short peptides derived from the HR2 sequence of gp41. Their binding to HR1 prevents gp41 from assuming the formation of the hairpin structure that mediates membrane fusion. The first generation fusion inhibitor T20 (Enfuvirtide/Fuzeon) is approved for clinical use. Second and third generation fusion inhibitors contain minor alterations in their amino acid sequences that result in improved antiviral potency with decreased probability of viral escape.26
Based on the ability of sCD4 to expose the HR1 region, we have previously covalently linked sCD4 to the third generation fusion inhibitor FIT4527 to generate a bifunctional entry inhibitor, sCD4-FIT45. We have shown that sCD4-FIT45 has an improved antiviral activity over sCD4, a fusion inhibitor FIT20, or combinations thereof.28 Expression of sCD4-FIT45 from gene-modified cells protected peripheral blood mononucleacytes (PBMCs) from infection and prevented viral spread from infected-to-uninfected PBMCs.28 Here, we extend these studies and show that sCD4-FIT45 is superior to sCD4, VRC01, and sCD4-scFv17b in protecting cells from HIV Env-mediated cell fusion and infection with several primary isolates.
Results
Design and Characterization of Bifunctional Entry Inhibitors
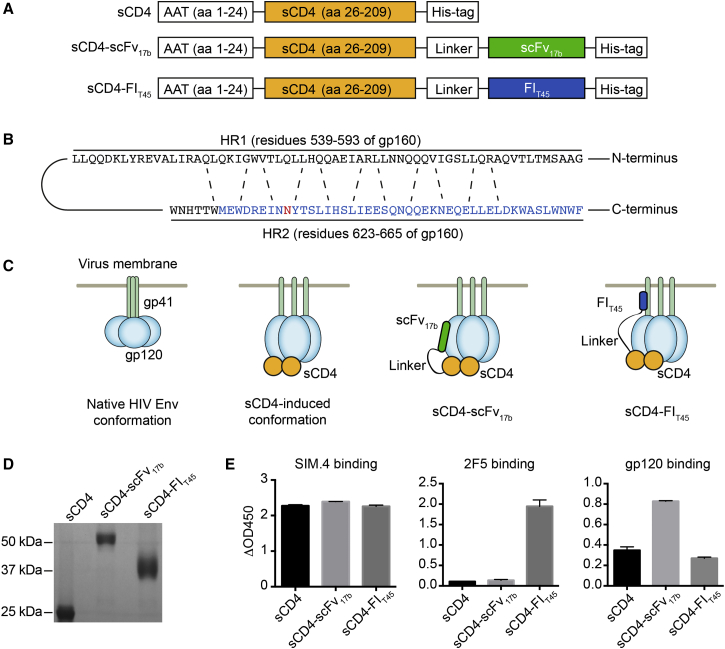
Genes encoding sCD4, sCD4-scFv17b, and sCD4-FIT45 were designed for expression and secretion from human cells. The signal peptide marks proteins for the secretory pathway. We have previously shown that substituting the native signal peptide sequence of sCD4 with the signal peptide of alpha-1 antitrypsin (AAT) increases secretion from HEK293T cells.29 Therefore, we used the AAT signal peptide instead of the native signal peptide to mediate secretion of all three proteins. sCD4 was comprised of amino acids 26–209 of CD4 and linked to scFv17b or FIT45 by a flexible linker consisting of seven GGGGS repeats. All proteins contained a C-terminal His-tag for detection and purification purposes. A schematic of the proteins is shown in Figure 1A. The sequence of FIT45 in relation to the HR1 and HR2 of HIV gp160 is depicted in Figure 2B. The anticipated mode of action of the bifunctional entry inhibitors, sCD4-scFv17b and sCD4-FIT45, is illustrated in Figure 1C.
Figure 1.
Bifunctional Entry Inhibitors
(A) Design of sCD4, sCD4-scFv17b, and sCD4-FIT45. A linker sequence consisting of seven GGGGS repeats was used to covalently link sCD4 to scFv17b or FIT45. All proteins were designed to contain the AAT signal peptide at the N terminus and a His-tag at the C terminus. Aa, amino acids. (B) Design of FIT45. The sequence of the HR1 and HR2 of HIVHXB2 (GenBank: AAB50262.1) is shown. The interactions between residues are indicated by dashed lines. The sequence of FIT45 is highlighted in blue. A potential glycosylation site is highlighted in red. (C) Anticipated mode of action. Binding of sCD4 to gp120 induces conformational changes that allow binding of scFv17b and FIT45. (D) SDS-PAGE analysis of purified proteins. The inhibitors were purified from the culture supernatant of gene-modified 293T cells. Equal volumes of the proteins were loaded onto a 12% gel. Following electrophoresis, the gel was stained with Coomassie brilliant blue. (E) Characterization of bifunctional entry inhibitors. Binding of the proteins to an anti-CD4 antibody (SIM.4), an anti-gp41 antibody (2F5), and gp120 was analyzed by ELISA. ΔOD450 values were generated by subtracting values of the negative control from test sample values. The data are means from two independent experiments. Error bars represent the SEM.
Figure 2.
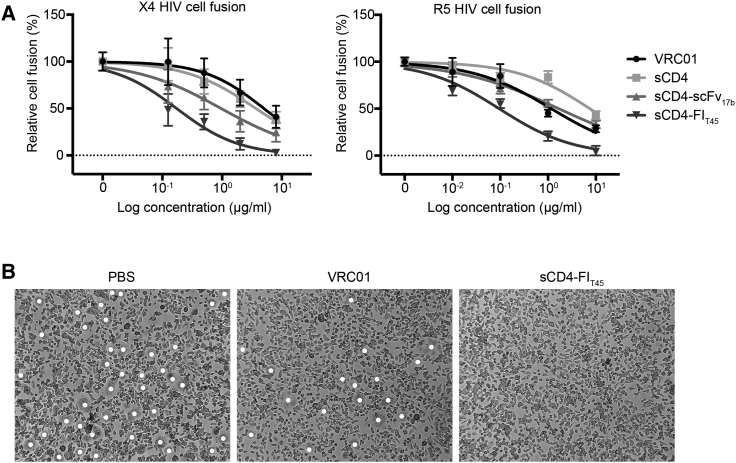
Inhibition of HIV Env-Mediated Cell Fusion
(A) CHO-WT cells expressing the HIVHXB2 Env proteins (X4 HIV cell fusion) or 293T cells expressing the HIVJRFL Env proteins (R5 HIV cell fusion) were cultured with TZM-bl cells in the presence of inhibitors at the indicated concentrations. The number of syncytia was determined by light microscopy. (B) Representative microscope images of R5 HIV Env-mediated cell fusion are shown for cells incubated with PBS, VRC01, or sCD4-FIT45. The concentration of VRC01 and sCD4-FIT45 was 10 μg/mL. Syncytia are marked with white dots. The data are means ± SEM from three independent experiments. The data for VRC01 are from two experiments.
We designed the lentiviral vectors LV-CMV-AAT-sCD4, LV-CMV-AAT-sCD4-scFv17b, and LV-CMV-AAT-sCD4-FIT45 to express the genes encoding these proteins under the control of the human cytomegalovirus immediate-early promoter/enhancer. The lentiviral vectors were used to generate 293T cells that constitutively express sCD4, sCD4-scFv17b, or sCD4-FIT45 The three proteins were purified from the culture supernatant of gene-modified 293T cells and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Coomassie blue staining revealed that sCD4, sCD4-scFv17b, and sCD4-FIT45 migrated at the expected size of 26 kDa, 54 kDa, and 35 kDa, respectively (Figure 1D). It is of note that a diffuse band was observed for sCD4-FIT45, which was potentially due to a glycosylation site present in the fusion inhibitor moiety (Figure 1D). We used enzyme-linked immunosorbent assays (ELISAs) to determine binding of sCD4 and the two bifunctional proteins to antibodies and gp120 (Figure 1E). All three proteins bound to the monoclonal anti-human CD4 antibody SIM.4 that targets the gp120-binding site on CD4 (Figure 1E, left panel). In contrast, only sCD4-FIT45 bound to the 2F5 antibody that recognizes the HR2 region of gp41 (Figure 1E, central panel), indicating that sCD4-FIT45 was correctly folded. In the binding assays with gp120, we observed equal binding of sCD4 and sCD4-FIT45 to gp120 (Figure 1E, right panel). In comparison, sCD4-scFv17b showed increased binding to gp120, which may indicate that sCD4-scFv17b could have a higher affinity to gp120 than sCD4. However, further experiments are required to determine whether the 17b moiety increases the affinity of sCD4 to gp120.
Inhibition of HIV Env-Mediated Cell Fusion
HIV can infect cells by virus-to-cell and cell-to-cell transmission. Interaction of HIV Env on the cell-free virus with cellular receptors mediates fusion of the viral and cellular membranes. Contrary to virus-to-cell infection, interaction of HIV Env on the surface of infected cells with receptors on uninfected cells leads to the formation of stable adhesive junctions termed virological synapses.30 Once the synapse has been established, virions bud preferentially at the cell contact sites into the synaptic space between the infected and uninfected cells.31 Engagement of HIV Env on the newly emerged virions with the receptors on the uninfected cell then mediates virus entry.32 It has previously been shown that entry inhibitors can prevent cell-to-cell transmission by interfering with the formation of the virological synapse and by inhibiting virus entry in the synaptic space between infected and uninfected cells.33 We tested the ability of VRC01, sCD4, sCD4-scFv17b, and sCD4-FIT45 to inhibit the interaction of cell surface-associated HIV Env with HIV target cells in a cell fusion assay. In this assay, co-culture of cells expressing HIV Env with target cells results in the formation of large multinucleated cells (syncytia). CHO-WT cells expressing X4 HIV EnvHXB2 or 293T cells expressing R5 HIV EnvJRFL were incubated with CD4+CXCR4+CCR5+ TZM-bl cells in the presence of the different inhibitors at graded concentrations. The formation of syncytia was assessed by light microscopy. All proteins inhibited cell fusion in a dose-dependent manner (Figure 2A). Representative microscope images are shown in Figure 2B. A summary of the IC50 of HIV Env-mediated cell fusion is shown in Table 1. IC50 values in the assay with X4 HIV Env were 30-, 20-, and 6-fold lower for sCD4-FIT45 than for VRC01, sCD4, and sCD4-scFv17b, respectively. A similar observation was made for R5 HIV Env-mediated cell fusion, where IC50 values for sCD4-FIT45 were 7-fold lower than for VRC01, 80-fold lower than for sCD4, and 20-fold lower than for sCD4-scFv17b.
Table 1.
Inhibition of HIV Env-Mediated Cell Fusion
| HIV Env | IC50 (μg/mL) |
|||
|---|---|---|---|---|
| sCD4-FIT45 | sCD4 | VRC01 | sCD4-scFv17b | |
| HXB2 | 0.173 | 3.676 | 5.003 | 0.961 |
| JRFL | 0.095 | 7.509 | 1.169 | 1.676 |
Cell fusion experiments were performed as described in Figure 2. The IC50 values for X4 HIV cell fusion (HXB2) and R5 HIV cell fusion (JRFL) are shown.
sCD4-Mediated Cell Fusion with CD4−CCR5+ Cells
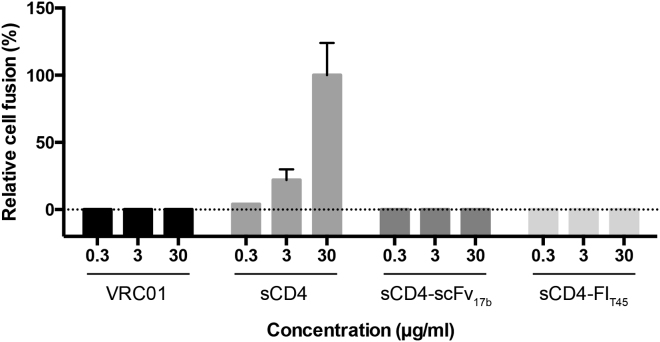
Initial clinical trials with sCD4 showed modest dose-dependent reductions of viral load in patients.34 Follow-up studies with higher concentrations of sCD4 showed a reduction of the cell-free virus to undetectable levels during a 2-week trial period.35 sCD4-immunoglobulin G fusion proteins (sCD4-IgGs) showed improved antiviral activity and stability in vitro,36, 37 but failed to show dose-dependent inhibition in patients, which was attributed to potential interactions between the immunoglobulin domain and Fc receptors.38, 39 Importantly, no adverse effects following administration of sCD4 or sCD4-IgGs have been reported. However, sCD4 has been shown to mediate infection of CD4−CCR5+ cells in vitro.15, 40, 41 To investigate whether sCD4-FIT45 would exhibit a similar activating effect, we incubated 293T cells expressing R5 HIV EnvJRFL with CD4−CCR5+ Cf2ThsynCCR5 cells in the presence of sCD4 and sCD4-FIT45. VRC01 and sCD4-scFv17b served as controls. Following incubation, the number of syncytia was determined by microscopy. sCD4 increased syncytia formation in a dose-dependent manner, showing that sCD4 could substitute for the CD4 receptor and cause HIV Env-mediated cell fusion (Figure 3). In contrast, no syncytia formation was observed in cells treated with VRC01, sCD4-scFv17b, or sCD4-FIT45, indicating that VRC01 and bifunctional proteins do not have the enhancing effect of sCD4 (Figure 3).
Figure 3.
sCD4-Mediated Cell Fusion
293T cells expressing the HIVJRFL Env protein were cultured with CD4−CCR5+ Cf2ThsynCCR5 cells in the presence of inhibitors at the indicated concentrations. The number of syncytia was determined by microscopy. The data are means from two independent experiments. Error bars represent the SEM.
Inhibition of HIV Entry
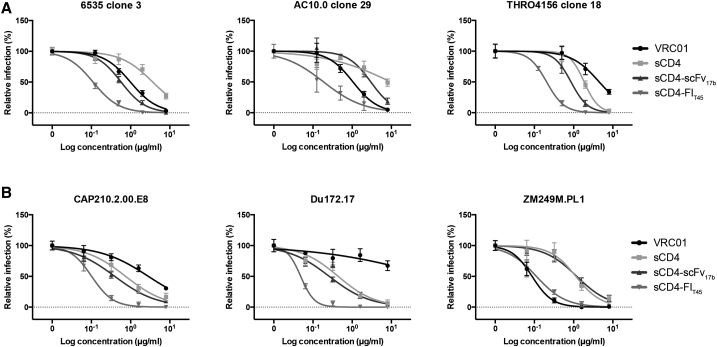
We characterized the ability of sCD4-FIT45 to neutralize several HIV isolates of clade B and C in single-round infection assays. In parallel, we assayed VRC01, sCD4, and sCD4-scFv17b. Examples of neutralization curves for primary isolates from clade B and C are shown in Figures 4A and 4B, respectively. A summary of the IC50 values for the different inhibitors is shown in Table 2. The results obtained in our assay were compared to published results where available (Table 2). The bifunctional protein sCD4-FIT45 substantially outperformed VRC01, sCD4, and sCD4-scFv17b. The neutralization potency of sCD4-FIT45 was 5- to 30-fold better in comparison to the other tested inhibitors. For some isolates, we observed over 100-fold improved neutralization. Importantly, all tested isolates were sensitive to inhibition by sCD4-FIT45, and neutralization by sCD4-FIT45 was superior to inhibition by sCD4 for every tested isolate. Additionally, we compared inhibition by sCD4-FIT45 and sCD4 in single-round infection assays and productive infection of PBMCs using replication-competent R5 HIVBaL (Table 2). In single-round infection assays, sCD4-FIT45 was 5-fold more potent than sCD4, whereas in the productive infection assay, sCD4-FIT45 was 10-fold better than sCD4. It is of note that significantly more virus and cells were used and the experimental endpoint was later (7 days post-infection) in the productive infection assay than in the single-round infection assay, resulting in higher IC50 values in the productive infection assay. However, the improved effect of sCD4-FIT45 was evident irrespective of the assay used.
Figure 4.
Inhibition of HIV Entry in Single-Round Infection Assays
TZM-bl cells were infected with HIV isolates in the presence of inhibitors at the indicated concentration. The details are provided in the Materials and Methods section. (A) Clade B isolates. (B) Clade C isolates. The results are representative of two independent experiments or triplicates. Data are represented as means ± SEM.
Table 2.
Inhibition of HIV Entry
| HIV Env | IC50 (μg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Clade | sCD4-FIT45 | sCD4 | sCD4a | VRC01 | VRC01a | sCD4-scFv17b | sCD4-scFv17ba | |
| 6535_3 | B | 0.11 | 3.68 | 1.05 | 0.99 | 1.18 | 0.58 | 1.66 |
| AC10.0_29 | B | 0.19 | >8.00 | 10.71 | 1.05 | 1.57 | 2.98 | 4.58 |
| CAAN5342 | B | 0.57 | >8.00 | 18.51 | NA | 0.99 | NA | 1.06 |
| PVO_4 | B | 0.50 | >8.00 | 8.18 | NA | 0.35 | NA | 0.67 |
| QH0692_42 | B | 0.18 | 1.87 | 0.65 | 1.02 | 1.4 | NA | 0.66 |
| REJO4541_67 | B | 0.12 | 3.59 | 1.50 | NA | 0.05 | NA | 1.05 |
| THRO4156_18 | B | 0.21 | 1.85 | 0.20 | 4.49 | 3.48 | 0.86 | 0.86 |
| WITO4160_33 | B | 0.26 | >8.00 | 6.42 | 0.12 | 0.12 | NA | 1.45 |
| CAP210.2.00.E8 | C | 0.02 | 0.75 | 1.96 | 2.84 | >50 | 0.40 | 1.06 |
| Du172.17 | C | 0.05 | 0.44 | 2.48 | >8 | >50 | 0.26 | 0.52 |
| ZM249M.PL1 | C | 0.11 | 1.14 | 12.65 | 0.09 | 0.06 | 1.2 | 1.82 |
| ZM53M.PB12 | C | 0.12 | 0.72 | 8.80 | 0.40 | 1.01 | 0.83 | 1.01 |
| HXB2 | B | 0.006 | 0.033 | 0.023 | NA | 0.033 | 0.008 | NA |
| JRFL | B | 0.016 | 2.000 | 1.52 | NA | 0.03 | 0.13 | NA |
| BaL_01b | B | 0.006 | 0.025 | 0.08 | 0.011 | 0.08 | 0.016 | 0.1 |
| BaL_01c | B | 0.430 | 4.237 | NA | NA | NA | NA | NA |
| Mean | 0.181 | 4.010 | 4.982 | 6.101 | 7.357 | 0.726 | 1.269 | |
| Median | 0.120 | 1.935 | 1.960 | 1.005 | 0.990 | 0.490 | 1.050 | |
Single-round infection assays were performed by infecting TZM-bl cells with HIV isolates from clade B or C in the presence of the indicated inhibitors at graded concentrations. The corresponding IC50 values are shown. Reduction of HIV entry in PBMCs was determined as described in the Materials and Methods section.
Single-round infection assay.
Productive infection of PBMCs.
Discussion
Our experiments reveal that the bifunctional protein sCD4-FIT45 is a potent inhibitor of HIV entry and HIV Env-mediated cell fusion. Unlike sCD4, sCD4-FIT45 inactivates the HIV Env upon binding and does not enhance infection of CD4−CCR5+ cells. While no adverse effects following sCD4 administration were observed in clinical trials,34, 35, 38, 39 it is not clear if sCD4-mediated infection of CD4− cells plays a role in vivo. Contrary to sCD4, binding of VRC01 does not induce conformational changes that expose conserved binding sites in HIV Env.42 Accordingly, we did not observe any syncytia formation when HIV Env-expressing cells were incubated with CD4−CCR5+ cells in the presence of VRC01. While the neutralization potential of sCD4-scFv17b is well documented, the ability to mediate infection of CD4− cells has never been reported. Like sCD4-FIT45, sCD4-scFv17b did not enhance infection, indicating that the bifunctional design leads to inactivation of the virus and inhibits events downstream of sCD4 binding.
HIV cell-to-cell transmission is an important mechanism of viral spread.43 It has previously been shown that VRC01-mediated inhibition of cell fusion is significantly worse than inhibition of the cell-free virus.44 sCD4-scFv17b has never been tested in cell fusion assays. Our results indicate that the neutralizing antibody VRC01, sCD4, and even the bifunctional protein sCD4-scFv17b are relatively inefficient at blocking HIV Env-mediated cell fusion. sCD4-FIT45 is the only inhibitor that mediated substantial reduction of cell fusion. The improved neutralization was likely due to the inclusion of the fusion inhibitor moiety. In comparison to inhibition of the cell-free virus, we observed that higher concentrations of VRC01, sCD4, sCD4-scFv17b, and sCD4-FIT45 were needed to inhibit cell fusion by HIV Env of the same isolates. Unless HIV Env assumes a different conformation on the cell surface, the inhibitors should have similar affinities for HIV Env on the cell and virion surface. The concentration of entry inhibitors at which efficient inhibition occurs should be dependent on the number and potential density of target molecules. Therefore, it is likely that there was a higher number or density of HIV Env in our cell fusion assays than in our single-round infection assays. The site where viral fusion occurs could also contribute to the observed differences. It has previously been shown for TZM-bl cells that HIV particles are internalized following receptor engagement and that fusion takes place in endosomes.45 In contrast, fusion occurs at the cell membrane in cell fusion assays. Nevertheless, the exact reason for the stronger inhibition of the cell-free virus is unclear and requires further investigation.
We have previously described the development of sCD4-FIT45 and examined its potential in a gene therapy approach.28 Lu et al.46 have developed a similar inhibitor by linking sCD4 to a different fusion inhibitor, T1144, and analyzed the mechanism of inhibition in detail. However, sCD4 linked to a fusion inhibitor has never been tested against a panel of primary isolates. We show here that sCD4-FIT45 can inhibit entry of primary isolates at consistently low concentrations, providing further evidence for the benefit of bifunctional inhibitors and exploiting the sCD4-induced conformational change. None of the tested isolates showed pre-existing resistance to sCD4-FIT45. Interestingly, HIV seems unable to escape binding to sCD4, and resistance to the latest generation of fusion inhibitors has not been observed.11, 15, 26 Therefore, escape from inhibition by sCD4-FIT45 may not happen without a severe toll on viral fitness. Nevertheless, the ability of HIV to develop resistance and escape from inhibition by sCD4-FIT45 needs to be addressed in the future.
Further improvements to the sCD4-fusion inhibitor design are possible. One-domain sCD4s have been described that contain only the domain that allows gp120 binding.47 The one-domain sCD4s showed higher affinity for gp120 than sCD4 and induced the same conformational changes. The two-domain sCD4 used in our study could be exchanged to a one-domain sCD4. Optimizing the length and composition of the linkers could also improve the potency of the bifunctional protein.48 Different fusion inhibitors could also be tested.
Administration of the latest generation of antibodies for the treatment of HIV-positive individuals is currently under investigation in clinical trials.7, 49 Passive immunization with antibodies has also shown promising results in preventing SHIV infection in macaques.50 Alternatively, sCD4-FIT45 could be delivered by gene therapy. Genetically modifying cells or tissues for the secretion of entry inhibitors via adeno-associated virus-derived vectors is currently under investigation for the prevention of HIV infection.51, 52 We have recently shown that secretion of sCD4 from gene-modified hematopoietic cell-lineages can control HIV infection in a mouse model of HIV infection (unpublished data). Since sCD4-FIT45 is highly effective against primary HIV isolates, it should be further evaluated for HIV treatment and prevention.
Materials and Methods
Cells
HEK293T cells were acquired from Dr. Jason Moffat (University of Toronto). The following cell lines were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Drs. Kappes and Wu and Tranzyme;53, 54, 55, 56, 57 CHO-WT from Drs. Carol Weiss and Judith White;58 and CfThsynCCR5 from Drs. Tajib Mirzabekov and Joseph Sodroski.59 PBMCs from healthy donors were obtained from Dr. Mario Ostrowski (University of Toronto). 293T, TZM-bl, and CfThsynCCR5 cells were cultured in DMEM (Thermo Fisher Scientific), supplemented with 10% fetal bovine serum (FBS). CHO-WT cells were cultured in RPMI (Thermo Fisher Scientific), supplemented with 10% FBS and 400 μM L-methionine sulfoximine (Sigma-Aldrich). PBMCs were activated by incubation in RPMI supplemented with 20% FBS, 40 units/mL interleukin-2 (BioShop), 2 mM L-glutamine (Thermo Fisher Scientific), and 1% phytohemagglutinin-M (PHA-M, Thermo Fisher Scientific) for 3 days. Activated PBMCs were cultured in the same media without PHA-M. All culture media contained 1% Antibiotic-Antimycotic (Thermo Fisher Scientific).
Plasmids
We previously described the generation of the self-inactivating lentiviral vector construct pLV-CMV-AAT-sCD4 encoding sCD4 with the AAT signal peptide.29 The vector pLV-CMV-AAT-sCD4-scFv17b encoding sCD4-scFv17b with the AAT signal peptide was generated by ligating the PspOMI-to-XmaI fragment of pLVX-sCD4-scFv17b28 to the XmaI-to-EcoRI and EcoRI-to-PspOMI fragments of pLV-CMV-AAT-sCD4. The vector pLV-CMV-AAT-sCD4-FIT45 encoding sCD4-FIT45 with the AAT signal peptide was generated by inserting the BamHI-to-NotI fragment of pLVX-sCD4-FIT4528 into the same sites in pLV-CMV-AAT-sCD4. Dr. Donald Branch (University of Toronto) provided pJRFL-Env and pHXB2-Env. The following plasmids were obtained through the NIH AIDS Reagent Program: psPAX2 and pMD2.G from Dr. Didier Trono; pSG3ΔEnv from Drs. Kappes and Wu;1, 55 panel of HIV subtype B Env clones from Drs. David C. Montefiori, Feng Gao, Ming Li, Beatrice H. Hahn, Jesus F. Salazar-Gonzalez, and Denise L. Kothe;1, 55, 60 and panel of HIV subtype C Env clones from Drs. Ramesh S. Paranjape, Smita S. Kulkarni, and Montefiori.61
Proteins
Lentiviral vector production, generation of gene-modified 293T cells, and purification of sCD4, sCD4-scFv17b, and sCD4-FIT45 from the culture media were performed as previously described.29 The concentration of purified and desalted proteins was determined by His-tag ELISA (GenScript) with an sCD4 protein standard of known concentration.29 The following proteins were obtained through the NIH AIDS Reagent Program: anti-Human CD4 monoclonal antibody (Sim.4) from Dr. James Hildreth; anti-HIV gp41 monoclonal antibody (2F5) from Dr. Hermann Katinger;62, 63, 64 anti-HIV gp120 monoclonal antibody (VRC01) from Dr. John Mascola;65 and HIV-1BaL gp120.
Binding Assays
In order to detect binding to the antibodies SIM.4 and 2F5, sCD4, sCD4-scFv17b, or sCD4-FIT45 were diluted in coating buffer (0.05 M sodium carbonate buffer; pH 9.6) and coated onto untreated 96-wells overnight at 4°C (100 μL and 10 μg/mL). Wells coated with scFv17b29 served as a control for no binding. The next day, the wells were washed four times with washing buffer (PBS containing 0.05% Tween 20) and blocked with blocking buffer (PBS containing 5% bovine serum albumin fraction V, BSA) for 2 hr at room temperature. Following four washes, SIM.4 or 2F5 was diluted in binding buffer (PBS containing 0.1% BSA and 0.05% Tween 20) and added to each well (100 μL and 1 μg/mL). After 1 hr at room temperature, the wells were washed four times and polyclonal goat anti-human antibodies conjugated to horseradish peroxidase (100 μL, 1:2,500 dilution in binding buffer; Promega) were added to the wells. After 30 min, the wells were washed four times and tetramethylbenzidine (TMB) substrate (GenScript) was added. The reaction was stopped after 5–10 min by adding Stop Solution (GenScript) and the optical density at 450 nm (OD450) was measured using a standard plate reader. Values from negative control wells that were coated with scFv17b, which should not bind to SIM.4 or 2F5, were subtracted from assay wells (ΔOD450).
To detect binding to gp120, purified HIVBaL gp120 was diluted in coating buffer and added to untreated 96-wells overnight at 4°C (100 μL per well and 2 μg/mL). Uncoated wells served as a control. The wells were washed and blocked for 2 hr at room temperature. Following washes, sCD4, sCD4-scFv17b, or sCD4-FIT45 diluted in binding buffer was added to all wells (50 μL and 2 μg/mL) and incubated overnight at 4°C. The wells were washed and incubated with a ready-to-use mouse anti-His-tag monoclonal antibody solution (GenScript) for 1 hr at room temperature. After washing, ready-to-use horseradish peroxidase-conjugated goat anti-mouse IgG solution (GenScript) was added to each well for 30 min. After the final wash step, TMB substrate was added, and the reaction was stopped after 5 min. The OD 450 was determined and values of uncoated control wells were subtracted from those of wells coated with gp120 (ΔOD450).
HIV Env-Mediated Cell Fusion
CHO-WT cells stably express X4 HIV Env. For the expression R5 HIV Env, 293T cells were transfected with 10.5 μg psPAX2 and 10.5 μg pJRFL-Env using the calcium phosphate method as previously described.29 The next day, the media were replaced by fresh media, and the cells were incubated for one additional day. On the day of the fusion assay, CHO-WT or transfected 293T cells were detached using PBS containing 1 mM ethylene-diamine tetraacetic acid (EDTA, Thermo Fisher Scientific) and washed twice with PBS supplemented with 1% FBS. HIV Env-expressing cells (1 × 105 cells) were seeded into a 24-well dish in the presence of inhibitors at the indicated concentrations. The cells were incubated for 30 min to allow the cells to attach and 2 × 105 TZM-bl cells were added. The cells were co-cultured for 6–8 hr, fixed with 1% formaldehyde (Sigma-Aldrich), and stained with Giemsa. Syncytia formation was analyzed by light microscopy. For each well, three microscope images were taken and the number of syncytia was counted. sCD4-mediated cell fusion into CD4−CCR5+ cells was analyzed by using CfThsynCCR5 cells instead of TZM-bl cells.
Inhibition of HIV Entry
A replication-incompetent virus was generated by calcium phosphate transfection of 293T cells with 10.5 μg of pSG3Δenv and 10.5 μg of an envelope encoding plasmid as previously described.29 The TCID50 and inhibition of HIV entry in single-round infection assays were determined in TZM-bl cells that express the lacZ gene under the control of HIV Tat. Virus (5 × 103 TCID50) was mixed with inhibitors at the indicated concentrations and added to 5 × 104 TZM-bl cells in the presence of polybrene (8 μg/mL final concentration, Sigma-Aldrich) in 24-well plates. The cells were incubated for 2 days, fixed with 1% formaldehyde for 5 min, and stained with X-Gal (0.4 mM potassium ferricyanide, 0.4 mM potassium ferrocyanide, 20 mM magnesium chloride, and 0.4 μg/mL X-gal) overnight at room temperature. Microscopy images were taken of each well and infected (blue) cells were counted.
For productive infection, replication-competent HIV (1 × 105 TCID50) and the inhibitors were added to 1 × 106 activated PBMCs, followed by 5 hr incubation. The cells were then washed to remove the unbound virus and the inhibitors. PBMCs were resuspended in 1 mL media and incubated for 1 week before the concentration of HIV p24 antigen in the supernatants was determined by ELISA (MyBioSource).
Author Contributions
A.F. designed and performed the experiments. S.J. initiated the study. A.F. and S.J. analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
Acknowledgments
This research was funded by the Canadian Institutes of Health Research and the Canadian Foundation for AIDS Research. A.F. was supported by the Laboratory Medicine and Pathobiology Program at the University of Toronto.
References
- 1.Wei X., Decker J.M., Wang S., Hui H., Kappes J.C., Wu X., Salazar-Gonzalez J.F., Salazar M.G., Kilby J.M., Saag M.S. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 2.Pancera M., Zhou T., Druz A., Georgiev I.S., Soto C., Gorman J., Huang J., Acharya P., Chuang G.Y., Ofek G. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.H., Ozorowski G., Ward A.B. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science. 2016;351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy L.E., van Gils M.J., Ozorowski G., Messmer T., Briney B., Voss J.E., Kulp D.W., Macauley M.S., Sok D., Pauthner M. Holes in the glycan shield of the native HIV envelope are a target of trimer-elicited neutralizing antibodies. Cell Rep. 2016;16:2327–2338. doi: 10.1016/j.celrep.2016.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheid J.F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T.Y., Pietzsch J., Fenyo D., Abadir A., Velinzon K. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., O’Dell S., Walker L.M., Wu X., Guenaga J., Feng Y., Schmidt S.D., McKee K., Louder M.K., Ledgerwood J.E. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J. Virol. 2011;85:8954–8967. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch R.M., Boritz E., Coates E.E., DeZure A., Madden P., Costner P., Enama M.E., Plummer S., Holman L., Hendel C.S., VRC 601 Study Team Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci. Transl. Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 8.Guo D., Shi X., Arledge K.C., Song D., Jiang L., Fu L., Gong X., Zhang S., Wang X., Zhang L. A single residue within the V5 region of HIV-1 envelope facilitates viral escape from the broadly neutralizing monoclonal antibody VRC01. J. Biol. Chem. 2012;287:43170–43179. doi: 10.1074/jbc.M112.399402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith D.H., Byrn R.A., Marsters S.A., Gregory T., Groopman J.E., Capon D.J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987;238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 10.Berger E.A., Fuerst T.R., Moss B. A soluble recombinant polypeptide comprising the amino-terminal half of the extracellular region of the CD4 molecule contains an active binding site for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA. 1988;85:2357–2361. doi: 10.1073/pnas.85.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch R.M., Wong P., Tran L., O’Dell S., Nason M.C., Li Y., Wu X., Mascola J.R. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J. Virol. 2015;89:4201–4213. doi: 10.1128/JVI.03608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daar E.S., Ho D.D. Relative resistance of primary HIV-1 isolates to neutralization by soluble CD4. Am. J. Med. 1991;90(4A):22S–26S. doi: 10.1016/0002-9343(91)90407-o. [DOI] [PubMed] [Google Scholar]
- 13.Ivey-Hoyle M., Culp J.S., Chaikin M.A., Hellmig B.D., Matthews T.J., Sweet R.W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc. Natl. Acad. Sci. USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brighty D.W., Rosenberg M., Chen I.S., Ivey-Hoyle M. Envelope proteins from clinical isolates of human immunodeficiency virus type 1 that are refractory to neutralization by soluble CD4 possess high affinity for the CD4 receptor. Proc. Natl. Acad. Sci. USA. 1991;88:7802–7805. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haim H., Si Z., Madani N., Wang L., Courter J.R., Princiotto A., Kassa A., DeGrace M., McGee-Estrada K., Mefford M. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog. 2009;5:e1000360. doi: 10.1371/journal.ppat.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starcich B.R., Hahn B.H., Shaw G.M., McNeely P.D., Modrow S., Wolf H., Parks E.S., Parks W.P., Josephs S.F., Gallo R.C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986;45:637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- 17.Kwong P.D., Wyatt R., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thali M., Moore J.P., Furman C., Charles M., Ho D.D., Robinson J., Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L., Gerard N.P., Wyatt R., Choe H., Parolin C., Ruffing N., Borsetti A., Cardoso A.A., Desjardin E., Newman W. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 20.Dey B., Del Castillo C.S., Berger E.A. Neutralization of human immunodeficiency virus type 1 by sCD4-17b, a single-chain chimeric protein, based on sequential interaction of gp120 with CD4 and coreceptor. J. Virol. 2003;77:2859–2865. doi: 10.1128/JVI.77.5.2859-2865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagenaur L.A., Villarroel V.A., Bundoc V., Dey B., Berger E.A. sCD4-17b bifunctional protein: extremely broad and potent neutralization of HIV-1 Env pseudotyped viruses from genetically diverse primary isolates. Retrovirology. 2010;7:11. doi: 10.1186/1742-4690-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peachman K.K., Wieczorek L., Polonis V.R., Alving C.R., Rao M. The effect of sCD4 on the binding and accessibility of HIV-1 gp41 MPER epitopes to human monoclonal antibodies. Virology. 2010;408:213–223. doi: 10.1016/j.virol.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 23.He Y., Vassell R., Zaitseva M., Nguyen N., Yang Z., Weng Y., Weiss C.D. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J. Virol. 2003;77:1666–1671. doi: 10.1128/JVI.77.3.1666-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshiba T., Chan D.C. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J. Biol. Chem. 2003;278:7573–7579. doi: 10.1074/jbc.M211154200. [DOI] [PubMed] [Google Scholar]
- 25.Si Z., Madani N., Cox J.M., Chruma J.J., Klein J.C., Schön A., Phan N., Wang L., Biorn A.C., Cocklin S. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc. Natl. Acad. Sci. USA. 2004;101:5036–5041. doi: 10.1073/pnas.0307953101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggink D., Langedijk J.P., Bonvin A.M., Deng Y., Lu M., Berkhout B., Sanders R.W. Detailed mechanistic insights into HIV-1 sensitivity to three generations of fusion inhibitors. J. Biol. Chem. 2009;284:26941–26950. doi: 10.1074/jbc.M109.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusch O., Kalyanaraman R., Tucker L.D., Wells J.M., Ramratnam B., Boden D. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS. 2006;20:1917–1922. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 28.Falkenhagen A., Ameli M., Asad S., Read S.E., Joshi S. A novel gene therapy strategy using secreted multifunctional anti-HIV proteins to confer protection to gene-modified and unmodified target cells. Gene Ther. 2014;21:175–187. doi: 10.1038/gt.2013.70. [DOI] [PubMed] [Google Scholar]
- 29.Falkenhagen A., Asad S., Read S.E., Joshi S. Lentiviral expression system for the purification of secreted proteins from human cell cultures. BMC Biotechnol. 2016;16:66. doi: 10.1186/s12896-016-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolly C., Kashefi K., Hollinshead M., Sattentau Q.J. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hübner W., McNerney G.P., Chen P., Dale B.M., Gordon R.E., Chuang F.Y., Li X.D., Asmuth D.M., Huser T., Chen B.K. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez R.A., Barría M.I., Chen B.K. Unique features of HIV-1 spread through T cell virological synapses. PLoS Pathog. 2014;10:e1004513. doi: 10.1371/journal.ppat.1004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin N., Welsch S., Jolly C., Briggs J.A., Vaux D., Sattentau Q.J. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J. Virol. 2010;84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schooley R.T., Merigan T.C., Gaut P., Hirsch M.S., Holodniy M., Flynn T., Liu S., Byington R.E., Henochowicz S., Gubish E. Recombinant soluble CD4 therapy in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase I-II escalating dosage trial. Ann. Intern. Med. 1990;112:247–253. doi: 10.7326/0003-4819-112-4-247. [DOI] [PubMed] [Google Scholar]
- 35.Schacker T., Collier A.C., Coombs R., Unadkat J.D., Fox I., Alam J., Wang J.P., Eggert E., Corey L. Phase I study of high-dose, intravenous rsCD4 in subjects with advanced HIV-1 infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995;9:145–152. [PubMed] [Google Scholar]
- 36.Byrn R.A., Mordenti J., Lucas C., Smith D., Marsters S.A., Johnson J.S., Cossum P., Chamow S.M., Wurm F.M., Gregory T. Biological properties of a CD4 immunoadhesin. Nature. 1990;344:667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- 37.Allaway G.P., Davis-Bruno K.L., Beaudry G.A., Garcia E.B., Wong E.L., Ryder A.M., Hasel K.W., Gauduin M.C., Koup R.A., McDougal J.S. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses. 1995;11:533–539. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 38.Hodges T.L., Kahn J.O., Kaplan L.D., Groopman J.E., Volberding P.A., Amman A.J., Arri C.J., Bouvier L.M., Mordenti J., Izu A.E. Phase 1 study of recombinant human CD4-immunoglobulin G therapy of patients with AIDS and AIDS-related complex. Antimicrob. Agents Chemother. 1991;35:2580–2586. doi: 10.1128/aac.35.12.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fletcher C.V., DeVille J.G., Samson P.M., Moye J.H., Jr., Church J.A., Spiegel H.M., Palumbo P., Fenton T., Smith M.E., Graham B., Pediatric AIDS Clinical Trials Group, Protocol 351 Study Group Nonlinear pharmacokinetics of high-dose recombinant fusion protein CD4-IgG2 (PRO 542) observed in HIV-1-infected children. J. Allergy Clin. Immunol. 2007;119:747–750. doi: 10.1016/j.jaci.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan N., Sun Y., Li J., Hofmann W., Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schutten M., Andeweg A.C., Bosch M.L., Osterhaus A.D. Enhancement of infectivity of a non-syncytium inducing HIV-1 by sCD4 and by human antibodies that neutralize syncytium inducing HIV-1. Scand. J. Immunol. 1995;41:18–22. doi: 10.1111/j.1365-3083.1995.tb03528.x. [DOI] [PubMed] [Google Scholar]
- 42.Falkowska E., Ramos A., Feng Y., Zhou T., Moquin S., Walker L.M., Wu X., Seaman M.S., Wrin T., Kwong P.D. PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. J. Virol. 2012;86:4394–4403. doi: 10.1128/JVI.06973-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigal A., Kim J.T., Balazs A.B., Dekel E., Mayo A., Milo R., Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 44.Malbec M., Porrot F., Rua R., Horwitz J., Klein F., Halper-Stromberg A., Scheid J.F., Eden C., Mouquet H., Nussenzweig M.C., Schwartz O. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J. Exp. Med. 2013;210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyauchi K., Kim Y., Latinovic O., Morozov V., Melikyan G.B. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L., Pan C., Li Y., Lu H., He W., Jiang S. A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains. Retrovirology. 2012;9:104. doi: 10.1186/1742-4690-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W., Feng Y., Gong R., Zhu Z., Wang Y., Zhao Q., Dimitrov D.S. Engineered single human CD4 domains as potent HIV-1 inhibitors and components of vaccine immunogens. J. Virol. 2011;85:9395–9405. doi: 10.1128/JVI.05119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L., Patel B., Ghanem M.H., Bundoc V., Zheng Z., Morgan R.A., Rosenberg S.A., Dey B., Berger E.A. Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J. Virol. 2015;89:6685–6694. doi: 10.1128/JVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caskey M., Klein F., Lorenzi J.C., Seaman M.S., West A.P., Jr., Buckley N., Kremer G., Nogueira L., Braunschweig M., Scheid J.F. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautam R., Nishimura Y., Pegu A., Nason M.C., Klein F., Gazumyan A., Golijanin J., Buckler-White A., Sadjadpour R., Wang K. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balazs A.B., Chen J., Hong C.M., Rao D.S., Yang L., Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gardner M.R., Kattenhorn L.M., Kondur H.R., von Schaewen M., Dorfman T., Chiang J.J., Haworth K.G., Decker J.M., Alpert M.D., Bailey C.C. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Platt E.J., Wehrly K., Kuhmann S.E., Chesebro B., Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derdeyn C.A., Decker J.M., Sfakianos J.N., Wu X., O’Brien W.A., Ratner L., Kappes J.C., Shaw G.M., Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei X., Decker J.M., Liu H., Zhang Z., Arani R.B., Kilby J.M., Saag M.S., Wu X., Shaw G.M., Kappes J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeuchi Y., McClure M.O., Pizzato M. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J. Virol. 2008;82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Platt E.J., Bilska M., Kozak S.L., Kabat D., Montefiori D.C. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J. Virol. 2009;83:8289–8292. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss C.D., White J.M. Characterization of stable Chinese hamster ovary cells expressing wild-type, secreted, and glycosylphosphatidylinositol-anchored human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 1993;67:7060–7066. doi: 10.1128/jvi.67.12.7060-7066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirzabekov T., Bannert N., Farzan M., Hofmann W., Kolchinsky P., Wu L., Wyatt R., Sodroski J. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J. Biol. Chem. 1999;274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 60.Li M., Gao F., Mascola J.R., Stamatatos L., Polonis V.R., Koutsoukos M., Voss G., Goepfert P., Gilbert P., Greene K.M. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulkarni S.S., Lapedes A., Tang H., Gnanakaran S., Daniels M.G., Zhang M., Bhattacharya T., Li M., Polonis V.R., McCutchan F.E. Highly complex neutralization determinants on a monophyletic lineage of newly transmitted subtype C HIV-1 Env clones from India. Virology. 2009;385:505–520. doi: 10.1016/j.virol.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchacher A., Predl R., Strutzenberger K., Steinfellner W., Trkola A., Purtscher M., Gruber G., Tauer C., Steindl F., Jungbauer A. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 63.Purtscher M., Trkola A., Gruber G., Buchacher A., Predl R., Steindl F., Tauer C., Berger R., Barrett N., Jungbauer A. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 64.Purtscher M., Trkola A., Grassauer A., Schulz P.M., Klima A., Döpper S., Gruber G., Buchacher A., Muster T., Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Wu X., Yang Z.Y., Li Y., Hogerkorp C.M., Schief W.R., Seaman M.S., Zhou T., Schmidt S.D., Wu L., Xu L. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon H., Macke J., West A.P., Jr., Foley B., Bjorkman P.J., Korber B., Yusim K. CATNAP: a tool to compile, analyze and tally neutralizing antibody panels. Nucleic Acids Res. 2015;43(W1) doi: 10.1093/nar/gkv404. W213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]