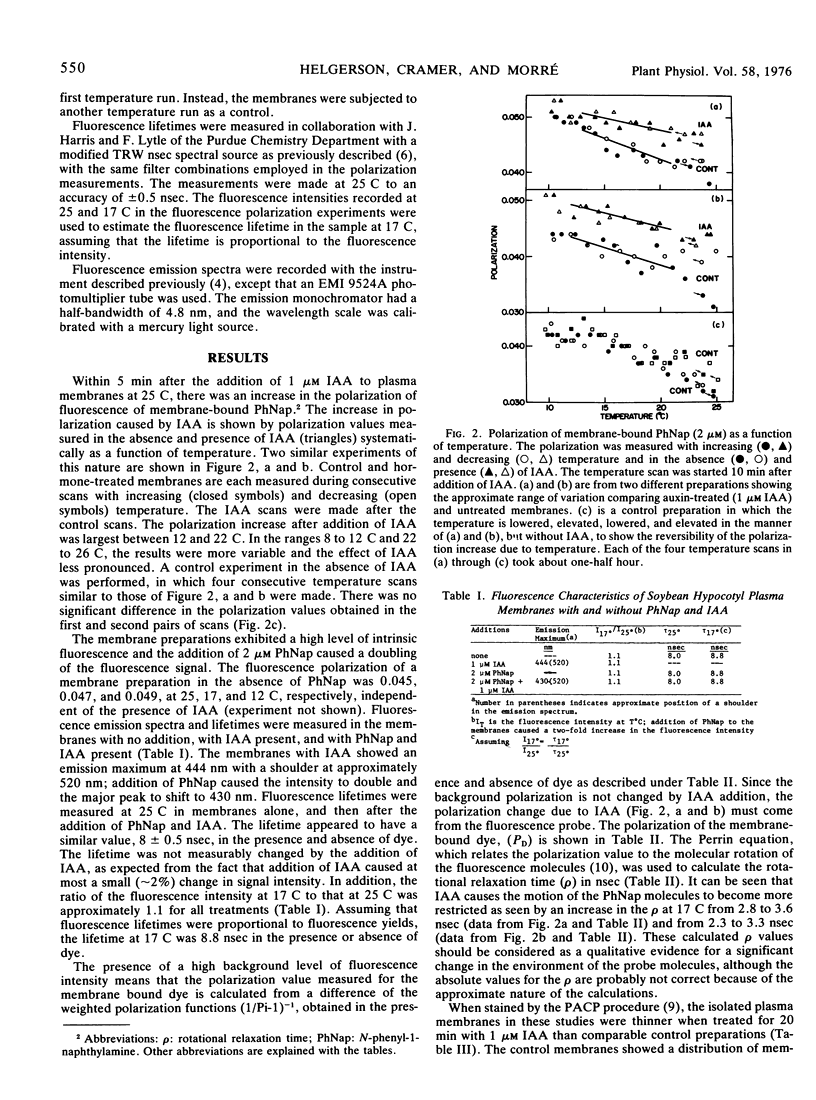
Abstract
The plant hormone indole-3-acetic acid (IAA or auxin) added at a concentration for half-maximal promotion of cell elongation (1 μm) caused an increase of 25% in the fluorescence polarization of the membrane-bound probe N-phenyl-1-naphthylamine, when added to fractions enriched in plasma membranes from soybean hypocotyls (Glycine max L. var. Wayne), with no measurable change in fluorescence lifetime. The amplitude of the polarization increase was maximal in the temperature range 12 to 22 C. The findings provide evidence for a cell-free response of isolated plasma membranes to the hormone and imply that the response involves an increase in the microviscosity of hydrocarbon regions of the membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colley C. M., Metcalfe J. C. The localisation of small molecules in lipid bilayers. FEBS Lett. 1972 Aug 15;24(3):241–246. doi: 10.1016/0014-5793(72)80364-8. [DOI] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Harris J. M., Lytle F. E. Evidence for a microviscosity increase in the Escherichia coli cell envelope caused by colicin E1. Biochemistry. 1974 Jul 16;13(15):3057–3061. doi: 10.1021/bi00712a010. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Bracker C. E. Ultrastructural alteration of plant plasma membranes induced by auxin and calcium ions. Plant Physiol. 1976 Oct;58(4):544–547. doi: 10.1104/pp.58.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Radda G. K. Enzyme and membrane conformation in biochemical control. Biochem J. 1971 May;122(4):385–396. doi: 10.1042/bj1220385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- WEBER G. Rotational Brownian motion and polarization of the fluorescence of solutions. Adv Protein Chem. 1953;8:415–459. doi: 10.1016/s0065-3233(08)60096-0. [DOI] [PubMed] [Google Scholar]



