Abstract
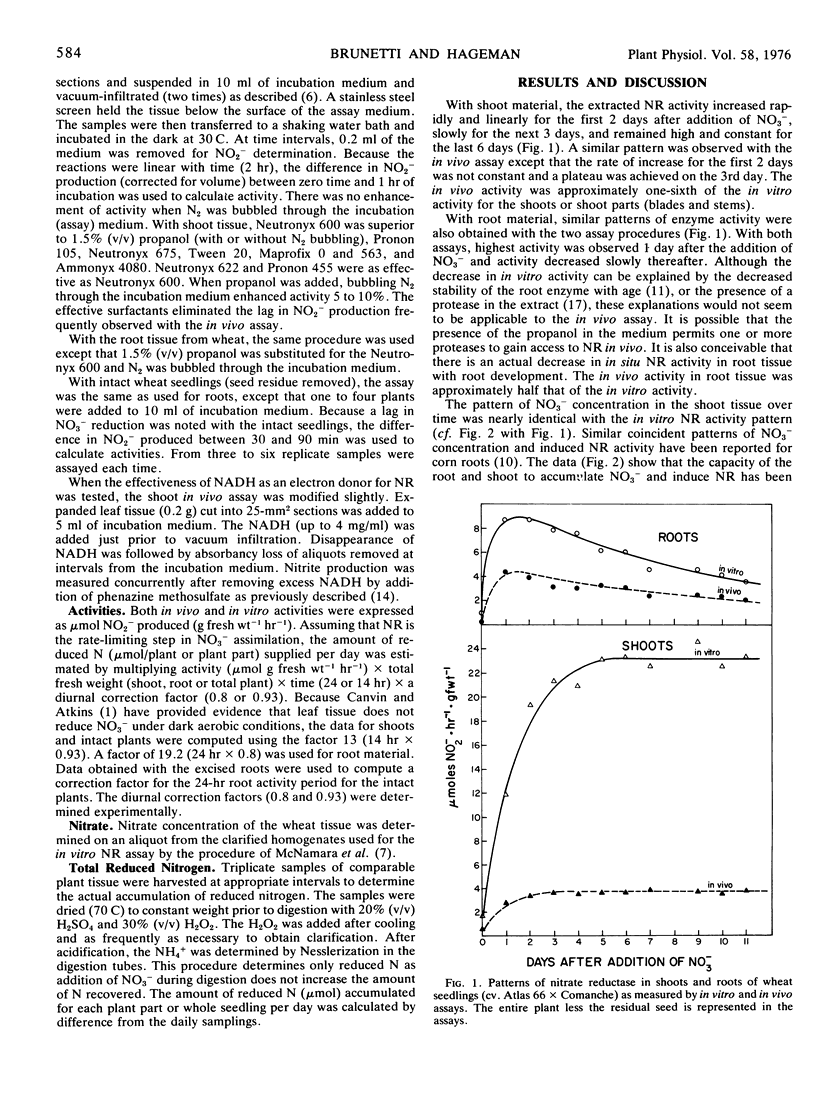
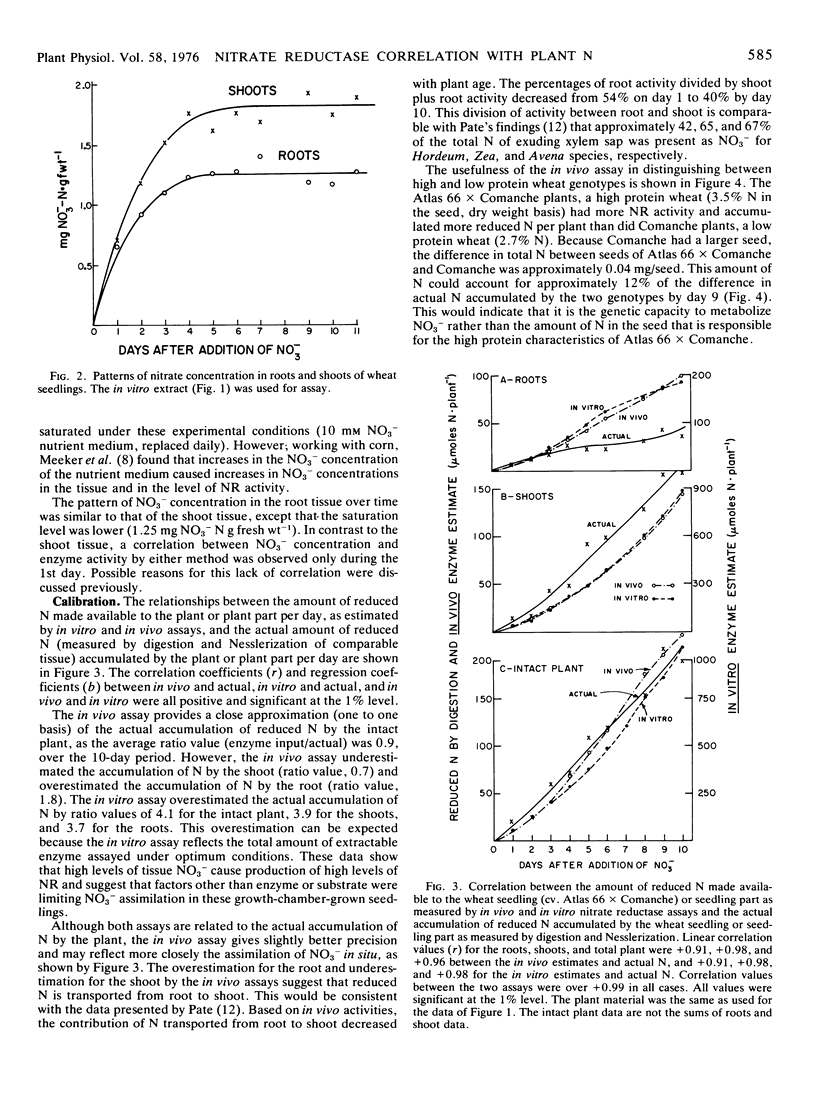
The effectiveness of the in vivo and in vitro assays for nitrate reductase (NR) in estimating the amounts of reduced N made available to plants was tested against the daily increases in reduced N (Nesslerization) actually accumulated by the plant. With growth-chamber-grown wheat seedlings, the average ratio values (input of reduced N as estimated by the in vitro assay to actual accumulation of N by the plant) were 3.9 for shoots, 3.7 for the roots, and 4.1 for the entire plant, over a 10-day period. With the in vivo assay, the average ratio values were 0.7 for the shoot, 1.8 for the root, and 0.9 for the entire plant. Although the linear regressions between the accumulated N in the plant and the estimated N input (by both in vitro and in vivo assays) were significant and positive, the in vivo assay provided the closest approximation of the actual amount of N accumulated.
The in vivo NR assay effectively distinguished between two wheat varieties. The variety known to have the higher percentage of seed protein also had the higher amounts of NR activity.
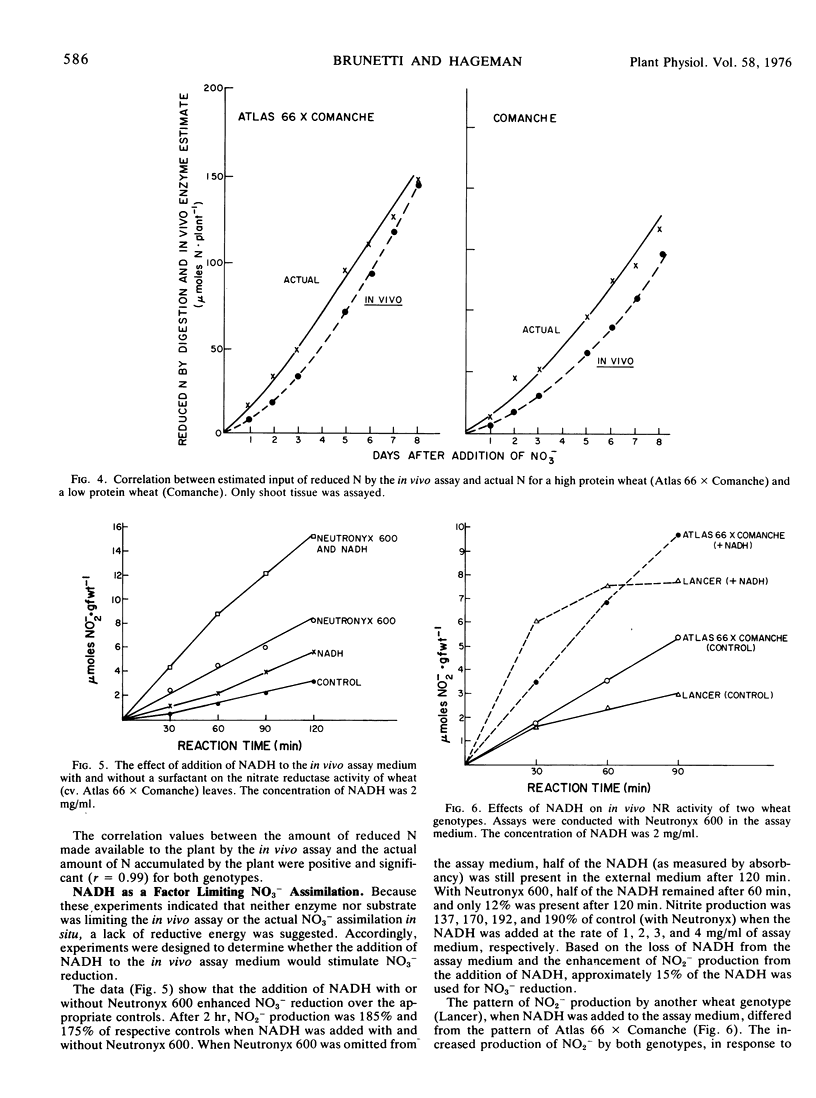
With seedling wheat leaves, the addition of NADH plus a surfactant increased in vivo NR activity approximately 2-fold over comparable controls. Because the tissue contained high levels of nitrate and enzyme, we concluded that reducing potential was the rate-limiting factor in nitrate reduction in situ in these growth-chamber-grown plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferrari T. E., Varner J. E. Control of nitrate reductase activity in barley aleurone layers. Proc Natl Acad Sci U S A. 1970 Mar;65(3):729–736. doi: 10.1073/pnas.65.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra C. A., Hageman R. H. Nitrate uptake and induction of nitrate reductase in excised corn roots. Plant Physiol. 1975 Nov;56(5):692–695. doi: 10.1104/pp.56.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A., Wallace W., Stevens D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972 Dec;50(6):649–654. doi: 10.1104/pp.50.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Cataldo D. A., Peterson D. M. Use of protein in extraction and stabilization of nitrate reductase. Plant Physiol. 1974 May;53(5):688–690. doi: 10.1104/pp.53.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G., Bosler M. E. Comparison of in vitro and in vivo assays for nitrate reductase in soybean leaves. Plant Physiol. 1972 Mar;49(3):448–450. doi: 10.1104/pp.49.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. A nitrate reductase inactivating enzyme from the maize root. Plant Physiol. 1973 Sep;52(3):197–201. doi: 10.1104/pp.52.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]


