Abstract
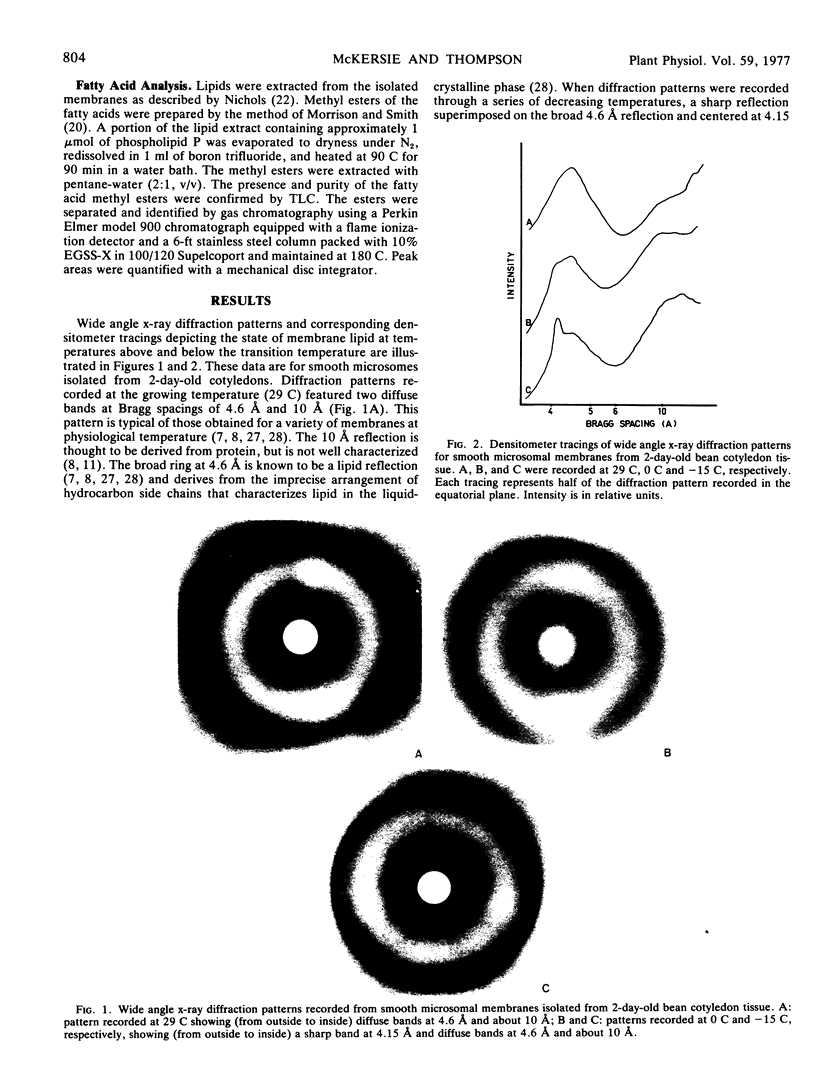
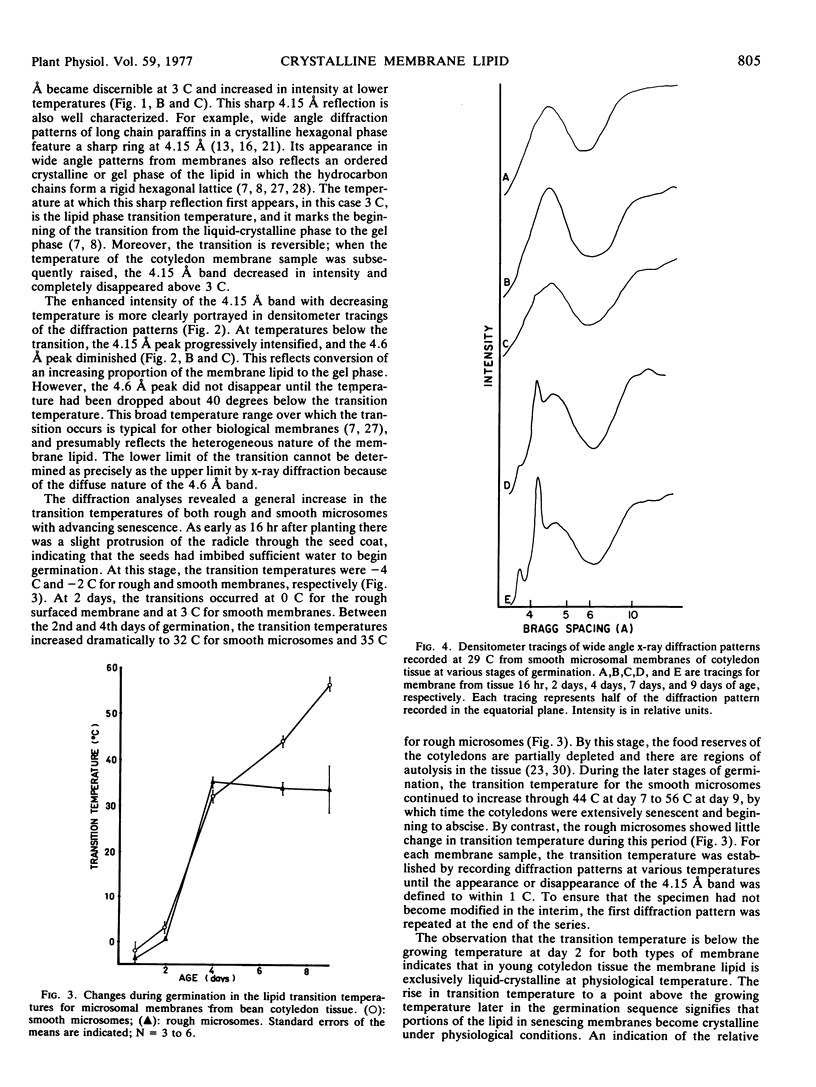
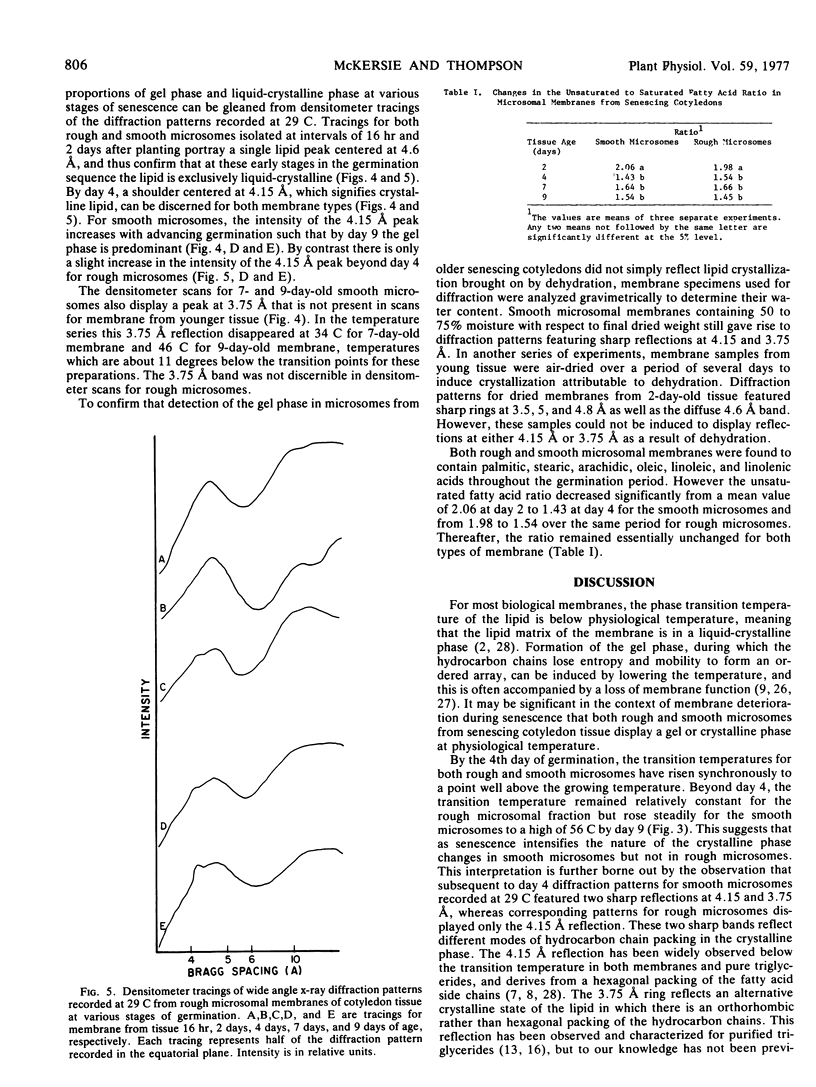
Lipid transition temperatures for rough and smooth microsomal membranes isolated from bean (Phaseolus vulgaris) cotyledon tissue at various stages of germination were determined by wide angle x-ray diffraction. The transition temperatures were established by recording diffraction patterns through a temperature series until a sharp x-ray reflection centered at a Bragg spacing of 4.15 Å and denoting the presence of crystalline lipid was discernible. For rough and smooth microsomes from 2-day-old tissue, the transitions occurred at 0 C and 3 C, respectively, indicating that at this early stage in the germination sequence the membrane lipid is entirely liquid-crystalline at physiological temperature. By the 4th day of germination, the transition temperatures had increased to 32 C for smooth microsomes and 35 C for rough microsomes, indicating that at 29 C, which was the growth temperature, portions of the membrane lipid were crystalline. During the later stages of germination, the transition temperature for smooth microsomes continued to rise through 44 C at day 7 to 56 C at day 9, by which time the cotyledons were extensively senescent and beginning to abscise. There was also a dramatic increase in the proportion of membrane lipid in the crystalline phase at 29 C. By contrast, the rough microsomes showed little change in transition temperature and only a slight increase in the proportion of crystalline lipid during this late period in germination. The data indicate that substantial amounts of the lipid is senescing membranes are crystalline even at physiological temperature. Moreover, there is a temporal correlation between the appearance of this crystallinity and loss of membrane function, suggesting that the two may be causally related.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok M. C., van der Neut-Kok E. C., van Deenen L. L., de Gier J. The effect of chain length and lipid phase transitions on the selective permeability properties of liposomes. Biochim Biophys Acta. 1975 Oct 6;406(2):187–196. doi: 10.1016/0005-2736(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Dallner G., Ernster L. Subfractionation and composition of microsomal membranes: a review. J Histochem Cytochem. 1968 Oct;16(10):611–632. doi: 10.1177/16.10.611. [DOI] [PubMed] [Google Scholar]
- Davidson S. J., Song S. W. A thermally induced alteration in lysosome membranes: salt permeability at 0 and 37 degrees C. Biochim Biophys Acta. 1975 Jan 28;375(2):274–285. doi: 10.1016/0005-2736(75)90195-9. [DOI] [PubMed] [Google Scholar]
- Eletr S., Williams M. A., Watkins T., Keith A. D. Perturbations of the dynamics of lipid alkyl chains in membrane systems: effect on the activity of membrane-bound enzymes. Biochim Biophys Acta. 1974 Mar 15;339(2):190–201. doi: 10.1016/0005-2736(74)90317-4. [DOI] [PubMed] [Google Scholar]
- Eletr S., Zakim D., Vessey D. A. A spin-label study of the role of phospholipids in the regulation of membrane-bound microsomal enzymes. J Mol Biol. 1973 Aug 5;78(2):351–362. doi: 10.1016/0022-2836(73)90121-6. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías R. N., Bloj B., Morero R. D., Siñeriz F., Trucco R. E. Regulation of allosteric membrane-bound enzymes through changes in membrane lipid compostition. Biochim Biophys Acta. 1975 Jun 30;415(2):231–251. doi: 10.1016/0304-4157(75)90003-9. [DOI] [PubMed] [Google Scholar]
- Finean J. B., Coleman R., Green W. G., Limbrick A. R. Low-angle x-ray diffraction and electron-microscope studies of isolated cell membranes. J Cell Sci. 1966 Sep;1(3):287–296. doi: 10.1242/jcs.1.3.287. [DOI] [PubMed] [Google Scholar]
- Finean J. B., Coleman R., Knutton S., Limbrick A. R., Thompson J. E. Structural studies of cell membrane preparations. J Gen Physiol. 1968 May;51(5 Suppl):19S+–19S+. [PubMed] [Google Scholar]
- Lai Y. F., Thompson J. E. Effects of Germination on NA-K-stimulated Adenosine 5'-Triphosphatase and ATP-dependent Ion Transport of Isolated Membranes from Cotyledons. Plant Physiol. 1972 Oct;50(4):452–457. doi: 10.1104/pp.50.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K. Classification of glyceride crystal forms. Acta Chem Scand. 1966;20(8):2255–2260. doi: 10.3891/acta.chem.scand.20-2255. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- McElhaney R. N., Souza K. A. The relationship between environmental temperature, cell growth and the fluidity and physical state of the membrane lipids in Bacillus stearothermophilus. Biochim Biophys Acta. 1976 Sep 7;443(3):348–359. doi: 10.1016/0005-2736(76)90455-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Rothman J. E. The molecular basis of mesomorphic phase transitions in phospholipid systems. J Theor Biol. 1973 Jan;38(1):1–16. doi: 10.1016/0022-5193(73)90221-x. [DOI] [PubMed] [Google Scholar]
- Shechter E., Letellier L., Gulik-Krzywicki G. Relations between structure and function in cytoplasmic membrane vesicles isolated from an Escherichia coli fatty-acid auxotroph. High-angle x-ray diffraction, freeze-etch electron microscopy and transport studies. Eur J Biochem. 1974 Nov 1;49(1):61–76. doi: 10.1111/j.1432-1033.1974.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Shires T. K., McLaughlin C. M., Pitot H. C. The selectivity and stoicheiometry of membrane binding sites for polyribosomes, ribosomes and ribosomal subunits in vitro. Biochem J. 1975 Mar;146(3):513–526. doi: 10.1042/bj1460513a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijck P. W., Ververgaert P. H., Verkleij A. J., Van Deenen L. L., De Gier J. Influence of Ca2+ and Mg2+ on the thermotropic behaviour and permeability properties of liposomes prepared from dimyristoyl phosphatidylglycerol and mixtures of dimyristoyl phosphatidylglycerol and dimyristoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Nov 3;406(4):465–478. doi: 10.1016/0005-2736(75)90025-5. [DOI] [PubMed] [Google Scholar]



