Abstract
The mechanism of carbon transport in Zea mays leaves was investigated using carbon 11 which is a short lived (half-life 20.4 min) positronemitting isotope. The gamma radiation produced on annihilation allows in vivo or nondestructive measurement of the isotope and the short half-life allows many measurements of translocation to be made on the same leaf within the same day.
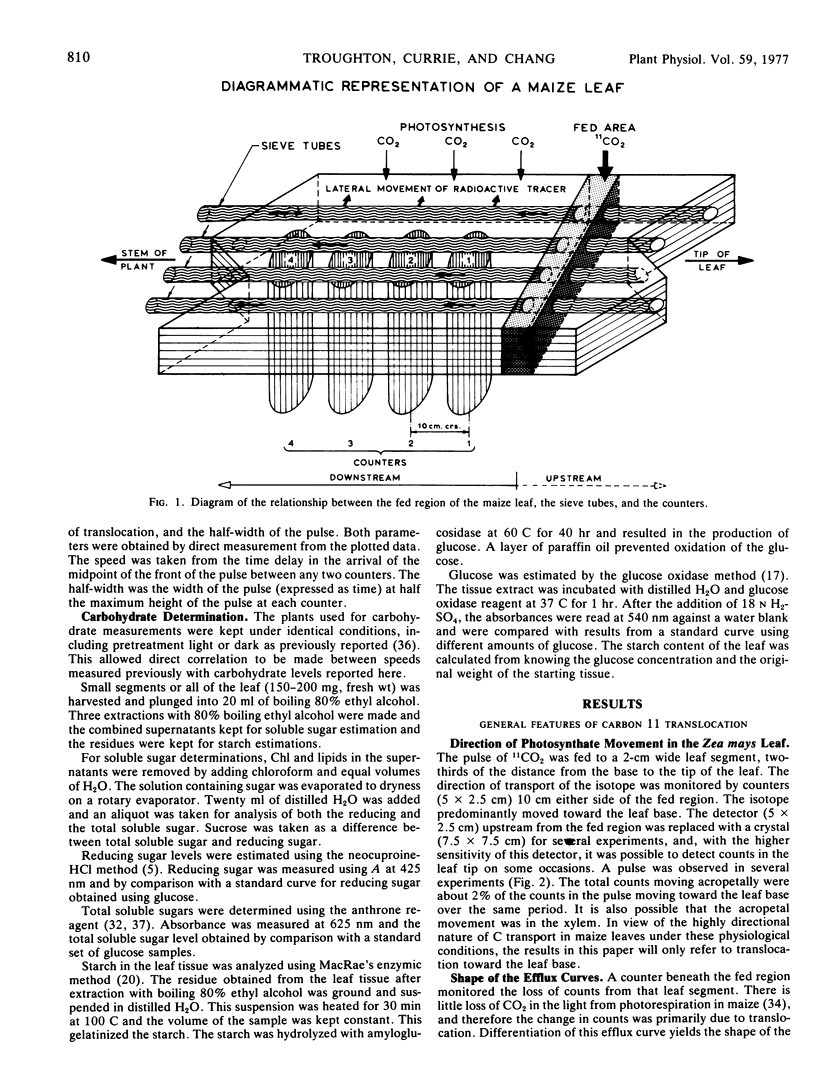
Carbon 11 produced by the 10B (d,n)11C nuclear reaction was converted to 11CO2, fed to a leaf as a short pulse, and assimilated during photosynthesis. The progress of the radioactive pulse along the leaf in the phloem was monitored in several positions simultaneously with counters. The counters were NaI crystals with photomultipliers and the output was amplified, passed to single channel analyzers, and the counts accumulated for 20 seconds every 30 seconds. Corrections were made for the half-life and background radiation by computer, and the results were displayed on a high speed plotter. Information derived from the corrected data included the speed of translocation, the shape of the radioactive carbon pulse, and the influence of light and distance along the leaf on these parameters. The plants were kept under controlled environment conditions during all measurements.
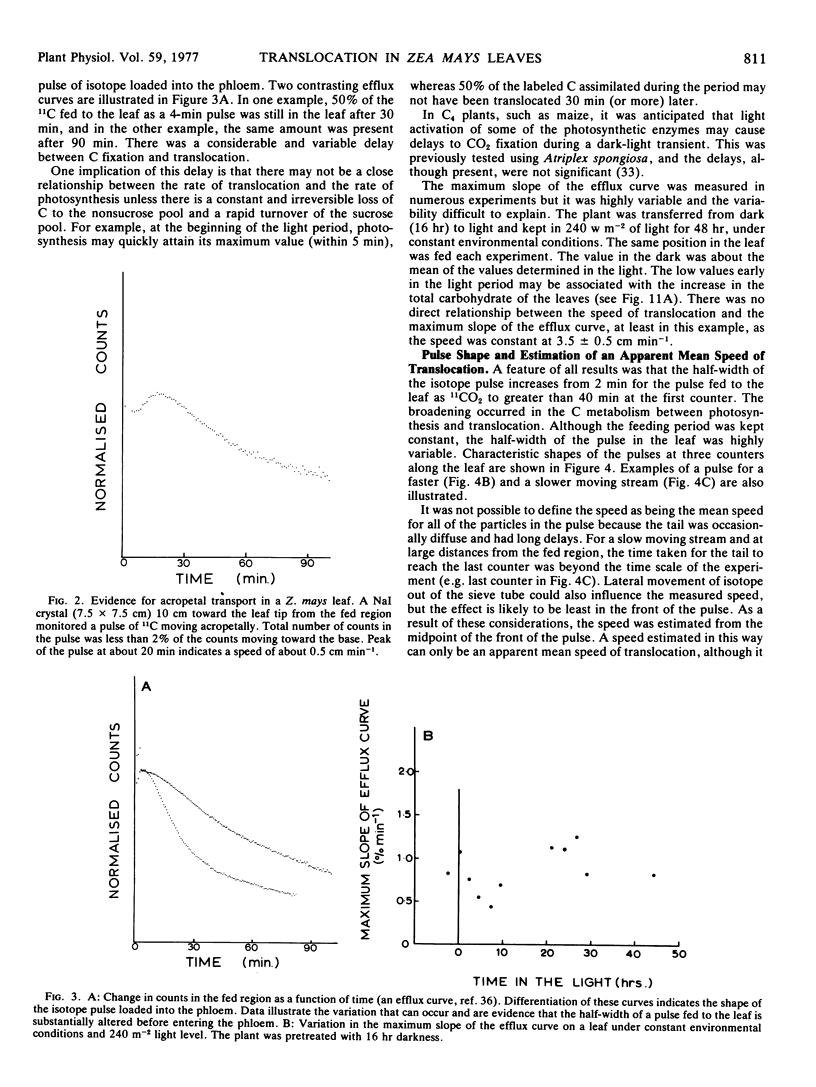
A speed was derived from the time displacement of the midpoint of the front of the pulse, measured at two positions along the leaf. This was an apparent mean speed of translocation because it averaged a variation in speed with distance, variation in speed between or within sieve tubes, and it averaged the mean speed of all of the particles in the pulse.
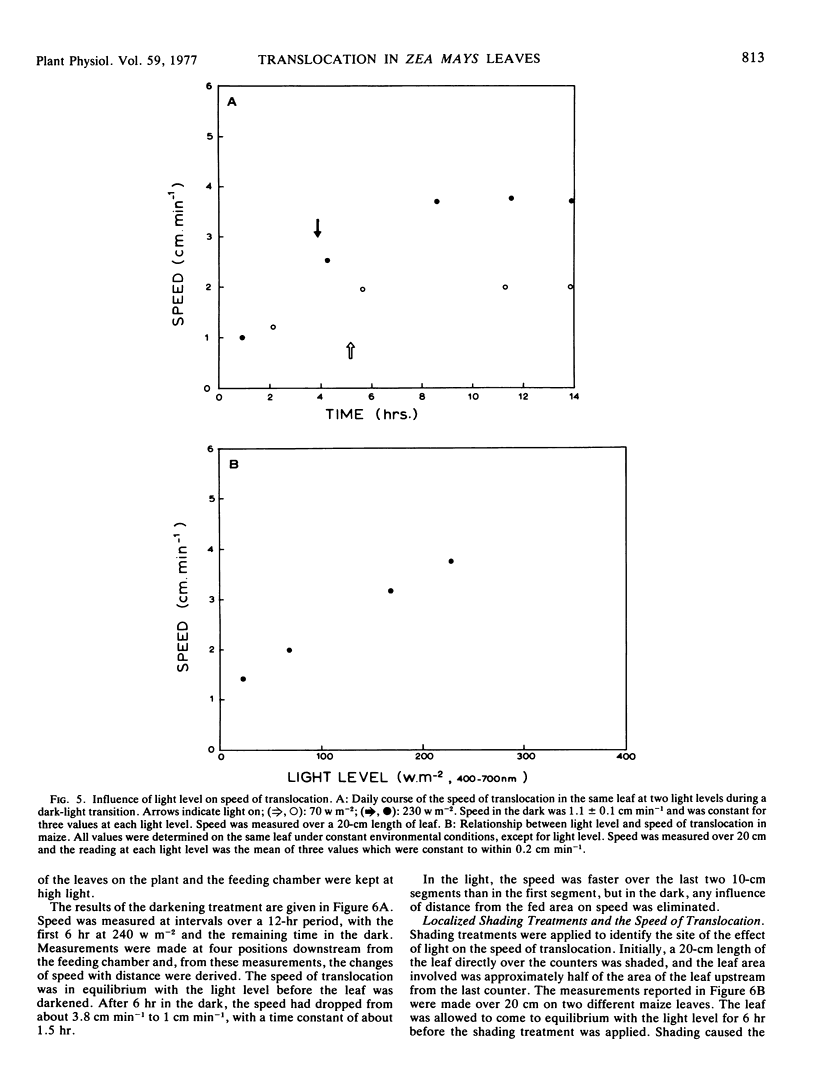
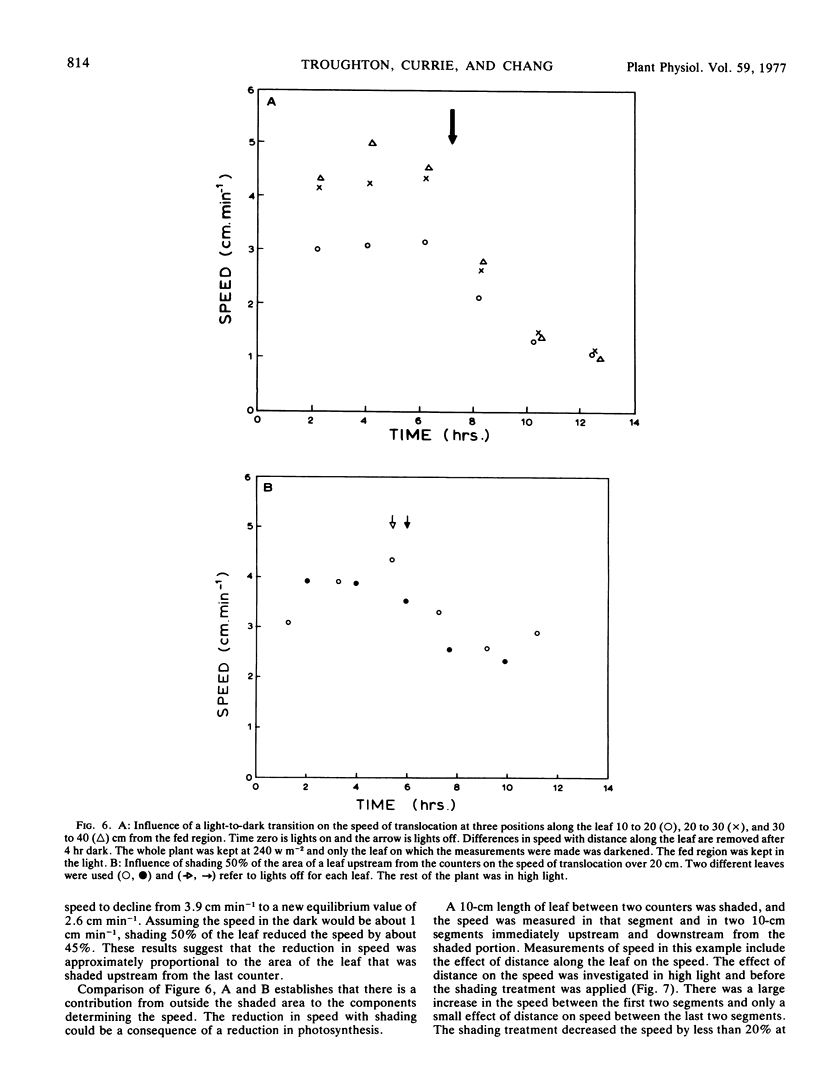
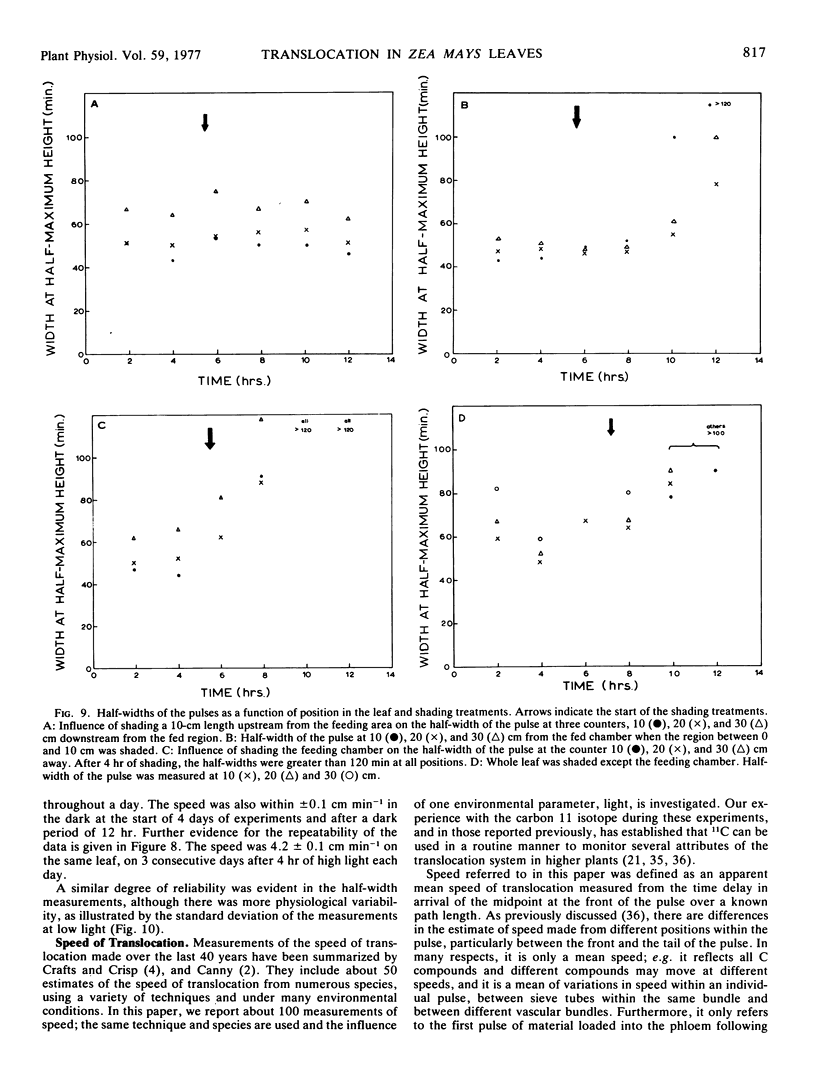
A wide range of speeds of translocation from 0.25 to 11 cm min−1 was observed but most of the variability was due to the variation in light available to the leaf. For example, the speed of translocation was proportional to the light level on either the whole plant or individual leaf. Shading of the leaf established that the light effect was not localized in either the feeding area or in the portion of the leaf on which the measurements were made. It was proposed that the speed was dependent on the proportion of the leaf in the light upstream from the last counter. The speed of translocation was relatively independent of the stage of growth of the plant, age of the leaf, and the time during the diurnal light cycle.
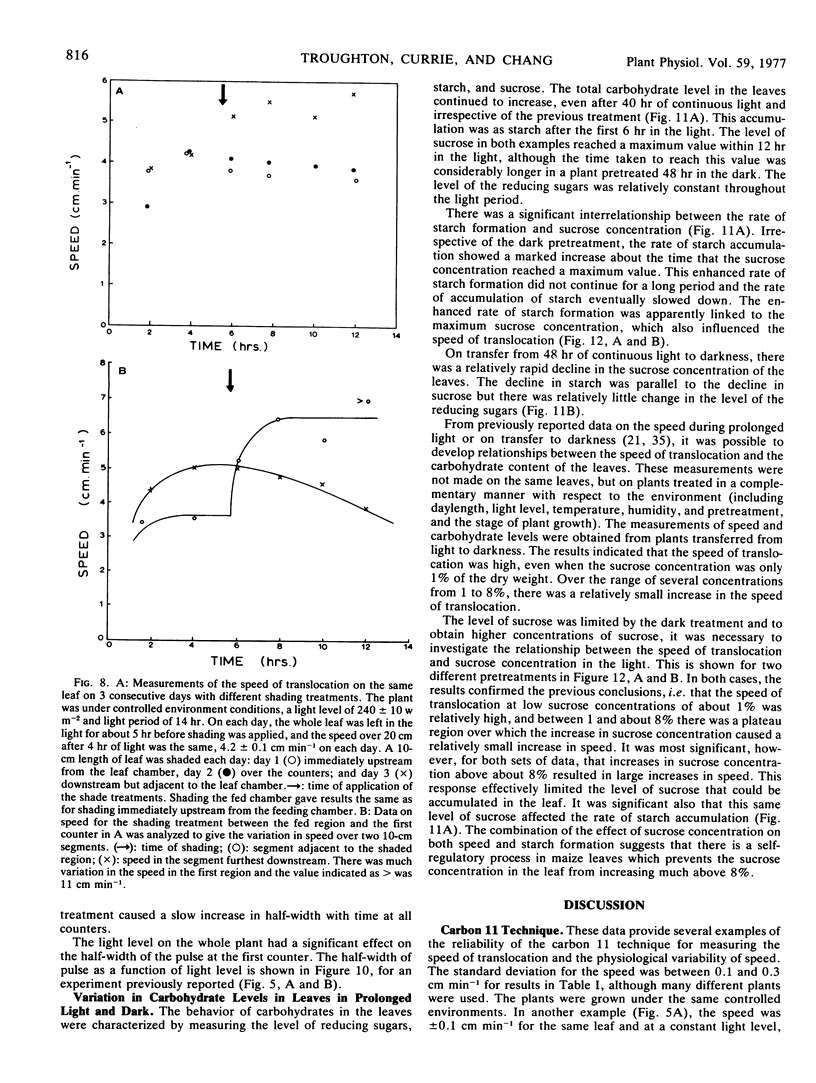
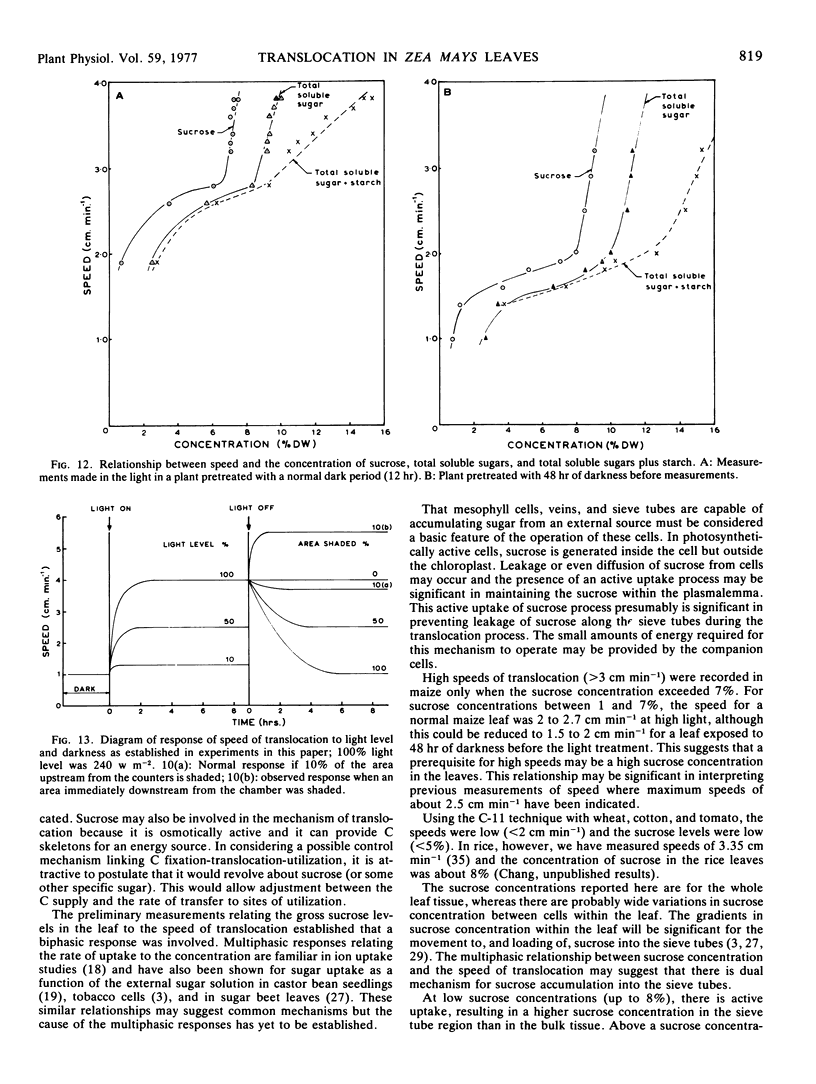
Data obtained on the level of the reducing sugars, starch, and sucrose in the leaf were related to the speed of translocation. A biphasic relationship between speed and sucrose concentration in the leaf was established and the high speeds measured during experiments only occurred when sucrose concentrations in the leaf exceeded 8% of the dry weight.
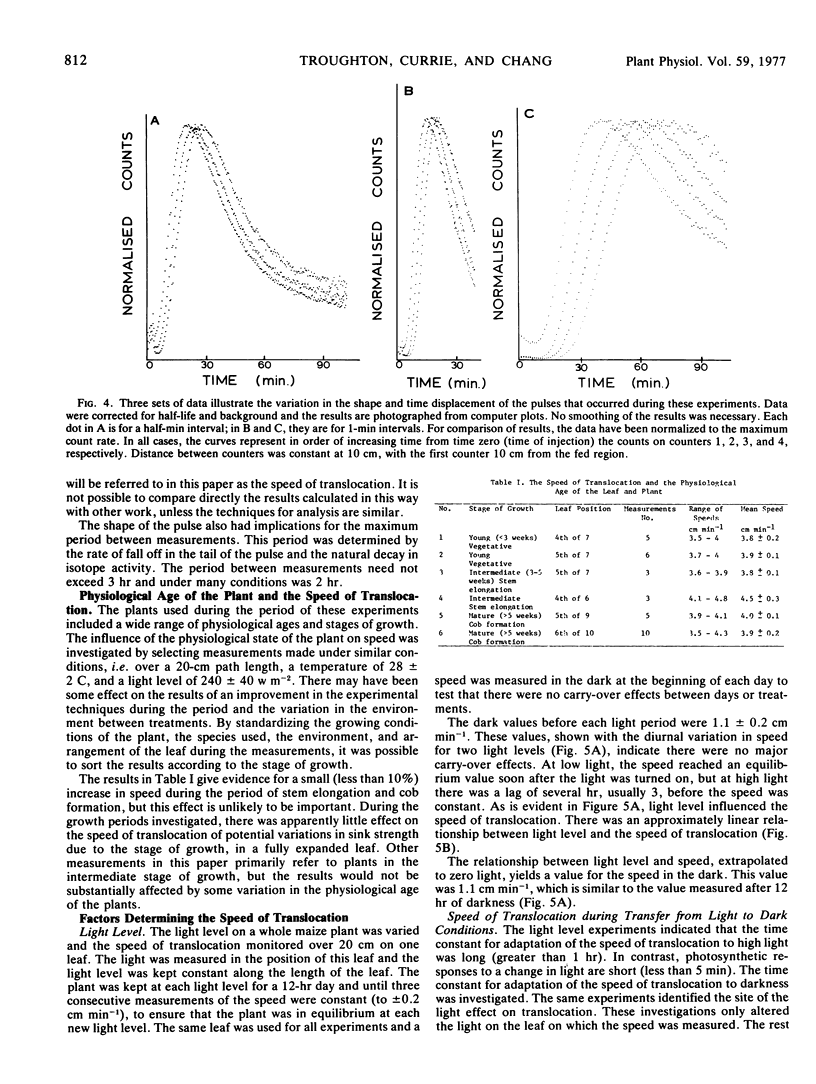
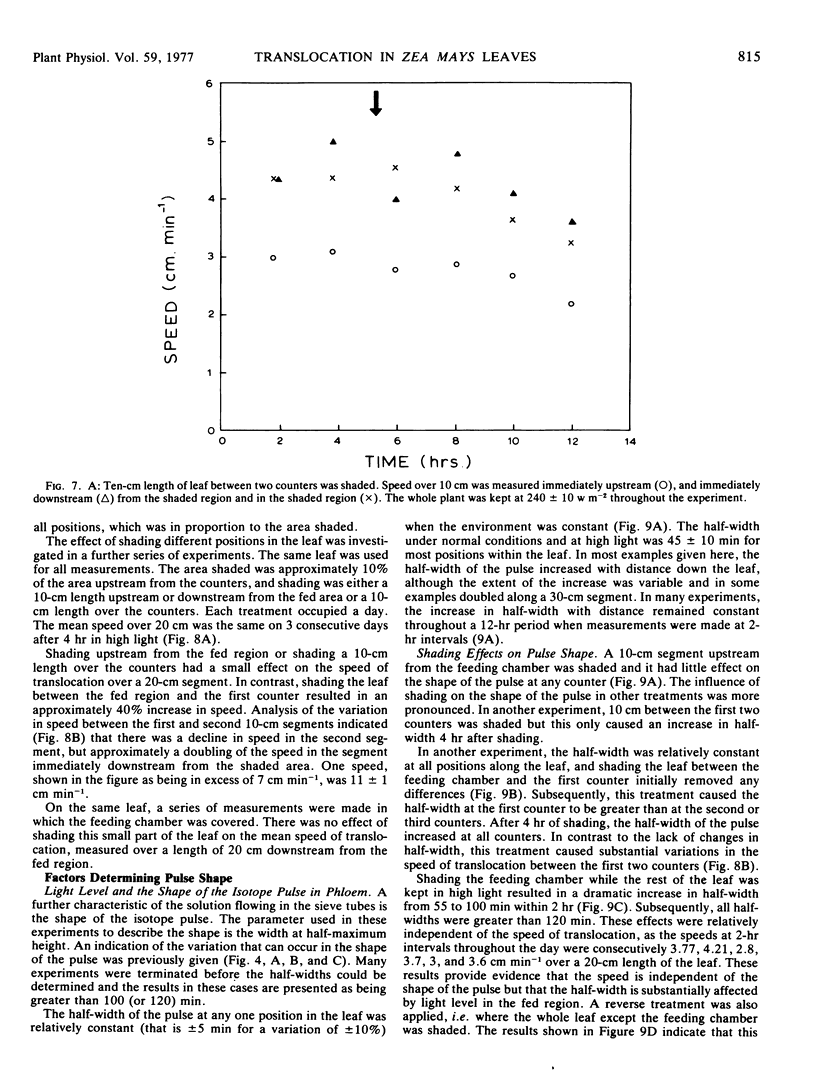
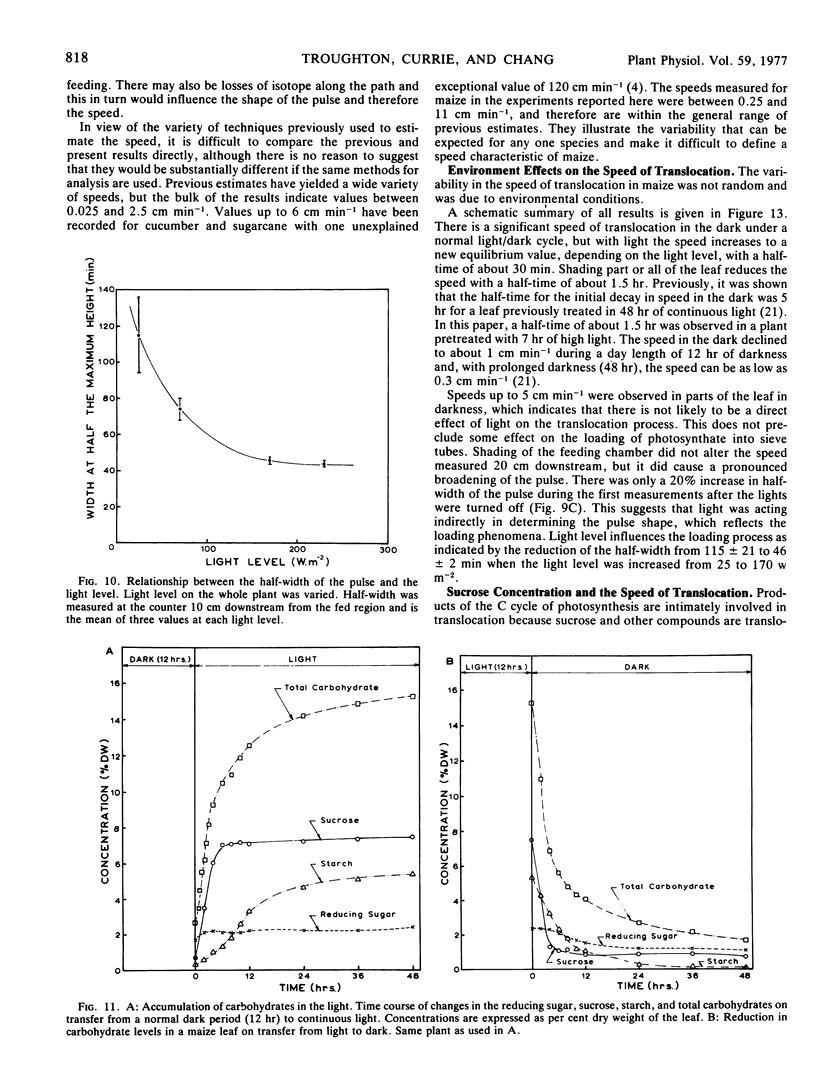
The shape of the pulse loaded into and translocated in the phloem was estimated from the half-width of the pulse. The half-width was primarily determined by loading phenomena which resulted in an increase in the half-width from 2 minutes when fed to the leaf to more than 40 minutes in the phloem. In many examples, the pulse continued to broaden with distance along the leaf from the fed region. The half-width was independent of the speed but highly dependent on the light level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A. Vein Loading: The Role of the Symplast in Intercellular Transport of Carbohydrate between the Mesophyll and Minor Veins of Tobacco Leaves. Plant Physiol. 1974 Jun;53(6):912–917. doi: 10.1104/pp.53.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Fisher D. B. Kinetics of C-14 Translocation in Soybean: II. Kinetics in the Leaf. Plant Physiol. 1970 Feb;45(2):114–118. doi: 10.1104/pp.45.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. Kinetics of C-14 Translocation in Soybean: III. Theoretical Considerations. Plant Physiol. 1970 Feb;45(2):119–125. doi: 10.1104/pp.45.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B. Kinetics of C-14 translocation in soybean: I. Kinetics in the stem. Plant Physiol. 1970 Feb;45(2):107–113. doi: 10.1104/pp.45.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel' S. Ia, Kukhareva L. V., Ginzburg B. M., Gasparian K. A., Vorob'ev V. I. Vliianie nagruzki na perekhod poriadok--besporiadok v nativnykh kollagenovykh voloknakh. Biofizika. 1965;10(5):735–742. [PubMed] [Google Scholar]
- Geiger D. R., Batey J. W. Translocation of C Sucrose in Sugar Beet during Darkness. Plant Physiol. 1967 Dec;42(12):1743–1749. doi: 10.1104/pp.42.12.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J. Salt transport in Valonia: inhibition of potassium uptake by small hydrostatic pressures. Science. 1968 Apr 5;160(3823):68–70. doi: 10.1126/science.160.3823.68. [DOI] [PubMed] [Google Scholar]
- Hartt C. E., Kortschak H. P., Forbes A. J., Burr G. O. Translocation of C in Sugarcane. Plant Physiol. 1963 May;38(3):305–318. doi: 10.1104/pp.38.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartt C. E. Light and Translocation of C in Detached Blades of Sugarcane. Plant Physiol. 1965 Jul;40(4):718–724. doi: 10.1104/pp.40.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn D. M., Taylor P. M. Effect of sulfhydryl reagents on glucose determination by the glucose oxidase method. Anal Biochem. 1969 Mar;27(3):555–558. doi: 10.1016/0003-2697(69)90069-4. [DOI] [PubMed] [Google Scholar]
- Kriedemann P., Beevers H. Sugar uptake and translocation in the castor bean seedling I. Characteristics of transfer in intact and excised seedlings. Plant Physiol. 1967 Feb;42(2):161–173. doi: 10.1104/pp.42.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask J., Laties G. G. Multiphasic absorption of glucose and 3-o-methyl glucose by aged potato slices. Plant Physiol. 1973 Feb;51(2):289–294. doi: 10.1104/pp.51.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson I. W., Licko V., Bartoli E. The nature of passive flows through tightly folded membranes. The influence of microstructure. J Membr Biol. 1973;11(4):293–308. doi: 10.1007/BF01869827. [DOI] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]


