Abstract
Cyanide and azide inhibit photosynthesis and catalase activity of isolated, intact spinach (Spinacia oleracea) chloroplasts. When chloroplasts are illuminated in the presence of CN− or N3−, accumulation of H2O2 is observed, parallel to inhibition of photosynthesis. Photosynthetic O2 evolution is inhibited to the same extent, under saturating light, whether CO2 or phosphoglycerate is present as electron acceptor.
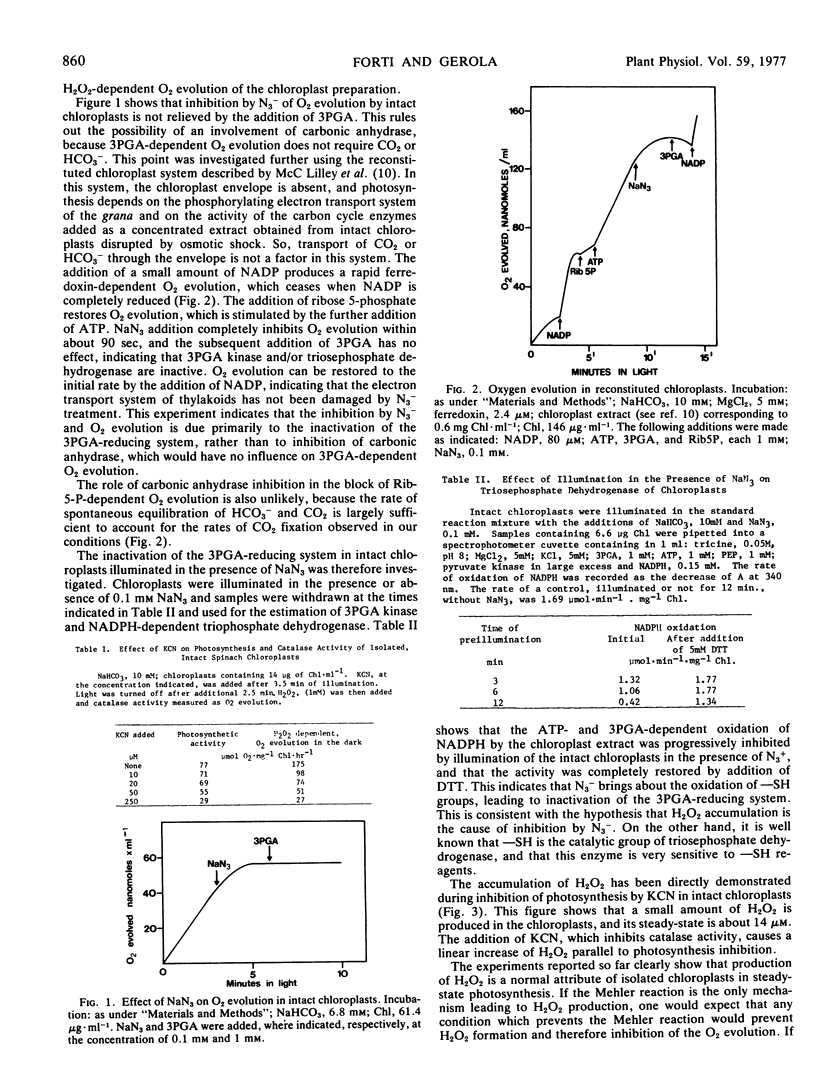
The illumination of chloroplasts with CN− or N3− inactivates the NADPH- and ATP-dependent phosphoglycerate reduction. This enzyme system can be reactivated by dithiothreitol. In reconstituted, envelope-less chloroplasts, the phosphoglycerate-dependent and the ribose 5-phosphate-dependent O2 evolution are inhibited to the same extent, while electron transport to NADP is unaffected.
It is concluded that the inhibition of photosynthesis by CN− and N3− is due to H2O2 accumulation, which is a consequence of catalase inhibition.
The inhibition of phosphoglycerate reduction, but not of CO2 reduction, is abolished under conditions where ATP is available in excess of NADPH (low light, supply of ATP). This is taken as an indication that electron flow from photosystem I is diverted to O2 (Mehler reaction, which produces H2O2) when the unavailability of ATP is limiting the rate of reoxidation of NADPH. The Mehler reaction is considered a physiological process supplying ATP for photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Avron M. Site of action of inhibitors of carbon dioxide assimilation by whole lettuce chloroplasts. Plant Physiol. 1975 Oct;56(4):481–485. doi: 10.1104/pp.56.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- FORTI G., JAGENDORF A. T. Photosynthetic phosphorylation in the absence of redox dyes: oxygen and ascorbate effects. Biochim Biophys Acta. 1961 Dec 9;54:322–330. doi: 10.1016/0006-3002(61)90372-9. [DOI] [PubMed] [Google Scholar]
- Graham D., Reed M. L. Carbonic anhydrase and the regulation of photosynthesis. Nat New Biol. 1971 May 19;231(20):81–83. doi: 10.1038/newbio231081a0. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Patterson C. O., Myers J. Photosynthetic Production of Hydrogen Peroxide by Anacystis nidulans. Plant Physiol. 1973 Jan;51(1):104–109. doi: 10.1104/pp.51.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]


