Abstract
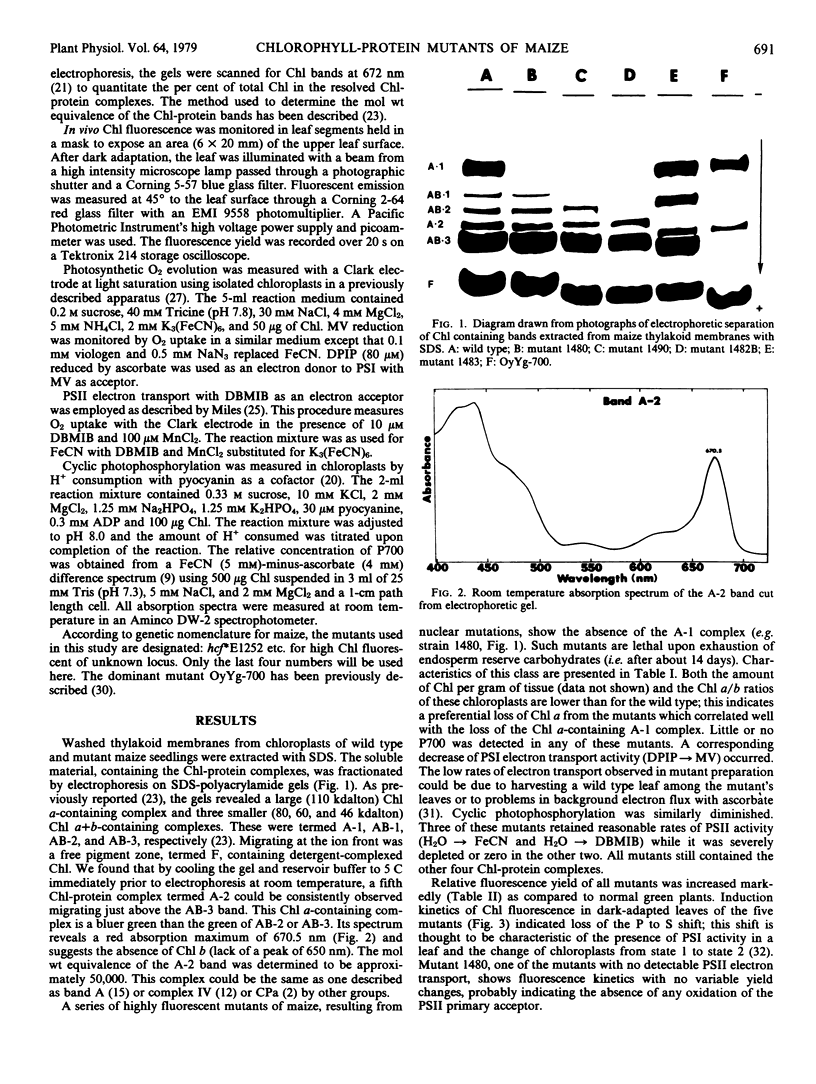
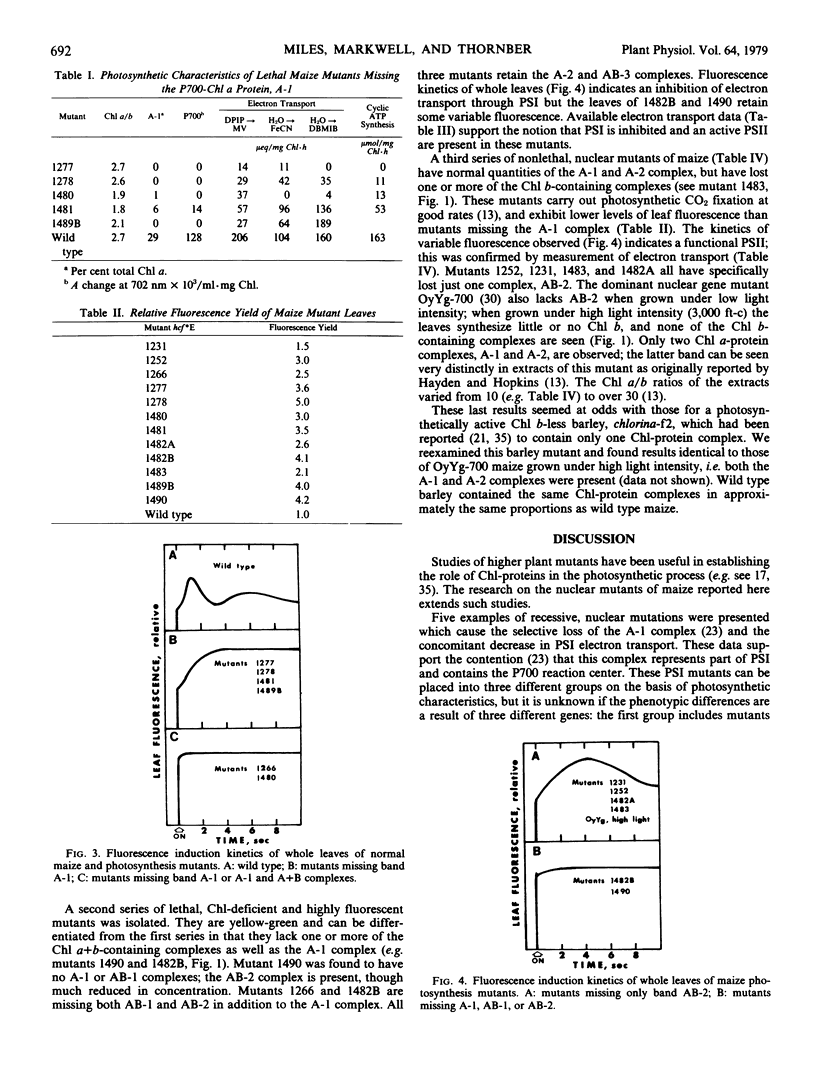
A number of new nuclear mutants have been isolated from maize by selection for high chlorophyll (Chl) fluorescence. These mutants show reduced rates of photosynthesis and/or are deficient in Chl. Electrophoretic examination of wild type thylakoid membranes revealed five Chl-protein complexes, two containing only Chl a and three containing Chl a and Chl b. A class of nonviable, photosystem I-deficient mutants was found to be lacking one (A-1) of the two Chl a-protein complexes. A second class of nonviable, photosystem I-lacking mutants was found to be missing not only this A-1 complex but also one or more of the three Chl a and b-containing, light-harvesting Chl-protein complexes. Viable mutants were obtained which appeared to have lost just one of the Chl b-containing complexes, whereas a second class of viable mutants was missing all three of the Chl b-complexes. The results confirm that the A-1 band is associated with the P700-Chl a-protein complex characterized previously. The data also indicate the existence of structurally different forms of the light-harvesting Chl a- and b-containing complexes. The results also show a lower molecular weight band (A-2) containing primarily Chl a and which appears to be required for viability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Bar-Nun S., Ohad I. Presence of Polypeptides of Cytoplasmic and Chloroplastic Origin in Isolated Photoactive Preparations of Photosystems I and II in Chlamydomonas reinhardi y-1. Plant Physiol. 1977 Feb;59(2):161–166. doi: 10.1104/pp.59.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Matlin K., Bennoun P. A chlorophyll-protein complex lacking in photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol. 1975 Nov;67(2PT1):361–377. doi: 10.1083/jcb.67.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck J. H., Lien S., San Pietro A. Isolation and characterization of a subchloroplast particle enriched in iron-sulfur protein and P700. Arch Biochem Biophys. 1977 Jan 15;178(1):140–150. doi: 10.1016/0003-9861(77)90178-3. [DOI] [PubMed] [Google Scholar]
- Gregory R. P., Raps S., Bertsch W. Are specific chlorophyll-protein complexes required for photosynthesis? Biochim Biophys Acta. 1971 Jun 15;234(3):330–334. doi: 10.1016/0005-2728(71)90199-x. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Characterization of three new chlorophyll-protein complexes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1113–1118. doi: 10.1016/0006-291x(78)91251-2. [DOI] [PubMed] [Google Scholar]
- Herrmann F. Genetic control of pigment-protein complexes I and Ia of the plastid mutant en:alba-1 of Antirrhinum majus. FEBS Lett. 1971 Dec 15;19(3):267–269. doi: 10.1016/0014-5793(71)80530-6. [DOI] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Reinman S., Thornber J. P. Chlorophyll-protein complexes from higher plants: a procedure for improved stability and fractionation. Arch Biochem Biophys. 1978 Sep;190(1):136–141. doi: 10.1016/0003-9861(78)90260-6. [DOI] [PubMed] [Google Scholar]
- Miles C. D., Daniel D. J. Chloroplast Reactions of Photosynthetic Mutants in Zea mays. Plant Physiol. 1974 Apr;53(4):589–595. doi: 10.1104/pp.53.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C. D. Manganese stimulation of oxygen consumption in chloroplasts with dibromothymoquinone. FEBS Lett. 1976 Jan 15;61(2):251–254. doi: 10.1016/0014-5793(76)81050-2. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Miller G. J., McIntyre K. R. The light-harvesting chlorpohyll-protein complex of photosystem II. Its location in the photosynthetic membrane. J Cell Biol. 1976 Nov;71(2):624–638. doi: 10.1083/jcb.71.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: V. Phosphorylation Efficiencies (P/e(2)) Associated with Aerobic Photooxidation of Artificial Electron Donors. Plant Physiol. 1974 Mar;53(3):370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy R., Hoarau J., Leclerc J. C. Electrophoretic and spectrophotometric studies of chlorophyll-protein complexes from tobacco chloroplasts. Isolation of a light harvesting pigment protein complex with a molecular weight of 70,000. Photochem Photobiol. 1977 Aug;26(2):151–158. doi: 10.1111/j.1751-1097.1977.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Alberte R. S., Hunter F. A., Shiozawa J. A., Kan K. S. The organization of chlorophyll in the plant photosynthetic unit. Brookhaven Symp Biol. 1976 Jun 7;(28):132–148. [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Wessels J. S., Borchert M. T. Polypeptide profiles of chlorophyll . protein complexes and thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):78–93. doi: 10.1016/0005-2728(78)90163-9. [DOI] [PubMed] [Google Scholar]


