Abstract
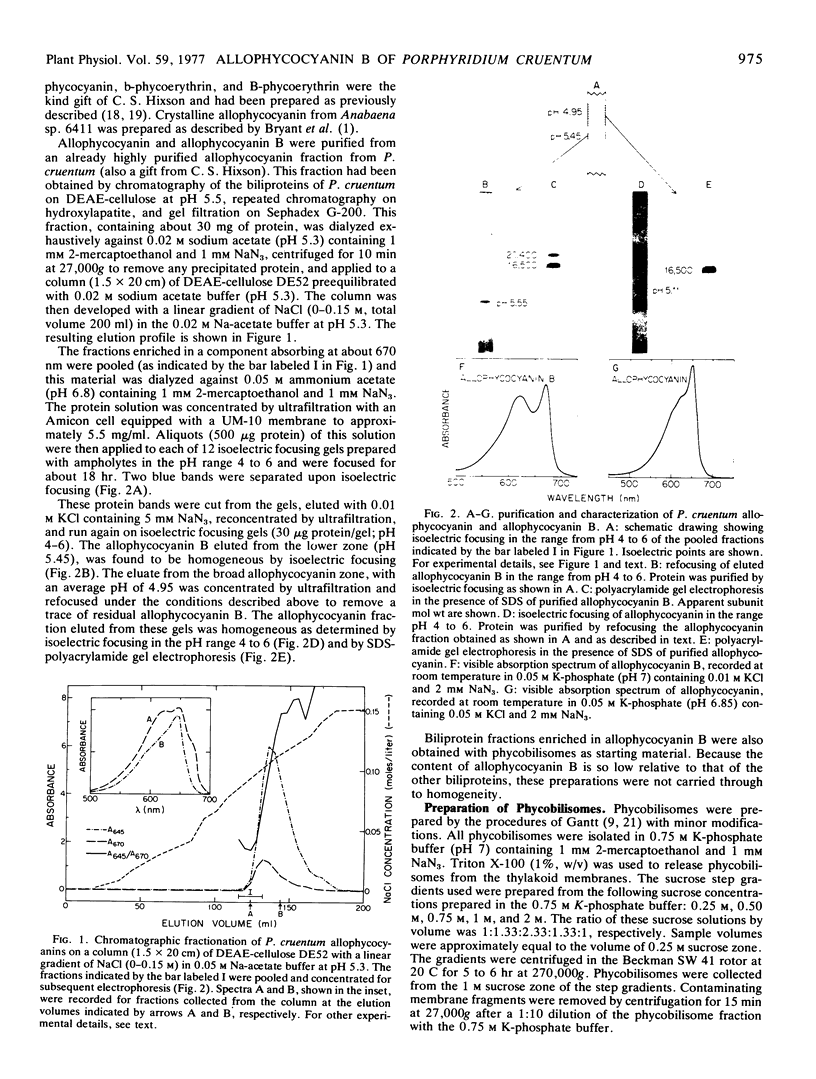
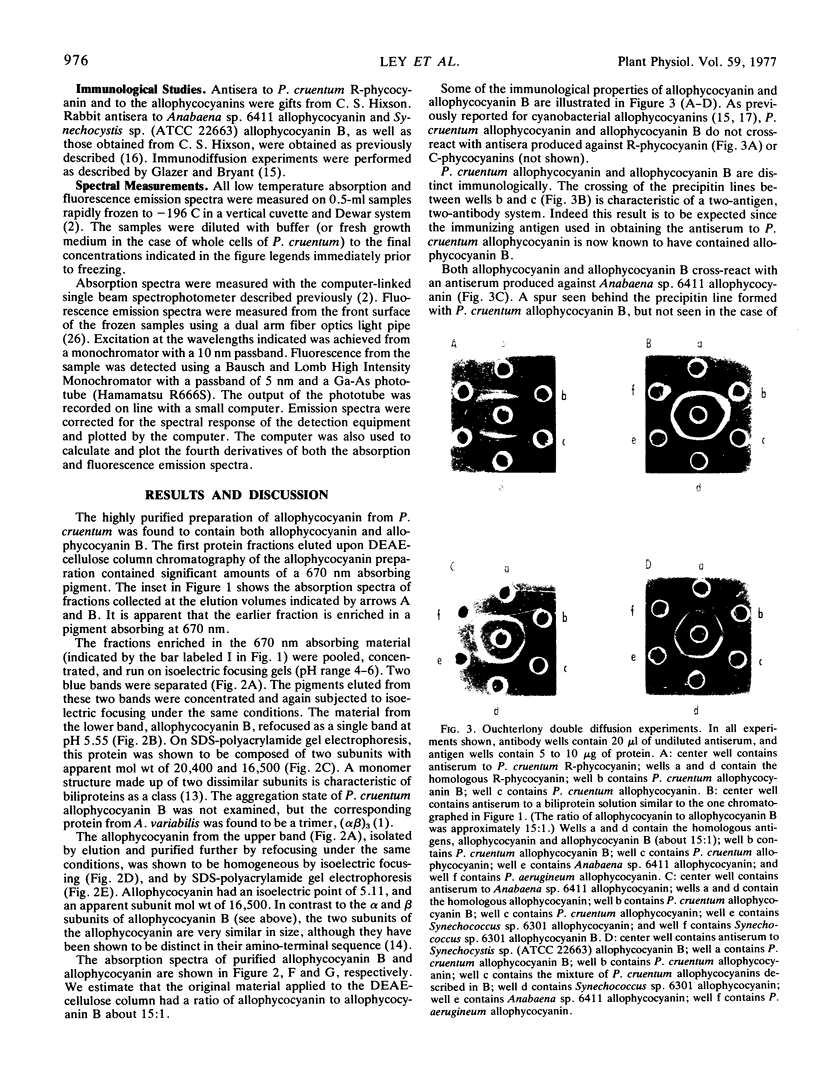
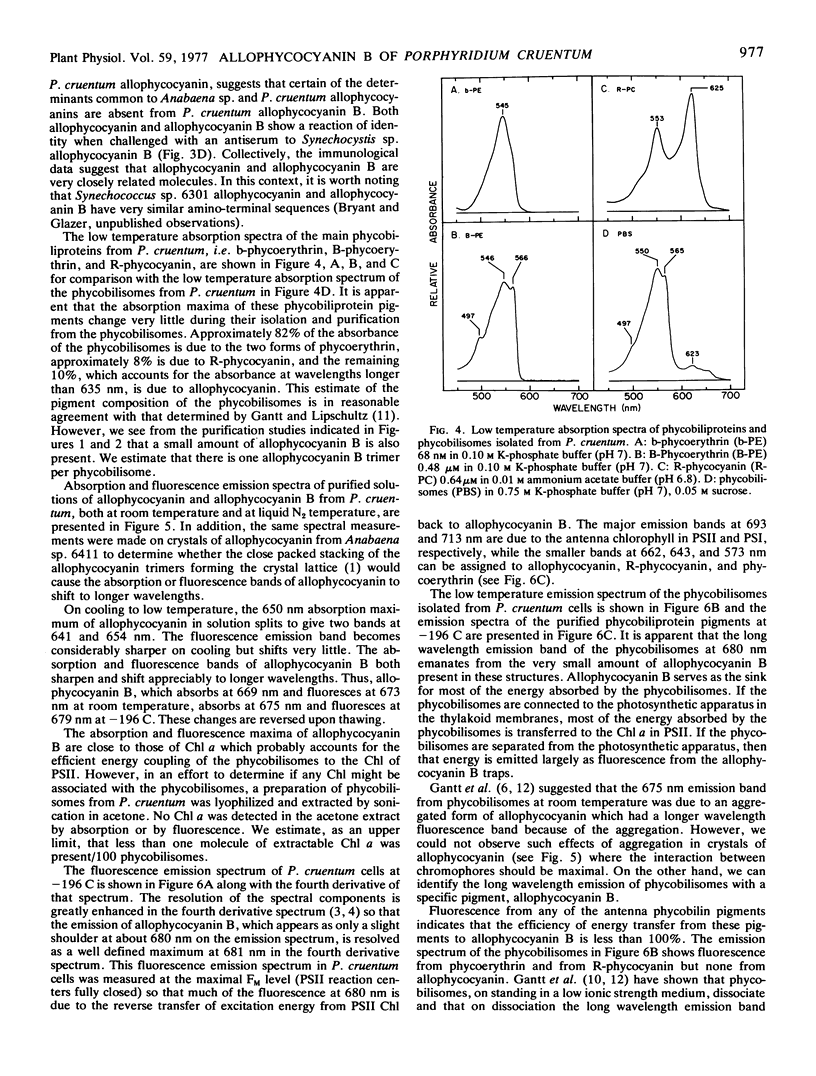
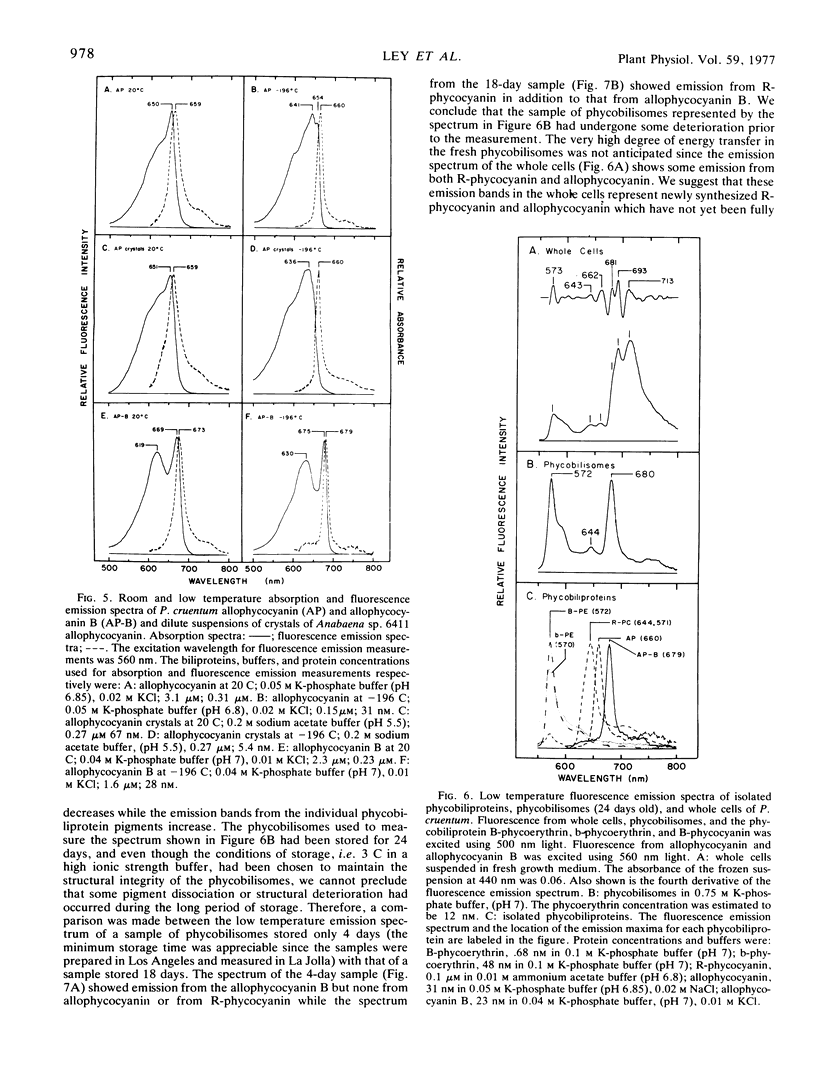
Allophycocyanin B was purified to homogeneity from the eukaryotic red alga Porphyridium cruentum. This biliprotein is distinct from the allophycocyanin of P. cruentum with respect to subunit molecular weights, and spectroscopic and immunological properties. The purified allophycocyanin B has a long wavelength absorption maximum at 669 nm at room temperature and at 675 nm at −196 C while the fluorescence emission maximum is at 673 nm at room temperature and 679 nm at −196 C. The emission spectrum of allophycocyanin shifted only 1 nm, from 659 to 660 nm, on cooling to −196 C, and was the same with allophycocyanin crystals as it was with pure solutions of the pigment. Phycobilisomes from P. cruentum have a major fluorescence emission band at 680 nm at −196 C which emanates from the small amount of allophycocyanin B present in the phycobilisomes. Light energy absorbed by the bulk of the biliprotein pigments is transferred to allophycocyanin B with high efficiency.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant D. A., Glazer A. N., Eiserling F. A. Characterization and structural properties of the major biliproteins of Anabaena sp. Arch Microbiol. 1976 Oct 11;110(1):61–75. doi: 10.1007/BF00416970. [DOI] [PubMed] [Google Scholar]
- Butler W. L. Absorption spectroscopy of biological materials. Methods Enzymol. 1972;24:3–25. doi: 10.1016/0076-6879(72)24052-6. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Phycobiliprotein localization in algae. Brookhaven Symp Biol. 1966;19:393–405. [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Energy transfer in phycobilisomes from phycoerythrin to allophycocyanin. Biochim Biophys Acta. 1973 Apr 5;292(3):858–861. doi: 10.1016/0005-2728(73)90036-4. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum: pigment analysis. Biochemistry. 1974 Jul 2;13(14):2960–2966. doi: 10.1021/bi00711a027. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Zilinskas B. Further evidence for a phycobilisome model from selective dissociation, fluorescence emission, immunoprecipitation, and electron microscopy. Biochim Biophys Acta. 1976 May 14;430(2):375–388. doi: 10.1016/0005-2728(76)90093-1. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Apell G. S., Hixson C. S., Bryant D. A., Rimon S., Brown D. M. Biliproteins of cyanobacteria and Rhodophyta: Homologous family of photosynthetic accessory pigments. Proc Natl Acad Sci U S A. 1976 Feb;73(2):428–431. doi: 10.1073/pnas.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Bryant D. A. Allophycocyanin B (lambdamax 671, 618 nm): a new cyanobacterial phycobiliprotein. Arch Microbiol. 1975 Jun 20;104(1):15–22. doi: 10.1007/BF00447294. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G., Stanier R. Y. Comparative immunology of algal biliproteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3005–3008. doi: 10.1073/pnas.68.12.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G. Subunit structure of the phycobiliproteins of blue-green algae. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1398–1401. doi: 10.1073/pnas.68.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Hixson C. S. Characterization of R-phycocyanin. Chromophore content of R-phycocyanin and C-phycoerythrin. J Biol Chem. 1975 Jul 25;250(14):5487–5495. [PubMed] [Google Scholar]
- Gray B. H., Lipschultz C. A., Gantt E. Phycobilisomes from a blue-green alga Nostoc species. J Bacteriol. 1973 Oct;116(1):471–478. doi: 10.1128/jb.116.1.471-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. C., Butler W. L. Efficiency of energy transfer from photosystem II to photosystem I in Porphyridium cruentum. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3957–3960. doi: 10.1073/pnas.73.11.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




