Abstract
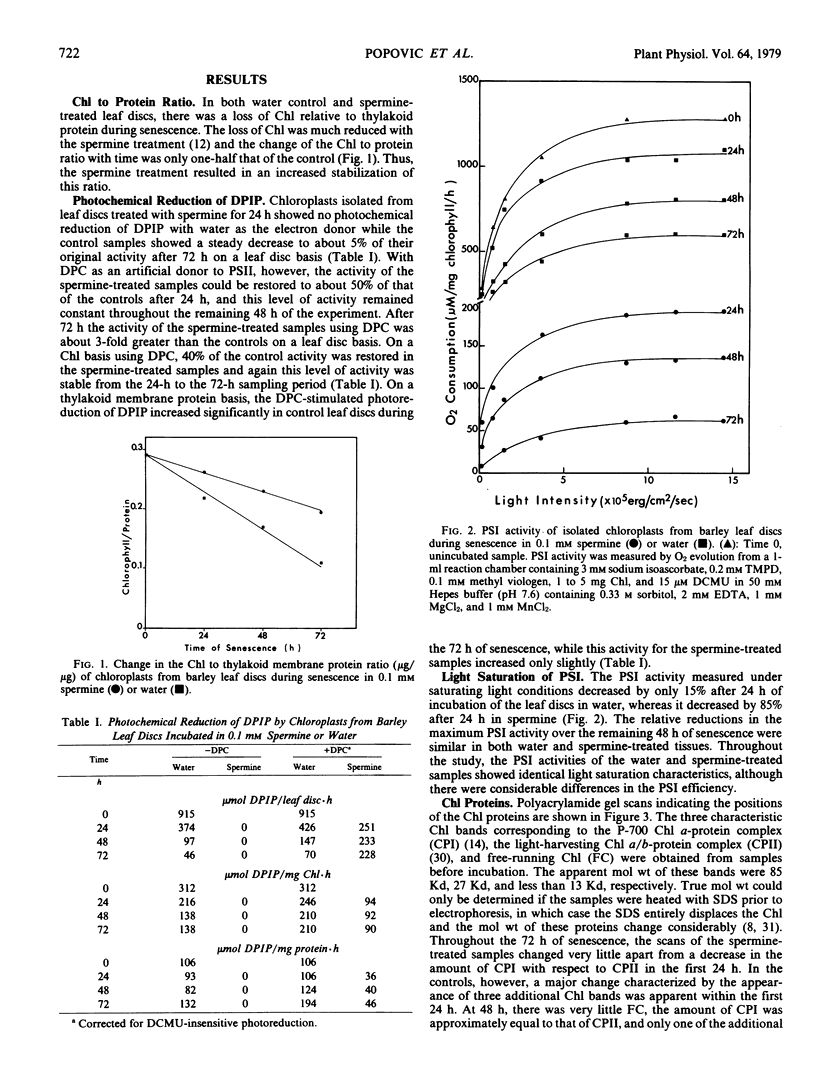
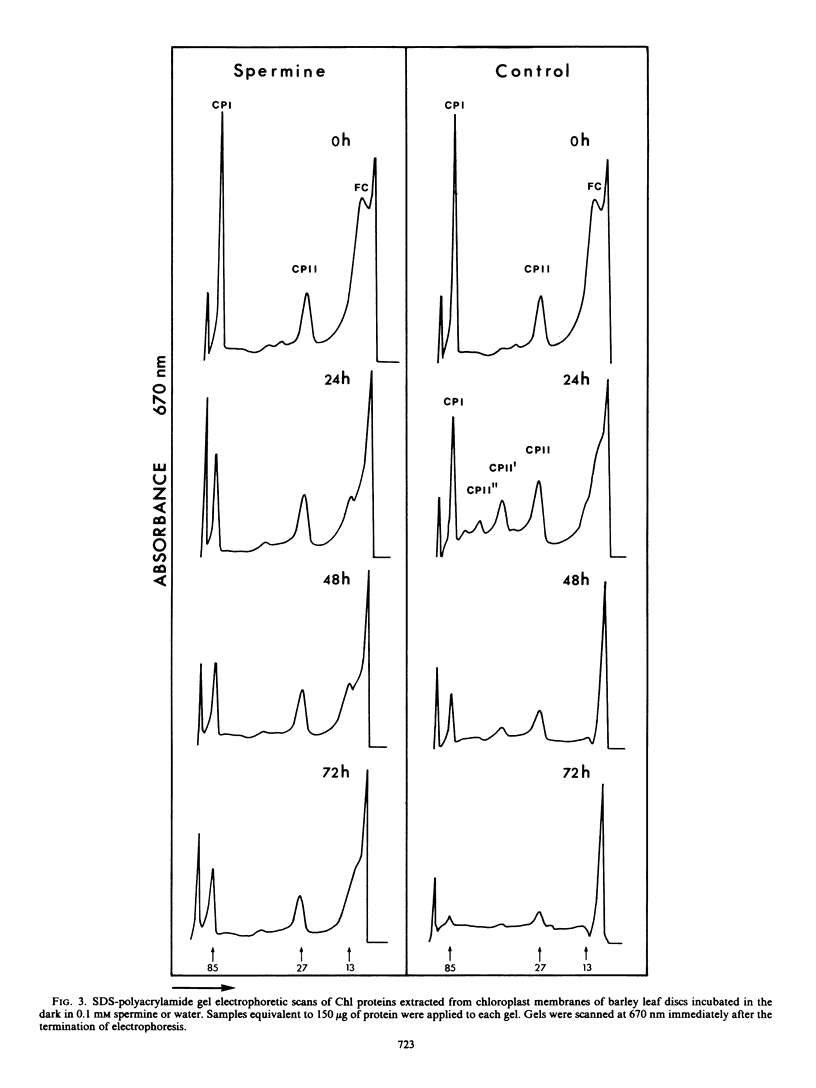
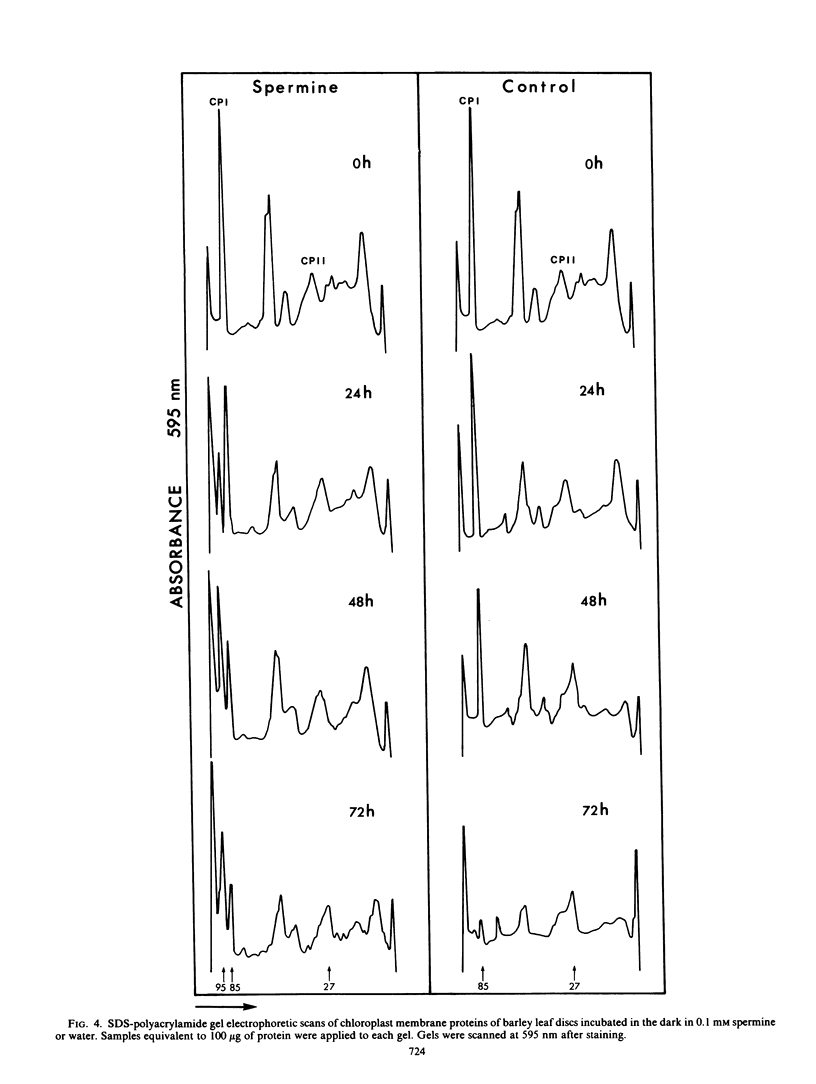
The effect of spermine on photochemical activity and polypeptide composition of chloroplasts from barley leaf discs during senescence in the dark was studied. Chloroplast membranes did not show photosystem II activity after spermine treatment when water was the electron donor, but in the presence of diphenylcarbazide, this activity was observed. The diphenylcarbazide-stimulated photoreduction of dichloroindophenol was 3-fold greater in leaf discs incubated for 72 hours in spermine than in water. Photosystem I activity was reduced by about 90% within the first 24 hours in the spermine-treated samples. This reduction, however, was not due to a decrease in the photosynthetic unit size. A preferential loss of polypeptides other than those associated with photosystem II was observed during senescence of the leaf discs in water, but this loss was reduced by spermine. Spermine treatment also prevented the appearance of several additional chlorophyll proteins found in the controls during senescence. The results have been interpreted on the basis of the interaction of spermine with thylakoid membranes resulting in stabilization of membrane function during senescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J. Localization and Characterization of Photosystem II in Grana and Stroma Lamellae. Plant Physiol. 1977 Mar;59(3):398–404. doi: 10.1104/pp.59.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Vernotte C., Briantais J. M., Armond P. Lactoperoxidase-catalyzed iodination of chloroplast membranes. II. Evidence for surface localization of photosystem II reaction centers. Biochim Biophys Acta. 1974 Oct 18;368(1):39–53. doi: 10.1016/0005-2728(74)90095-4. [DOI] [PubMed] [Google Scholar]
- Bose S., Arntzen C. J. Reversible inactivation of photosystem II reaction centers in cation-depleted chloroplast membranes. Arch Biochem Biophys. 1978 Jan 30;185(2):567–575. doi: 10.1016/0003-9861(78)90202-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caldarera C. M., Rossoni C., Casti A. Involvement of polyamines in ribonucleic acid synthesis as a possible biological function. Ital J Biochem. 1976 Jan-Feb;25(1):33–55. [PubMed] [Google Scholar]
- Cohen A. S., Popovic R. B., Zalik S. Effects of Polyamines on Chlorophyll and Protein Content, Photochemical Activity, and Chloroplast Ultrastructure of Barley Leaf Discs during Senescence. Plant Physiol. 1979 Nov;64(5):717–720. doi: 10.1104/pp.64.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. E., Jr, Thornber J. P. The P700-chlorophyll -protein of a blue-green alga. Biochim Biophys Acta. 1971 Sep 7;245(2):482–493. doi: 10.1016/0005-2728(71)90164-2. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Moudrianakis E. N. Function of the "quantasome" in photosynthesis: structure and properties of membrane-bound particle active in the dark reactions of photophosphorylation. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1261–1268. doi: 10.1073/pnas.58.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Altman A., Galston A. W. Dual Mechanisms in Polyamine-mediated Control of Ribonuclease Activity in Oat Leaf Protoplasts. Plant Physiol. 1978 Jul;62(1):158–160. doi: 10.1104/pp.62.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchanski S. J., Park R. B. Comparative Studies of the Thylakoid Proteins of Mesophyll and Bundle Sheath Plastids of Zea mays. Plant Physiol. 1976 Sep;58(3):345–349. doi: 10.1104/pp.58.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Reinman S., Thornber J. P. Chlorophyll-protein complexes from higher plants: a procedure for improved stability and fractionation. Arch Biochem Biophys. 1978 Sep;190(1):136–141. doi: 10.1016/0003-9861(78)90260-6. [DOI] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Armond P. A., Miller K. R. Chloroplast membrane organization at the supramolecular level and its functional implications. Brookhaven Symp Biol. 1976 Jun 7;(28):278–315. [PubMed] [Google Scholar]
- Staehelin L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol. 1976 Oct;71(1):136–158. doi: 10.1083/jcb.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Wessels J. S., Borchert M. T. Polypeptide profiles of chlorophyll . protein complexes and thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):78–93. doi: 10.1016/0005-2728(78)90163-9. [DOI] [PubMed] [Google Scholar]


