Abstract
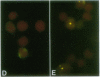
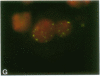
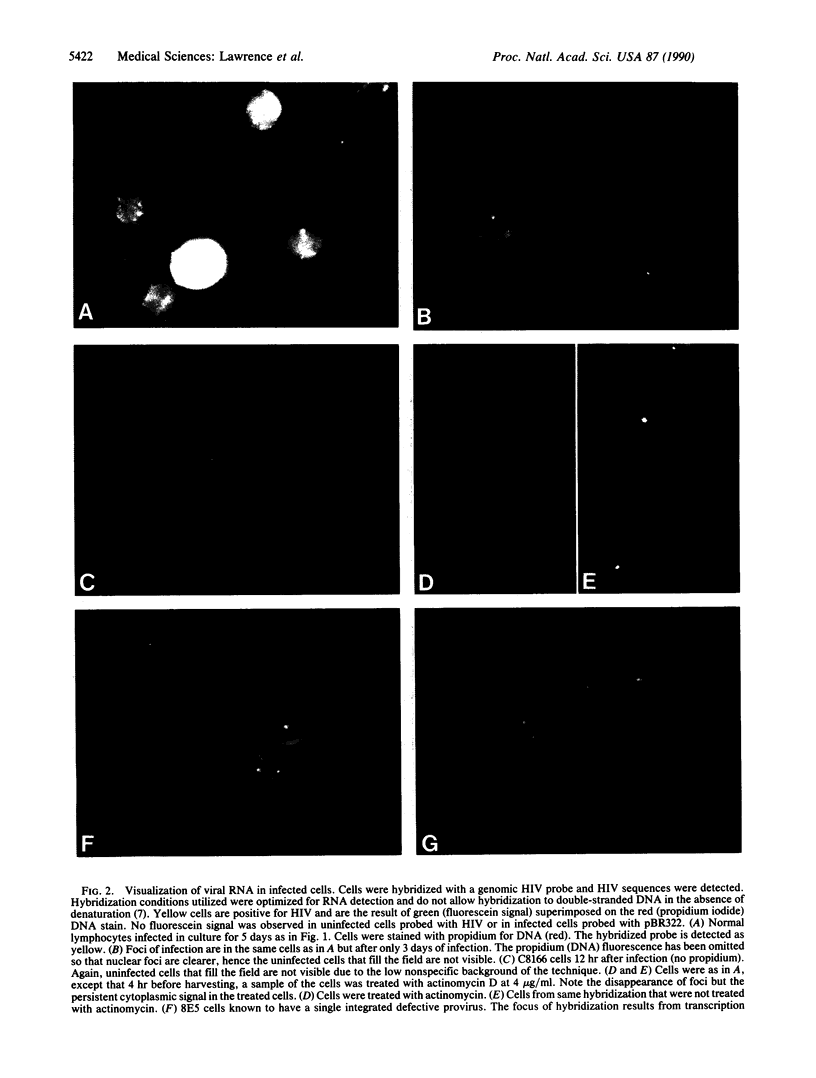
Detection and subcellular localization of human immunodeficiency virus (HIV) were investigated using sensitive high-resolution in situ hybridization methodology. Lymphocytes infected with HIV in vitro or in vivo were detected by fluorescence after hybridization with either biotin or digoxigenin-labeled probes. At 12 hr after infection in vitro, a single intense signal appeared in the nuclei of individual cells. Later in infection, when cytoplasmic fluorescence became intense, multiple nuclear foci frequently appeared. The nuclear focus consisted of newly synthesized HIV RNA as shown by hybridization in the absence of denaturation and by susceptibility to RNase and actinomycin D. Virus was detected in patient lymphocytes and it was shown that a singular nuclear focus also characterizes cells infected in vivo. The cell line 8E5/LAV containing one defective integrated provirus revealed a similar focus of nuclear RNA, and the single integrated HIV genome was unequivocally visualized on a D-group chromosome. This demonstrates an extremely sensitive single-cell assay for the presence of a single site of HIV transcription in vitro and in vivo and suggests that it derives from one (or very few) viral genomes per cell. In contrast, productive Epstein-Barr virus infection exhibited many foci of nuclear RNA per cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Busch M. P., Rajagopalan M. S., Gantz D. M., Fu S., Steimer K. S., Vyas G. N. In situ hybridization and immunocytochemistry for improved assessment of human immunodeficiency virus cultures. Am J Clin Pathol. 1987 Dec;88(6):673–680. doi: 10.1093/ajcp/88.6.673. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H., Marselle L. M. Highly localized tracks of specific transcripts within interphase nuclei visualized by in situ hybridization. Cell. 1989 May 5;57(3):493–502. doi: 10.1016/0092-8674(89)90924-0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Villnave C. A., Singer R. H. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell. 1988 Jan 15;52(1):51–61. doi: 10.1016/0092-8674(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Scott G. B., Buck B. E., Leterman J. G., Bloom F. L., Parks W. P. Acquired immunodeficiency syndrome in infants. N Engl J Med. 1984 Jan 12;310(2):76–81. doi: 10.1056/NEJM198401123100202. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Byron K. S., Lawrence J. B., Sullivan J. L. Detection of HIV-1-infected cells from patients using nonisotopic in situ hybridization. Blood. 1989 Nov 1;74(6):2295–2301. [PubMed] [Google Scholar]
- Somasundaran M., Robinson H. L. Unexpectedly high levels of HIV-1 RNA and protein synthesis in a cytocidal infection. Science. 1988 Dec 16;242(4885):1554–1557. doi: 10.1126/science.3201245. [DOI] [PubMed] [Google Scholar]