Abstract
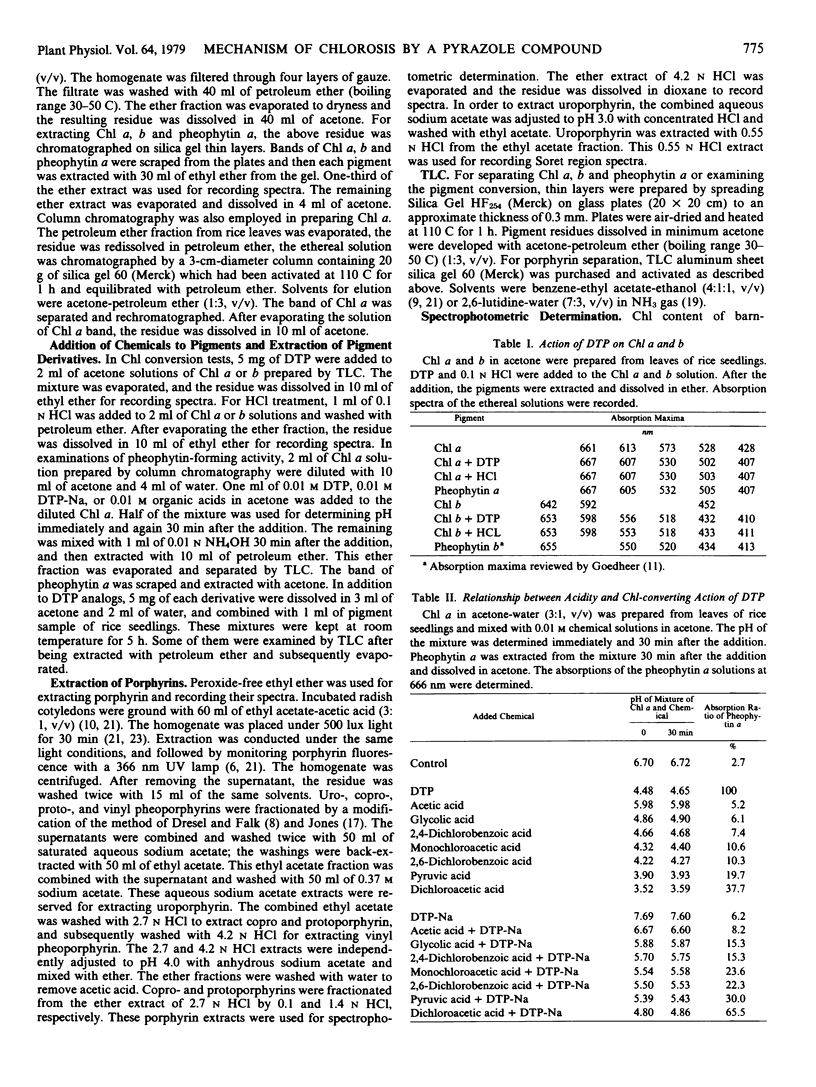
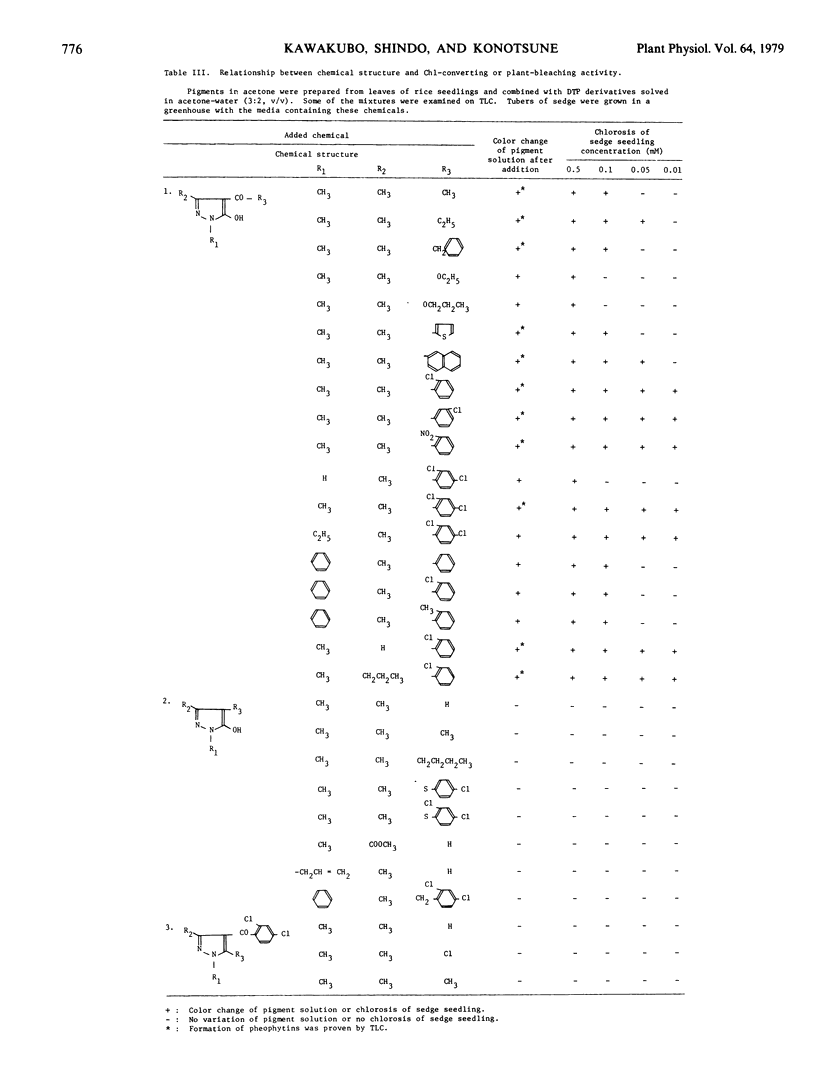
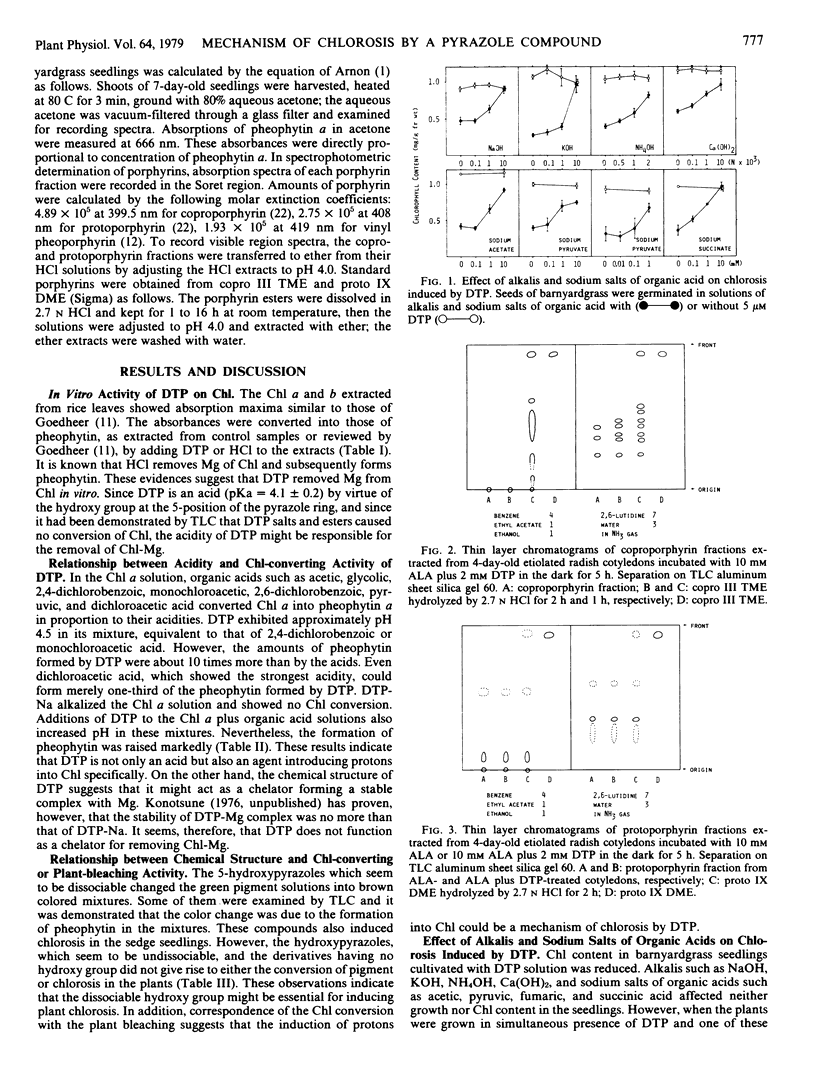
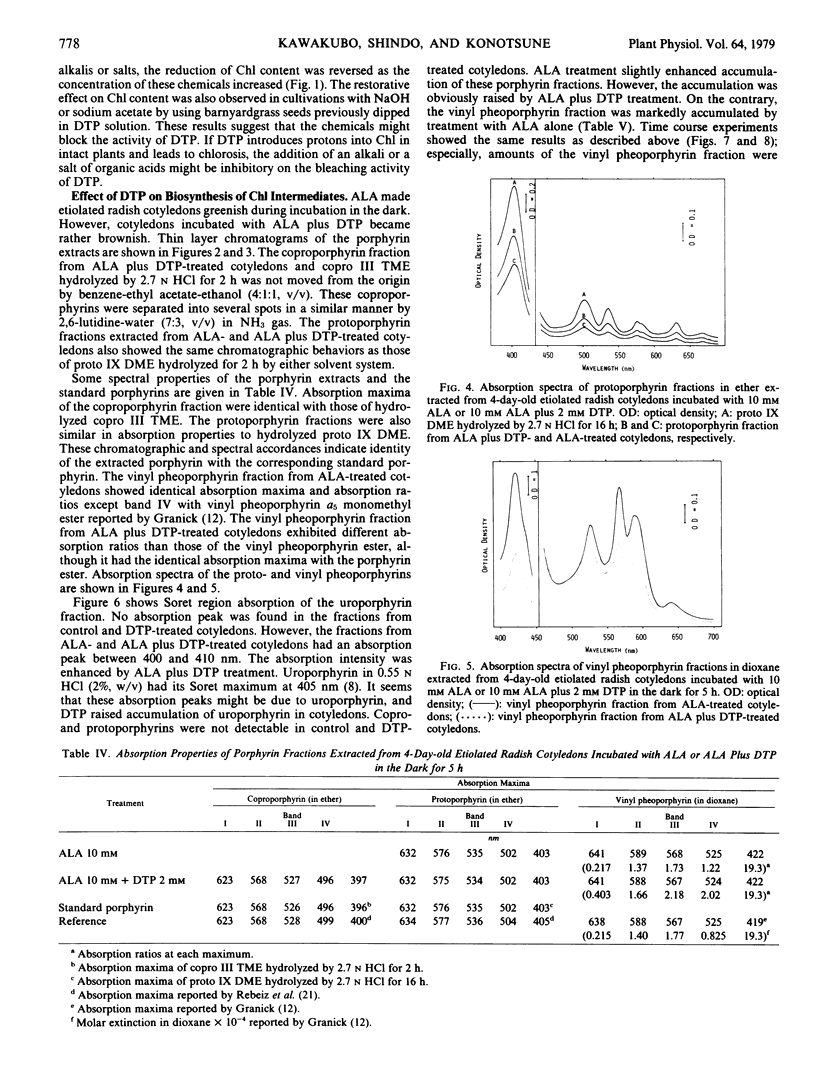
In organic solvents, 1,3-dimethyl-4-(2,4-dichlorobenzoyl)-5-hydroxypyrazole (DTP) converted chlorophyll a and b extracted from rice seedlings (Oryza sativa L. `Kinmaze') into pheophytin a and b, respectively. On comparing the chlorophyll-converting activity of DTP to those of acetic, glycolic, 2,4-dichlorobenzoic, monochloroacetic, 2,6-dichlorobenzoic, pyruvic, and dichloroacetic acids, it was demonstrated that DTP induced H+ into chlorophyll specifically. 5-Hydroxypyrazoles, which seem to be dissociable, converted chlorophyll into pheophytin in vitro. These compounds also induced chlorosis in sedge seedlings (Cyperus serotinus Rottb.), when the seedlings were grown in media containing these compounds. However, 5-hydroxypyrazoles, which seem to be undissociable, and analogs having no hydroxy group caused neither the chlorophyll conversion in vitro nor chlorosis in the seedlings. Chlorosis in barnyardgrass seedlings (Echinochloa crus-galli Beauv. var. oryzicola Ohwi) induced by DTP was reversed by cultivating the seedlings in media containing DTP plus NaOH, KOH, NH4OH, Ca(OH)2, sodium acetate, sodium pyruvate, sodium succinate, or sodium fumarate. Accumulation of the vinyl pheoporphyrin fraction in 4- day-old etiolated radish cotyledons (Raphanus sativus L. `Minowase 2') was enhanced by incubating the cotyledons with δ-aminolevulinic acid in the dark. However, simultaneous treatment with δ-aminolevulinic acid and DTP reduced accumulation of the fraction and promoted formation of the uro, copro, and protoporphyrin fractions. These results suggest that DTP blocks the synthesis of protochlorophyllide in intact plants and induces consequent chlorosis, and the H+ -donating activity of DTP might cause the reduction of protochlorophyllide biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., Hyde A. Chloroplast Development in 4-Chloro-5-(dimethylamino)-2-(alpha,alpha,alpha-trifluoro-m-tolyl)-3 (2H)-pyridazinone (Sandoz 6706)-treated Wheat Seedlings: A Pigment, Ultrastructural, and Ultracentrifugal Study. Plant Physiol. 1970 Jun;45(6):807–810. doi: 10.1104/pp.45.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns E. R., Buchanan G. A., Carter M. C. Inhibition of carotenoid synthesis as a mechanism of action of amitrole, dichlormate, and pyriclor. Plant Physiol. 1971 Jan;47(1):144–148. doi: 10.1104/pp.47.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Jones O. T. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975 Mar;55(3):485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESEL E. I., FALK J. E. Studies on the biosynthesis of blood pigments. 2. Haem and porphyrin formation in intact chicken erythrocytes. Biochem J. 1956 May;63(1):72–79. doi: 10.1042/bj0630072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK J. E. Chromatography of porphyrins and metalloporphyrins. J Chromatogr. 1961 Apr;5:277–299. doi: 10.1016/s0021-9673(01)92860-2. [DOI] [PubMed] [Google Scholar]
- JONES O. T. MAGNESIUM 2,4-DIVINYLPHAEOPORPHYRIN A5 MONOMETHYL ESTER, A PROTOCHLOROPHYLL-LIKE PIGMENT PRODUCED BY RHODOPSEUDOMONAS SPHEROIDES. Biochem J. 1963 Nov;89:182–189. doi: 10.1042/bj0890182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas spheroides. Biochem J. 1963 Mar;86:429–432. doi: 10.1042/bj0860429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Haidar M. A., Yaghi M. Porphyrin Biosynthesis in Cell-free Homogenates from Higher Plants. Plant Physiol. 1970 Oct;46(4):543–549. doi: 10.1104/pp.46.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960 Jun;75(3):620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]


