Abstract
Ayahuasca is a hallucinogen brew traditionally used for ritual and therapeutic purposes in Northwestern Amazon. It is rich in the tryptamine hallucinogens dimethyltryptamine (DMT), which acts as a serotonin 5-HT2A agonist. This mechanism of action is similar to other compounds such as lysergic acid diethylamide (LSD) and psilocybin. The controlled use of LSD and psilocybin in experimental settings is associated with a low incidence of psychotic episodes, and population studies corroborate these findings. Both the controlled use of DMT in experimental settings and the use of ayahuasca in experimental and ritual settings are not usually associated with psychotic episodes, but little is known regarding ayahuasca or DMT use outside these controlled contexts. Thus, we performed a systematic review of the published case reports describing psychotic episodes associated with ayahuasca and DMT intake. We found three case series and two case reports describing psychotic episodes associated with ayahuasca intake, and three case reports describing psychotic episodes associated with DMT. Several reports describe subjects with a personal and possibly a family history of psychosis (including schizophrenia, schizophreniform disorders, psychotic mania, psychotic depression), nonpsychotic mania, or concomitant use of other drugs. However, some cases also described psychotic episodes in subjects without these previous characteristics. Overall, the incidence of such episodes appears to be rare in both the ritual and the recreational/noncontrolled settings. Performance of a psychiatric screening before administration of these drugs, and other hallucinogens, in controlled settings seems to significantly reduce the possibility of adverse reactions with psychotic symptomatology. Individuals with a personal or family history of any psychotic illness or nonpsychotic mania should avoid hallucinogen intake.
Keywords: ayahuasca, dimethyltryptamine, hallucinogens, psychosis
Introduction
Ayahuasca is a natural hallucinogen traditionally used by several indigenous groups from the Northwestern Amazon for ritual and therapeutic purposes [Schultes and Hofmann, 1992]. It is usually prepared by the prolonged decoction of the vine Banisteriopsis caapi together with the leaves of the shrub Psychotria viridis [Schultes and Hofmann, 1992]. B. caapi contains β-carbolines alkaloids such as harmine, tetrahydroharmine (THH) and harmaline, which are reversible monoamine oxidase A (MAO-A) inhibitors, while P. viridis contains the serotonin2A/2C/1A receptor agonist hallucinogen N,N-dimethyltryptamine (DMT) [McKenna and Riba, 2015]. Although DMT by itself is orally inactive, in the case of ayahuasca, its β-carbolines inhibit the metabolism of DMT by peripheral MAO-A, thus allowing the alkaloid to reach cortical 5-HT2A receptors [Riba et al. 2015].
In the last 25 years, ayahuasca use has expanded from the Amazon to the United States, Europe, Africa and Asia, raising concerns about its possible toxic effects and hopes on its therapeutic potentials [Labate et al. 2009; Labate and Feeney, 2012]. Laboratory studies involving oral administration of single ayahuasca doses to healthy volunteers show that this botanical hallucinogen induces perceptual alterations, introspection, increases in autobiographical memories, positive mood and wellbeing [dos Santos et al. 2016a]. These studies also suggest that ayahuasca has an acceptable tolerability, with nausea and vomiting as the most frequent adverse reactions. Likewise, long-term ingestion of ayahuasca in ritual settings is not associated with increases in cognitive deficits or psychopathology [dos Santos et al. 2016a].
The subjective and neurophysiological effects of acute ayahuasca intake are apparently mediated by the agonist action of DMT on 5-HT2A receptors expressed in paralimbic and frontal brain areas including the default mode network (DMN) [Riba et al. 2006; de Araujo et al. 2012; Palhano-Fontes et al. 2015]. Therefore, ayahuasca shares, at least in part, the same mechanism of action of classic 5-HT2A agonist hallucinogens such as lysergic acid diethylamide (LSD), psilocybin, and mescaline [Vollenweider and Kometer, 2010; Nichols, 2016]. The agonism of these compounds at cortical 5-HT2A receptors also seems to depend on metabotropic glutamate receptors (mGluR) [Gonzalez-Maeso et al. 2008; Moreno et al. 2011].
During the 1950s–1970s, when the use of classic/serotonergic hallucinogens such as LSD and psilocybin was allowed both in therapeutic/clinical and experimental settings, one of the most prominent concerns regarding the use of these compounds was their possible association with prolonged psychotic reactions [Cohen, 1960; Cohen and Ditman, 1962; Smart and Bateman, 1967; Malleson, 1971; Strassman, 1984; Johnson et al. 2008]. However, studies from that time reported that the incidence of such cases in controlled settings was rare both in healthy volunteers and in psychiatric patients [Cohen 1960; Cohen and Ditman, 1962; Smart and Bateman, 1967; Malleson, 1971; Strassman, 1984; Johnson et al. 2008]. In one of the most cited studies, Cohen reported the following estimated rates of psychotic reactions lasting longer than 48 hours in both experimental subjects and patients undergoing therapy: 0.8/1000 and 1.8/1000, respectively [Cohen, 1960]. However, the nature of these psychotic reactions was not characterized.
Regarding noncontrolled/recreational use of classic hallucinogens, although case reports of psychotic experiences have been described since the 1960s [Klock et al. 1974; Smith et al. 2014], these reports often involved individuals with preexisting psychiatric disorders and possibly instances of poor preparation, guidance, and integration of drug effects, thus making it difficult to establish a causal relationship with hallucinogen use in many cases [Strassman, 1984; Johnson et al. 2008; Smith et al. 2014; Garcia-Romeu et al. 2016]. According to the fifth edition of the Diagnostic Statistical Manual of the American Psychiatric Association [DSM-V; American Psychiatric Association, 2013], hallucinogen-induced disorders are among the rarest of all substance use disorders, and this also seems to be valid regarding drug-induced psychosis [Vallersnes et al. 2016]. Moreover, hallucinogens are considered one of the least toxic classes of drugs [Nutt et al. 2010; van Amsterdam et al. 2011, 2013, 2015], and recent population studies did not find significant associations between lifetime use of classic hallucinogens (LSD, psilocybin, mescaline) and increases in mental health problems [Krebs and Johansen, 2013; Johansen and Krebs, 2015], including nonaffective psychosis and mania [Krebs and Johansen, 2013].
Indeed, these and other population studies suggested that hallucinogen consumption is associated with reductions in mental health problems and in problematic behavior [Krebs and Johansen, 2013; Hendricks et al. 2014, 2015; Walsh et al. 2016]. These potential benefic effects were previously reported in both controlled and uncontrolled studies from the 1950s–1970s [Johnson et al. 2008; Vollenweider and Kometer, 2010; Smith et al. 2014; dos Santos et al. 2016b; Garcia-Romeu et al. 2016], and are being corroborated by recent open-label studies suggesting positive effects in drug dependence and mood and anxiety disorders [dos Santos et al. 2016b; Garcia-Romeu et al. 2016]. Importantly, there are no reports describing prolonged psychotic reactions in the volunteers participating in these recent studies [dos Santos et al. 2016b; Garcia-Romeu et al. 2016]. The picture seems to be somewhat different regarding new synthetic hallucinogens, such as phenylethylamine and tryptamine derivatives, which appear to be associated with more adverse reactions, including psychotic experiences [Araujo et al. 2015; Tittarelli et al. 2015; Vallersnes et al. 2016]. However, these drugs are not the topic of the present article and will not be further discussed.
The possible relationship between ayahuasca and psychotic experiences is poorly understood. In experimental and clinical studies involving single or few ayahuasca doses, transient (<6 hours) dysphoric reactions with anxiety and possibly psychotic-like features such as modifications on perceptions and thought content may occur [dos Santos et al. 2016a], as with other hallucinogens [Strassman, 1984; Johnson et al. 2008; Vollenweider and Kometer, 2010; Studerus et al. 2011; Garcia-Romeu et al. 2016]. However, these cases are usually rare and transient, with verbal support reducing distress in most instances [Strassman, 1984; Johnson et al. 2008; Studerus et al. 2011; Garcia-Romeu et al. 2016]. Indeed, Riba and colleagues [Riba et al. 2001; Riba and Barbanoj, 2006] reported the case of a volunteer that experienced an intense and transient (around 20 minutes) dysphoric reaction with disorientation, anxiety, and feelings of suspiciousness and menace that was effectively handled only with verbal support, disappearing completely after the expected time of action of ayahuasca (<6 hours) without the need of any kind of medical intervention. Likewise, long-term ingestion of ayahuasca in controlled/ritual settings is not usually associated with an increased incidence of psychotic disorders [dos Santos et al. 2016a].
Therefore, the aim of the present work is to present a systematic review of the cases in which psychotic events occurred after the acute effects of ayahuasca and DMT.
Methods
The data for this systematic review were obtained according to the systematic reviews and meta-analysis guidelines from the PRISMA group [Moher et al. 2009].
Data acquisition
We attempted to identify all studies available to review up to 16 August 2016 in which a possible association between ayahuasca/DMT intake and psychotic disorders was reported.
Search strategy
Electronic searches were performed using the PubMed (1 January 1966–16 August 2016), LILACS (1 January 1982–16 August 2016) and SciELO (1 January 1998–16 August 2016) database. The following key words were used: ayahuasca OR dimethyltryptamine AND psychosis OR psychotic OR schizophrenia OR mania OR psychotic depression OR psychotic mania OR psychotic bipolar. References were retrieved by searching the aforementioned electronic databases and handsearching of reference lists of the identified literature. All studies published in English up to 16 August 2016 were included.
Eligibility criteria
The following inclusion and exclusion criteria were established prior to the literature search:
Article type
Original research reports, reviews, qualitative studies, case series and case reports, books and book chapters, abstracts, letters, conference abstracts, comments and editorials were included. Animal studies were excluded.
Study design
The review only included studies where a possible association between ayahuasca/DMT intake and psychotic disorders was reported.
Participants/sample
Healthy volunteers and clinical populations.
Interventions
Ayahuasca/DMT intake.
Comparisons
No comparators were considered.
Outcomes
Psychotic disorders.
Data extraction
All studies were screened by two independent reviewers with discrepancies resolved by a third reviewer. Names of authors, year of publication, study type, intervention, and outcome measures were recorded for all included articles.
Results
Study selection
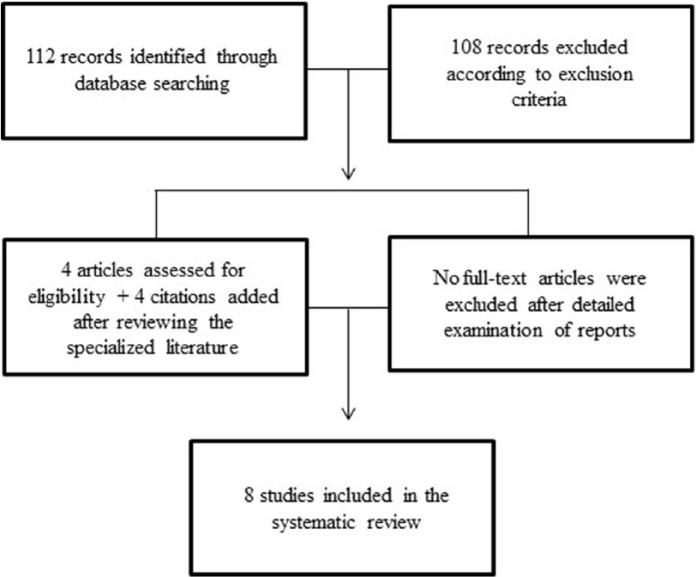
A flow diagram illustrating the different phases of the systematic review is presented in Figure 1.
Figure 1.
Flow diagram illustrating the different phases of the systematic review.
The electronic database search yielded 112 separate references that were reviewed for abstract screening. Following this first pass, four potentially relevant references were identified [Gable, 2007; Warren et al. 2013; Paterson et al. 2015; Szmulewicz et al. 2015]. Four additional citations were added after reviewing both the specialized and grey literature [Lima et al. 2002; dos Santos and Strassman, 2008; Lima and Tófoli, 2011; Umut et al. 2011]. Full-text reports of these eight citations were obtained for a more detailed evaluation. Following detailed examination of the reports, all eight citations were included. Selected publications included three studies related to DMT use (all case reports): [Umut et al. 2011; Warren et al. 2013; Paterson et al. 2015] and five studies related to ayahuasca use (two case reports): [dos Santos and Strassman, 2008; Szmulewicz et al. 2015]; and three case series/observational studies: [Lima et al. 2002; Gable, 2007; Lima and Tófoli, 2011]. The main information of the studies included in the review is presented in Table 1.
Table 1.
Psychotic reactions associated with ayahuasca and DMT.
| Reference | Study design | Sample/drug | Main findings |
|---|---|---|---|
| Lima et al. [2002] * | Case series/observational 1996–2000 |
Brazilian UDV members AYA (oral) |
Seven cases of psychotic disorders$ |
| Gable [2007] * | Case series/observational 5 years (?) |
Brazilian UDV members AYA (oral) |
13–24 cases of psychotic disorders |
| Dos Santos and Strassman [2008] | Case report | 21-year-old Brazilian man AYA (oral) |
Two psychotic episodes separated by 1 year |
| Lima and Tófoli, [2011] * | Case series/observational 1994–2007 |
Brazilian UDV members AYA (oral) |
29 cases of psychotic disorders¶ |
| Umut et al. [2011] | Case report | 19-year-old North American man DMT (smoked?) |
Psychotic episode |
| Warren et al. [2013] | Case report | 24-year-old South Australian man DMT (smoked) |
Psychotic episode |
| Szmulewicz et al. [2015] | Case report | 30-year-old Argentinian man AYA (oral) |
Psychotic episode |
| Paterson et al. [2015] | Case report | 42-year-old North American (?) man DMT (smoked) |
Psychotic episode |
| Described in the present review | Case report | 40-year-old Spanish woman AYA (oral) |
Psychotic episode |
AYA, ayahuasca; DMT, dimethyltryptamine; UDV, União do Vegetal.
Interrogation: not specified.
Included subjects from the same sample.
Two cases did not present relation with ayahuasca, three cases were relapses of previous psychotic episodes, in one case, ayahuasca was associated with other factors (no details were given), and only one case presented immediate temporal relation with ayahuasca and there were no psychotic antecedents.
Schizophrenia (n = 9), acute and transient psychotic disorders (n = 4), unspecified nonorganic (n = 2), severe depressive episode with psychotic symptoms (n = 4), substance-induced psychosis (n = 6), and bipolar affective disorder with psychotic manic episode (n = 4). In 10 cases there was no immediate temporal relation between ayahuasca intake and the psychotic episode, and 19 of the 29 cases (65.5%) presented some relation with ayahuasca: in four cases, there was an immediate temporal relation between ayahuasca intake and the psychotic episode, and subjects had no psychiatric history; in five cases, there was an immediate temporal relation between ayahuasca intake and the psychotic episode, but subjects had a psychiatric history with or without an active symptomatology; and in 10 cases there was no immediate temporal relation between ayahuasca intake and the psychotic episode, but ayahuasca may have contributed with others factors for the development of the case.
Brief background information on the different settings within which ayahuasca/N,N-dimethyltryptamine were administered
It is important to consider the different types of settings where ayahuasca or DMT were administered or ingested, since they can be an important variable when evaluating the occurrence of the psychotic episodes reported in the present review. These settings could be basically divided between controlled and uncontrolled contexts. The controlled contexts include ritual or religious ayahuasca use, both within the setting of any of the Brazilian ayahuasca religions (Santo Daime and União do Vegetal, for example) and in organized retreats and workshops; the controlled settings also include experimental and clinical contexts where ayahuasca or DMT are administered. In the controlled settings, some form of screening, preparation, guidance, and integration are usually present. The uncontrolled or recreational settings could be characterized in those contexts where ayahuasca or DMT are used outside a religious, ritual or experimental/clinical framework, and usually do not have any form of screening, preparation, guidance, and integration.
N,N-dimethyltryptamine
Umut and colleagues described the case of a 19-year-old North American male who experienced sudden and dramatic psychotic symptoms immediately after consuming a solution of DMT and cannabis (route of administration not specified, but probably smoked) [Umut et al. 2011]. He had no personal or family psychiatric history, but had been using cannabis for the last 3 years before the episode: in the first 1.5 years, he used cannabis ‘rarely and irregularly’, but in the last 1.5 years he was using 1–2 joints/day. The subject lived in Turkey and had just returned from a 3-month period living abroad with his father, 40 days before the episode. In the evening when he returned home, the subject experienced an episode of psychotic mania characterized by continuous swearing, delusional ideas (such as believing that he was a king), increased speech, excessive money spending and joyfulness, inappropriate dressing and behavior (such as dancing in the streets and being rapidly familiar and getting friendly with people he did not know before). Because he had no psychiatric history, this psychotic mania episode was later associated with his 3-year period of continuous cannabis use. His mother thought that these symptoms were related to his happiness in returning home, and they did not seek any treatment. After this episode, the subject used cannabis a few more times and 15 days later, a friend offered him a DMT/cannabis solution (probably smoked). After using this solution, the subject experienced intense psychotic symptoms such as feeling that he was being directed by another power, seeing musical sounds in the sky, contacting creatures from outer space, and believing that people could read his thoughts and were saying numbers to him while he was walking in the street, among other symptoms. His mother got worried with these sudden and dramatic symptoms and started looking for help, taking him to a private hospital where cannabis metabolites were detected in the subject’s urine 20 days after DMT consumption. It is not clear in the report when exactly the subject went to this hospital or what happened in those 20 days, but it seems that the subject did not receive any treatment in this period. The subject received a prescription (not specified) in the private hospital, but did not use the medication. After being convinced, he was brought to another hospital 3 days later, where he received a 12-day inpatient antipsychotic treatment (haloperidol, risperidone). After being discharged, the subject continued to take his medications (risperidone) regularly and was followed for approximately 2.5 months, and the psychotic symptoms gradually remitted. The authors concluded that DMT exacerbated the psychotic symptoms of a previous ongoing cannabis-induced psychotic mania.
Warren and colleagues reported a brief description (letter) of a case of a 24-year-old man from rural South Australia that was admitted to hospital after suffering a psychotic episode associated with continuous use of a smokable powder made of DMT-containing plants [Warren et al. 2013]. After being introduced to DMT by his friends and encouraged to investigate about the drug on the internet, the subject collected the leaves, bark, and seeds from two DMT-rich plants (Phalaris aquatica and an unidentified Acacia species), dried and grounded the botanical material into a fine powder, and then added the powder to a pipe where he regularly used tobacco and cannabis. According to the authors, in the year before his admission the subject was smoking this material with increasing frequency, and in the last 6 months before the admission, the subject developed ‘a complex delusional spiritual belief system and was pursuing enlightenment.’ This pattern of increased use and delusional thinking led the subject to a hospital admission for presenting positive symptoms of schizophrenia. Importantly, the subject had a family history of psychotic disorder (not specified), and also an extensive prior experience with tobacco, cannabis, methamphetamines, and DMT. These factors complicate the assessment of the possible role of DMT in this episode.
Paterson presented the case of a 42-year-old man (apparently North American, not specified) that suffered a psychotic episode associated with repeated use of smoked DMT [Paterson et al. 2015]. The subject had no personal psychiatric history, but had an extensive history of multiple substance use disorders [alcohol, tobacco, cannabis, 3, 4-methylenedioxymethamphetamine (MDMA, ecstasy), hydrocodone] associated with legal problems (e.g. driving while intoxicated, fined for cannabis possession). Moreover, he had a family history of alcoholism, bipolar disorder, and obsessive–compulsive disorder. When the subject was 39-years old, he had successfully completed a drug-treatment program, but resumed cannabis use afterwards. Just 3 weeks before his hospitalization (at age 42 years), he began smoking DMT, later informing that he had smoked DMT no more than 10 times. At the time of his hospitalization, the subject had several stressors occurring in his life, such as eviction from his apartment, unemployment, and his mother’s death. He arrived at the emergency department, brought by the police, presenting agitated, bizarre, and disinhibited behavior, time disorientation, disorganized thought, and delusions (e.g. being ‘navigated by the stars’). Due to his agitation, he need emergency medication (benzodiazepines) and was admitted to an inpatient psychiatry unit. Over the next 12 days, the subject was hyperverbal and intrusive, and presented paranoid and grandiose delusions (e.g. being able to read minds, interact with ‘aliens’, and control distant events and persons by adopting specific body postures). These body postures were performed by the subject before and for several days after his admission, and were possibly associated with the transient (5 days) elevated creatinine kinase level (2732 units/l) observed upon admission. During these 12 days, the subject was treated with antipsychotics (quetiapine, olanzapine, risperidone) and drugs for controlling impulsivity (divalproex sodium), anxiety (gabapentin), and to improve sleep (hydroxyzine). By day 14, he showed improved insight and judgment, and was discharged on day 21 to a residential drug-treatment program, with no further psychotic symptoms. At 6 months after discharge, he remained treatment compliant and started to reduce his antipsychotic treatment (quetiapine), and was drug and symptom free. Importantly, although the subject stated the he was a long-term cannabis user, and recent cannabis use could have contributed to the psychotic episode, urine toxicology performed 3 days after admission was negative for cannabinoids. According to the authors, since cannabinoids usually persist in the urine of chronic users for several days, this negative result could indicate that the subject had a lower level of cannabis use or was not using cannabis in the weeks before the psychotic episode. Moreover, the negative result suggests that DMT was the main drug associated with the patient’s psychotic symptoms.
Ayahuasca
Lima and colleagues reported the incidence of psychiatric occurrences from data collected in an institutional study of the União do Vegetal (UDV) to monitor the psychological health of its members [Lima et al. 2002; Lima and Tófoli, 2011]. The UDV is a Brazilian syncretic religion that regularly uses ayahuasca in a ritual setting twice monthly, but often as frequently as several times per week [Labate et al. 2009].
In a conference abstract, Lima and colleagues reported results from UDV members from the period of 1996–2000 [Lima et al. 2002]. Lima and Tófoli reviewed and updated the previous data, presenting results from 1994 to 2007 [Lima and Tófoli, 2011]. Lima and colleagues reported seven cases of psychotic disorders in the UDV context [Lima et al. 2002]. According to their report, two cases did not present any relation with ayahuasca; in three cases, ayahuasca apparently increased symptoms of previous psychotic episodes; in one case, ayahuasca was associated with other factors (not specified), and only one case presented immediate temporal relation with ayahuasca and there were no psychotic antecedents. The authors affirmed that this incidence of psychotic disorders is similar to that of the general population, although they did not inform the sample size of the study nor how they calculated this incidence.
Lima and Tófoli reported data from 1994 to 2007 and stated that there were 51 cases of psychiatric occurrences among UDV members, 29 of which were psychotic disorders: schizophrenia (n = 9), acute and transient psychotic disorders (n = 4), unspecified nonorganic (n = 2), severe depressive episode with psychotic symptoms (n = 4), substance-induced psychosis (n = 6), and bipolar affective disorder with psychotic manic episode (n = 4) [Lima and Tófoli, 2011]. Until 2007, 18 of these cases (62%) were subjects that were no longer participating in ayahuasca rituals, while 11 were still participating. Moreover, detailed evaluation of the cases showed that in only 19 of the 29 (65.5%) ayahuasca seemed to be the main contributing factor. In the other 10 cases, there was no immediate temporal relation between ayahuasca intake and the psychotic episode, suggesting that ayahuasca might not have significantly contributed for the development of the case. Among the cases related to ayahuasca intake, in four cases there was an immediate temporal relation between ayahuasca consumption and the psychotic episode, and subjects had no psychiatric history; in five cases there was an immediate temporal relation between ayahuasca intake and the psychotic episode, but subjects had a psychiatric history with or without an active symptomatology; in 10 cases there was no immediate temporal relation between ayahuasca consumption and the psychotic episode, but ayahuasca may have contributed with others factors for the development of the case. It is important to note that, according to the authors, even in the cases were ayahuasca may have produced a psychotic episode in subjects without a psychiatric history, the detailed examination of the cases suggested the presence of traces of premorbid personality factors that could also influence the occurrence of a psychotic episode.
Gable made a comment on the data presented by the UDV in the legal battle that this group won regarding their right to use ayahuasca in the United States [Supreme Court of the United States, 2005; Gable, 2007]. Gable reported that over a period of 5 years, the UDV documented between 13 and 24 cases in which ayahuasca might have been a contributing factor in a psychotic incident. Although the exact years were not specified, it seems that the data were obtained from 2000 to 2005, since the Supreme Court report was published in 2005. Thus, it seems highly probable that at least part of this sample was previously reported by Lima and Tófoli, since they reported data from within the UDV context in this same period (from 1994 until 2007) [Lima and Tófoli, 2011]. According to the document from the Supreme Court of the United States [Supreme Court of the United States, 2005], the United States ‘government claims that hoasca (note from the authors: hoasca is the name of ayahuasca within the UDV context) has caused 24 psychotic incidents in Brazil over a period of 5–6 years’. Nevertheless, ‘a review of the entire record, however, reveals that only 8–13 arguably psychotic incidents have been documented’. These incidents occurred from an estimated total of 25 000 servings of ayahuasca according to Gable, but the document from the Supreme Court of the United States informed the total of 250 000 servings [Gable, 2007]. Both references failed to inform how these numbers were estimated. Gable reported that the rate of psychotic episodes in the UDV context is under 1% (0.052–0.096%, considering 13–24 episodes in 25.000 servings) [Gable, 2007], which is similar to the estimated prevalence rate of psychosis/schizophrenia in the general population [Stilo and Murray, 2010]. If we consider the 8–13 ‘arguably psychotic incidents’ occurring reported in 250 000 servings, as reported by the document from the Supreme Court of the United States, the rate is even lower: 0.0032–0.0052%. Furthermore, the document from the Supreme Court of the United States stated that ‘many or most of these psychological problems were transient and resolved’, and that ‘a review of the case histories in the record reveals that in many of those, either no truly psychotic incident was identified or no causal link to hoasca was found’.
Dos Santos and Strassman reported the case of a 21-year-old Brazilian male who experienced two consecutive psychotic episodes after participation in ayahuasca rituals [dos Santos and Strassman, 2008]. The episodes were separated by 1 year from each other, and both occurred during the rituals but endured several days/weeks afterwards. Neither the subject nor his parents had a history of psychosis. The subject had used other hallucinogens (LSD and psilocybin) on several occasions, but did not report any adverse effects associated with these experiences. He was also a nearly daily cannabis user for the preceding 6 years before the first psychotic episode, with no significant adverse effects associated with this pattern of cannabis use. Before the first psychotic episode, the subject had already used ayahuasca ‘more or less twice per month, for about 2 years’, without incident. Sometimes he used cannabis concurrently, also without incident. However, during one particular ayahuasca ritual, the subject ingested ayahuasca and combined its use with cannabis, and sometime later (not specified) he experienced very intense paranoid and suicidal ideas. Moreover, the subject also superficially cut himself with a sharp-edged ceremonial item during the ritual. Psychotic/paranoid symptoms persisted for 2–3 weeks, and only subsided and resolved after a 1-year antipsychotic treatment (risperidone). During this year, the subject did not use ayahuasca, cannabis, or other drugs, and remained symptom free. At 1 year later, after the treatment had finished, the subject wished to continue participation in ayahuasca rituals. He ingested ayahuasca again in three separated ceremonies, and was not using cannabis any more. Although no adverse reactions occurred in the first two rituals, during the third one, he again experienced paranoid and suicidal ideation. As in the first episode, symptoms persisted for 2–3 weeks and only resolved after another year of risperidone treatment. The previous use of other hallucinogens and the concomitant use of cannabis by this subject with no personal or family history of psychotic disorders makes it difficult to establish the exact role of ayahuasca in this case, especially regarding the first episode. In the second one, although it happened a year later and there was no concomitant use of cannabis, the subject might have developed a sensibility or predisposition to psychotic experiences after his first episode.
We had the opportunity to follow-up this case until 2016. After the second episode and treatment, the subject continued to use cannabis daily and occasionally used other hallucinogenic [LSD, psilocybin, ketamine, 2, 5-dimethoxy-4-iodophenethylamine (2C-I)] and nonhallucinogenic drugs [MDMA, γ-hydroxybutyric acid (GHB), alcohol, tobacco, amphetamines, cocaine, heroin], but did not use ayahuasca anymore. Approximately 1 year after the second treatment, the subject experimented with MDMA on four occasions separated by 3–4 months, and had another paranoid/psychotic episode in the fourth occasion, followed by another year of successful risperidone treatment. Some months after this third treatment, the subject experimented the hallucinogenic phenethylamine 2C-I and had another paranoid episode, again followed by a year of risperidone treatment. A last psychotic episode occurred some months after the last treatment, and this time it was apparently associated with excessive alcohol intake. This episode was also successfully treated with risperidone for another year. The subject did not use any hallucinogen after this last episode and did not have other psychotic symptoms afterwards. Interestingly, he continued to use cannabis daily until 2016, including during all antipsychotic treatments, apparently without increases in psychotic symptoms.
Szmulewicz and colleagues reported the case of a 30-year-old Argentinian man who developed a manic episode after participating in a 4-day ayahuasca retreat [Szmulewicz et al. 2015]. The subject had traveled to Brazil for 3 months before to learn about South American tribes, and 2 weeks before the travel he experienced a 10-day period compatible with a hypomanic episode: increased energy, self-esteem, and goal-directed activity, sleep disorder, pressured speech, and running thoughts. Although there was no previous diagnosis of manic or depressive episodes, there was a prior history, as the subject stated that he had experienced this kind of hypomanic episodes several times before. Moreover, his father had been diagnosed with bipolar affective disorder type I. According to the subject and his mother, he did not present any manic symptoms before the ayahuasca ritual. At 2 days after the last ayahuasca use (the number of ayahuasca doses was not specified), the subject began to experience mystical and paranoid delusional ideas, auditory hallucinations, racing thoughts, disorganized behavior, elevated energy, and euphoria. Afterwards (time not specified), the subject was admitted to a psychiatric hospital in Brazil, where he received antipsychotic/benzodiazepine treatment (risperidone and clonazepam) for a month. After this period, he was symptom-free, was discharged with the same medications, and traveled back to Argentina to continue treatment. When he arrived in a hospital in Argentina, the subject had a depressive episode characterized by significant anhedonia, hopelessness, apathy, ideas of ruin, and clinophilia (tendency to spend extra time in bed, without necessarily sleeping). Surprisingly, the authors suggested that this was not a case of a psychotic/mania episode induced by ayahuasca, but an ‘antidepressant-induced mania due to excessively prolonged use of a substance with antidepressant properties’ in a man with a personal history of hypomania and a family history of bipolar disorder. Interestingly, the authors stated that this ‘substance with antidepressant properties’ was harmine, one of the main ayahuasca components [McKenna and Riba, 2015]. It is not clear why the authors suggested that ayahuasca and harmine were not part of the same ‘substance’.
One last unpublished case was reported by phone to one of us and involved a 40-year-old woman who suffered a psychotic crisis during an ayahuasca weekend retreat She attended the retreat for self-improvement purposes following the advice of a friend that told her that ayahuasca was a potential tool for helping to solve daily difficulties and that she could experience beneficial effects by participating in the retreat. The subject had no history of mental health problems nor had psychiatric family antecedents. She had a history of occasional cannabis use years before the episode, always in small quantities. Also, some months before the ayahuasca retirement, she experimented with a medium dose of MDMA in a house setting with her partner, having a good experience. The subject took ayahuasca on two occasions: on Friday night and in Saturday evening. The subject did not experience side effects during the Friday session and she spent all Saturday in a normal state. But just before taking ayahuasca in the Saturday session, she started to manifest an incoherent discourse, according to the friend that was with her at the retreat. About 10–15 minutes after taking ayahuasca, before the psychoactive effects have begun, she started to develop paranoid ideas, delusional thinking and aberrant behavior. The content of her speech was related with personal events involving aspects of her life and aspects of the life of some of her relatives and near friends, including possible past traumatic experiences not remembered until that moment. She remained in that state for more than 24 hours. All Sunday night she stayed awake, talking endlessly in a constant and incoherent monologue with evident suffering and uncontrolled movements. On Monday, a psychologist attending the ceremony suggested to the guides to administer an antipsychotic (2 mg of risperidone). Less than 30 min after taking risperidone her psychotic symptomatology disappeared, and she asked to the people in the retreat what has happened to her. In the following hours, she slowly remembered the content of the session, and after being almost 48 hours awake and in a psychotic state she finally slept. After 7 hours of sleep, she woke up again in a psychotic state that lasted for 2 days, when she was finally taken to a hospital, where she received antipsychotic treatment (haloperidol) and her psychotic state was again interrupted. The antipsychotic treatment was maintained for a few months, and she did not experience psychotic symptoms anymore. One of us had the opportunity to talk with her by phone at some moment while she was in the psychotic state and to interview by phone some other people present in the retreat and her partner along all the psychotic process. After the haloperidol treatment, we lost contact with the patient, but 1 year later, we could interview her again. The psychotic symptoms never came back and she had a normal life, although she preferred not to talk about what happened in the ceremony since she just wanted to forget it. Our institution does not require ethics approval for reporting individual cases, and the subject provided written informed consent for including the reported information in this article.
Discussion
The present systematic review reported evidence from case reports and case series associating ayahuasca or DMT intake with psychotic episodes enduring more than the expected time of action of each drug.
To the best of our knowledge, there is no published report of prolonged psychotic reactions associated with the use of ayahuasca in controlled settings [dos Santos et al. 2016a]. In these settings, volunteers are screened for a possible psychiatric history or a current psychiatric diagnosis, including psychotic disorders, bipolar disorder, or a history of mania or hypomania induced by antidepressant or substance use [Riba et al. 2006; de Araujo et al. 2012; Osório et al. 2015; Palhano-Fontes et al. 2015; dos Santos et al. 2016b; Sanches et al. 2016]. The performance of a psychiatric screening before experimental or clinical use of hallucinogens is essential to reduce the possible occurrence of adverse reactions [Strassman, 1984; Johnson et al. 2008; Studerus et al. 2011; Garcia-Romeu et al. 2016]. Therefore, a possible explanation for the occurrence of psychotic episodes could be the presence of previous psychiatric disorders.
Indeed, in the UDV case series [Lima et al. 2002; Lima and Tófoli, 2011], several psychotic episodes associated with ritual ayahuasca intake were related to previous psychiatric diagnoses or current symptomatology, including psychotic symptoms/disorders. Moreover, in the manic episode associated with ayahuasca intake, the subject had a family history of bipolar disorder and a personal history of hypomanic episodes [Szmulewicz et al. 2015]. Interestingly, acute ayahuasca administration to depressed patients screened for bipolar disorder or a history of mania/hypomania was not associated with increases in manic symptomatology [Osório et al. 2015; Sanches et al. 2016].
In both the UDV case series [Lima et al. 2002; Lima and Tófoli, 2011] and in the case reports [dos Santos and Strassman, 2008] ayahuasca intake was also associated with psychotic episodes in people without a personal or family psychiatric history. However, in the case report, the use of other drugs (e.g. cannabis and other hallucinogens) complicates the assessment of the possible role of ayahuasca in the psychotic episode, and in the UDV case series no details are given regarding isolated cases, limiting their evaluation. In any case, the overall incidence of psychotic episodes in the UDV context seems to be rare. Gable reported that such cases represented a rate of less than 0.1% (0.052–0.096%) [Gable, 2007], which is comparable with the data reported by Cohen regarding controlled LSD administration [Cohen, 1960].
Regarding DMT, we are also not aware of any published reports describing prolonged psychotic reactions associated with experimental/controlled DMT administration [e.g. Strassman et al. 1994, 1996; Daumann et al. 2008, 2010]. Volunteers in these studies are also psychiatrically screened for psychotic disorders before drug administration, thus limiting the possible occurrence of adverse reactions with psychotic features [Strassman, 1984; Daumann et al. 2008; Johnson et al. 2008; Daumann et al. 2010; Studerus et al. 2011; Garcia-Romeu et al. 2016]. Like ayahuasca, in two of the case reports associated with smoked DMT, the subjects had a previous hypomanic/psychotic mania episode [Umut et al. 2011; Paterson et al. 2015], and in one case, the subject had a family history of psychotic disorder [Warren et al. 2013]. Furthermore, in all three cases the subjects used other drugs as well, especially cannabis. Like other hallucinogens, it seems that people with a personal or family psychiatric history of psychotic or manic episodes should avoid DMT intake [Strassman, 1984; Johnson et al. 2008; Studerus et al. 2011; Garcia-Romeu et al. 2016].
It is not clear why some people suffer prorogued psychotic reactions to ayahuasca or DMT. Mental health assessments of long-term ritual ayahuasca use do not report increased psychopathology in these population, and ayahuasca intake could even be related to better mental health [dos Santos et al. 2016a]. However, studies with experimented ayahuasca users could be biased by the fact that these people are already adapted to ayahuasca, having used this substance for several years or even decades in some cases [dos Santos et al. 2016a]. Therefore, it is possible that people that have adverse reactions to ayahuasca during their first experiences do not continue to use this hallucinogen anymore, and will not be present in studies assessing the mental health of long-term users. Previous prospective studies assessing the effects of a first ayahuasca intake in naïve users after 2 weeks [Barbosa et al. 2005] and 6 months [Barbosa et al. 2009] did not report adverse reactions with psychotic features, but observed reductions in psychiatric symptoms. More prospective studies following novice ayahuasca users for longer periods are need to better understand the effects of ayahuasca, both the negative and the therapeutic.
Nevertheless, the case reports describing the occurrence of psychotic episodes among the UDV members show that a minority of individuals may be predisposed to such experiences even in ritual settings. People with previous psychiatric diagnoses of psychotic disorders (schizophrenia or schizophreniform disorders, psychotic depression or mania) or with current psychotic symptomatology seem to be the individuals with more predisposition to an adverse reaction with psychotic features, both for ayahuasca and DMT intake. Therefore, both in experimental/clinical and ritual settings, a previous psychiatric screening should be performed and individuals with such characteristics should avoid ayahuasca intake. This is similar for other hallucinogens [Strassman, 1984; Johnson et al. 2008; Studerus et al. 2011; Garcia-Romeu et al. 2016]. Indeed, in the case report described by dos Santos and Strassman, the second psychotic episode could be the result of both sensitization (increased susceptibility to the psychoactive effects of ayahuasca/DMT) and predisposition (increased tendency to suffer another psychotic reaction after a first-episode psychosis) induced by the first episode 1 year before [dos Santos and Strassman, 2008].
The cases of people that had a psychotic episode with ayahuasca or DMT but that the subjects had no personal or family psychiatric history are more difficult to interpret. In the case of DMT, all reports described subjects with past or ongoing psychiatric symptomatology and use of other drugs that could also induce psychotic symptoms/disorders (e.g. cannabis and other hallucinogens). The case described by dos Santos and Strassman with ayahuasca also had an association with cannabis and other hallucinogens, even considering the absence of a personal or previous psychiatric history for the subject [dos Santos and Strassman, 2008]. Future studies should record not only the psychiatric history of the participants, but also the drug use history with detailed information on the type of drug used and the frequency and duration of such use. For instance, in two of the cases related to DMT the psychotic episode was apparently associated with an increase in the frequency/duration of DMT use, among other factors [Warren et al. 2013; Paterson et al. 2015]. Radiolabeled DMT remained for 7 days after injection in the rabbit brain [Vitale et al. 2011], suggesting the possibility that continuous use of DMT could increase brain levels of this compound over time, maybe reaching levels high enough to induce psychotic experiences, especially in predisposed individuals.
Unfortunately, although the UDV case series have described several psychotic episodes, they lack the detailed information present in the descriptions of the case reports. Thus, the UDV data is limited regarding the details of the psychiatric and drug use history of the subjects. It would be interesting to further explore the UDV data, and to assess if the religious setting of organized groups such as the UDV has any positive influence in how the person suffering a psychotic episode in that context copes with that experience.
Individuals predisposed to psychotic episodes after ayahuasca/DMT intake could share some genetic/metabolic characteristics that predispose them to such experiences. The so-called ‘transmethylation hypothesis’ suggests that an enzymatic/metabolic alteration could induce the organism to increase the synthesis of endogenous hallucinogenic compounds such as DMT, thus inducing psychosis/schizophrenia [Gillin et al. 1978; Barker et al. 1981; Ciprian-Ollivier and Cetkovich-Bakmas, 1997; Pomilio et al. 1999; Vitale et al. 2010; Barker et al. 2012; Grammenos and Barker, 2015]. However, although DMT is indeed an endogenous compound that has been detected in the human cerebrospinal fluid, blood, and urine [Barker et al. 2012], and some studies did find increased concentrations of DMT and related compounds (5-hydroxy-DMT, 5-methoxy-DMT) in the body fluids of psychotic/schizophrenic patients and other psychiatric patients [Barker et al. 2012], results are inconclusive and the transmethylation hypothesis is still under debate [Barker et al. 2012; Grammenos and Barker, 2015]. Moreover, although several authors have speculated on other possible physiological roles of endogenous DMT and related compounds in our organism, including regulation/production of dreams, near-death experiences, and mystical states [Callaway, 1988; Strassman, 2001], anxiolytic effects [Jacob and Presti, 2005], and tissue protection/regeneration and immune regulation [Frecska et al. 2013, 2016; Szabo et al. 2014], we still do not know what DMT is doing in our body. Additional research in this area is needed to better understand the presence of these compounds in humans.
There may be at least two explanations to understand the psychotic crisis reported in this paper, one psychological and another physiological. In the first case, maybe the subjects faced psychological content that unbalanced them. In the two cases in which we had personal contact with the subjects, they became paranoid, thinking that the people surrounding them wanted to harm them. At the same time, both subjects reacted quite well to treatment with atypical antipsychotics. In the case reported by dos Santos and Strassman, the subject apparently only became psychotic after taking drugs with hallucinogenic (ayahuasca, 2C-I) or hallucinogenic-like (MDMA) effects [dos Santos and Strassman, 2008]. Although MDMA is not considered a classic or serotonergic hallucinogen, it has hallucinogenic-like effects and may influence cortical 5-HT2A receptors [Mueller et al. 2016]. Moreover, the subject was successfully treated in all occasions with risperidone, an atypical antipsychotic that act as an antagonist of dopamine D1–2 receptors and of the serotonin 5-HT2A receptor. The unpublished case report involving ayahuasca also described the effective (although transitory) use of risperidone, as well as one of the case reports involving DMT [Umut et al. 2011]. Thus, although a possible involvement of the dopaminergic system in the psychotic effects of hallucinogens cannot be ruled out, it seems that the psychotic crisis induced by ayahuasca, DMT, and other serotonergic hallucinogens may have their cause in their action on cortical 5-HT2A receptors. Solid evidence supporting this idea includes the fact that three of the reviewed cases reported successful use of an atypical antipsychotic with mixed dopamine and serotonin antagonism, and that the subject described by dos Santos and Strassman did not become psychotic after taking neither D2 agonists (cocaine, amphetamines) nor cannabis, but only after using serotoninergic compounds that act as 5-HT2A agonists [dos Santos and Strassman, 2008]. This is also in accordance with studies in which 5-HT2A antagonists reduced the effects of classic/serotonergic hallucinogens, including ayahuasca [Vollenweider et al. 1998; Kometer et al. 2013; Valle et al. 2016].
However, the subject described by dos Santos and Strassman also described an extensive experience with other hallucinogens that act as 5-HT2A agonists (such as LSD and psilocybin) without psychotic incidents, and the last episode was apparently induced by excessive alcohol intake [dos Santos and Strassman, 2008]. It is not clear why these psychotic reactions occurred only with 2C-I, MDMA, and alcohol, but it is possible that after several psychotic episodes the subject may have developed an increased sensibility to psychotic symptoms that generalized to nonserotonergic drugs, or that the dose, intensity, or frequency of use of the other drugs was not sufficient to induce full-blown psychotic symptoms. Indeed, several of the cases reviewed described that the subjects had used several doses of ayahuasca/DMT before the psychotic episode, suggesting a possible dose-related or cumulative effect.
The hallucinogenic effects of ayahuasca do not seem to be mediated by harmine and related β-carboline alkaloids (THH and harmaline), since human studies about their possible psychoactive properties are inconclusive, with reports describing hallucinogenic effects [Shulgin and Shulgin, 1997; Naranjo, 1959; Pennes and Hoch, 1957], sedative-like effects [Ott, 1994, 1999], or lack of psychoactive effects [Slotkin et al. 1970]. However, a recent study suggested that the β-carbolines in ayahuasca could have specific effects in the human electroencephalogram (EEG), suggesting central/psychoactive effects [Schenberg et al. 2015]. Moreover, compounds such as harmine may have pro-dopaminergic effects [Nunes et al. 2016], and the possible role of these compounds in the psychotic effects of ayahuasca is not currently known. Thus, more studies are needed to better understand the effects of the β-carboline alkaloids in humans.
It is unknown if there are people vulnerable to hallucinogenic drugs, but in the two cases that we had direct contact subjects had no personal or familiar history of psychological problems, and were subjects perfectly adapted and with high qualified works and high educational levels. One of these subjects had a long history of ayahuasca use without having suffered any psychological side effect. Since he used cannabis and ayahuasca at the same time, it is possible cannabis could have potentiated the psychotic effects of DMT. In our field observations over the last 10–15 years, we have observed precisely this phenomenon [dos Santos, 2011]. Also, those two subjects suffered the psychotic crisis under the course of a structured ritual, and the UDV case series reported cases that occurred in this structured religious context. Thus, although the ritual may have some protective effect, it is not a total guarantee of complete safety [Lima, 1996–97; Lima and Tófoli, 2011]. Considering the good and fast response of those two subjects to antipsychotic medication, it could be possible that ayahuasca/DMT do not trigger a latent psychotic response in healthy individuals, but just induce a transitory psychotic crisis. But in people with a history of mental disorders, as was the case of some of the reports described in the present review, these substances could aggravate the premorbid condition. Therefore, although the psychological content of an ayahuasca experience can be sometimes difficult to face, it does not explain by itself a psychotic reaction. It is more probable that psychological side effects will appear in the form of transitory anxiety that disappears when the effects of ayahuasca fade away. But there may be conditions where the biochemical reactions induced by hallucinogens over 5-HT2A receptors induce a psychotic state that lasts more than the pharmacological effect of the drug, being necessary to rebalance the system/brain with a pharmacological/antipsychotic intervention that it is not dependent on the psychological content. Ayahuasca guides should be cautious in selecting people before giving them ayahuasca. Since ayahuasca use is being popularized internationally [Labate and Feeney, 2012], future studies should explore deeply the prevalence and incidence of psychiatric reactions in ayahuasca communities.
Limitations
Limitations of the present study include the small number of studies and the small sample sizes of most studies, which were case reports. Moreover, the case series reported data collected from a specific ritual/religious context (UDV), and the authors (Lima and Tófoli) were members of the UDV at the time of the publication of these reports. Thus, the extrapolation of the results to other settings might be limited due to potential conflict of interests. On the other hand, the publication of the UDV monitoring system has provided the researchers with information of ayahuasca-related adverse effects that otherwise would not be available. Moreover, UDV’s system reflects a concern about their followers’ wellbeing that is unique among ayahuasca religions.
Other important limitations regarding the assessment of a possible causal role of ayahuasca/DMT in the reviewed cases include personal or family psychiatric histories of psychotic or bipolar disorders (schizophrenia or schizophreniform disorders, psychotic depression or mania), ongoing psychotic or manic symptomatology, and concomitant use of other drugs with potential to induce psychotic symptoms/disorders such as cannabis and other hallucinogens.
Conclusion
It is estimated that nearly 20 000 people worldwide are members of some of the Brazilian ayahuasca religions (Santo Daime, UDV, Barquinha) [Labate et al. 2009]. The scientific literature suggests that acute ayahuasca administration to healthy volunteers has a good safety profile, and that long-term ritual ayahuasca consumption is not associated with cognitive or psychiatric problems [dos Santos et al. 2016a]. The psychotic episodes described in the present systematic review are apparently associated with several contributing factors, and not only ayahuasca or DMT intake. Many cases involved individuals with a personal or family history of psychosis or nonpsychotic bipolar disorder, or concomitant use of other drugs. Therefore, these individuals have a different profile from those that participate in controlled studies where a psychiatric screening is performed and use of other drugs is not allowed. Moreover, in controlled studies, only single or few ayahuasca/DMT doses are administered, and in some cases described, related to DMT use, the subjects have used several doses DMT before their psychotic episode.
Previous reviews of adverse effects of hallucinogens in noncontrolled/recreational settings demonstrated that in reports ‘from the field’ it is very difficult to tease apart preexisting psychopathology, drug/alcohol abuse, family history, and other important features such as proper preparation, guidance, and integration of drug effects. Thus, it is difficult to establish a causal relationship with hallucinogen use and most of these cases [Klock et al. 1974; Strassman, 1984; Johnson et al. 2008; Smith et al. 2014; Garcia-Romeu et al. 2016]. In the case reports described in the present review, it is similarly difficult to determine the significance of ayahuasca/DMT use in such complicated settings, especially considering the safety record of ayahuasca/DMT administration in controlled clinical trials where rigorous screening, supervision, and follow up are provided.
Taken together, these results suggest that the incidence of psychotic episodes associated with ayahuasca/DMT intake is a rare phenomenon, and these rare instances appear be associated with previous premorbid characteristics of the individuals, previous and possibly concurrent drug abuse, and lack of a supervised setting. Therefore, considering the possible influence of these and other factors, the causal relation between ayahuasca/DMT and these cases is not always clear. These data suggest that performance of a psychiatric and drug use history before ayahuasca or DMT administration in controlled settings may reduce the occurrence of psychotic experiences. Regarding noncontrolled/recreational use, individuals with personal or family history of schizophrenia or schizophreniform disorders, psychotic depression or mania, or with ongoing manic or psychotic symptomatology, should avoid ayahuasca/DMT intake.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: RGS is Fellow of the Programa Nacional de Pós-Doutorado, Brazil (PNPD/CAPES). JECH receives a CNPq (Brazil) Productivity Fellowship Award. Sponsors had no role in study design, data analysis, data interpretation, or writing of the report. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
Contributor Information
Rafael G. dos Santos, Department of Neurosciences and Behavior, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil; National Institute for Translational Medicine (INCT-TM), CNPq, Ribeirão Preto, Brazil; International Center for Ethnobotanical Education, Research and Service, ICEERS, Barcelona, Spain.
José Carlos Bouso, International Center for Ethnobotanical Education, Research and Service, ICEERS, Barcelona, Spain.
Jaime E. C. Hallak, Department of Neurosciences and Behavior, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil; National Institute for Translational Medicine (INCT-TM), CNPq, Ribeirão Preto, Brazil
References
- American Psychiatric Association. (2013) Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Araujo A., Carvalho F., Bastos Mde L., Guedes de, Pinho P., Carvalho M. (2015) The hallucinogenic world of tryptamines: an updated review. Arch Toxicol 89: 1151–1173. [DOI] [PubMed] [Google Scholar]
- Barbosa P., Cazorla I., Giglio J., Strassman R. (2009) A 6-month prospective evaluation of personality traits, psychiatric symptoms and quality of life in ayahuasca-naïve subjects. J Psychoactive Drugs 41: 205–212. [DOI] [PubMed] [Google Scholar]
- Barbosa P., Giglio J., Dalgalarrondo P. (2005) Altered states of consciousness and short-term psychological after-effects induced by the first-time ritual use of ayahuasca in an urban context in Brazil. J Psychoactive Drugs 37: 193–201. [DOI] [PubMed] [Google Scholar]
- Barker S., McIlhenny E., Strassman R. (2012) A critical review of reports of endogenous psychedelic N,N-dimethyltryptamines in humans: 1955–2010. Drug Test Anal 4: 617–635. [DOI] [PubMed] [Google Scholar]
- Barker S., Monti J., Christian S. (1981) N,N-dimethyltryptamine: an endogenous hallucinogen. Int Rev Neurobiol 22: 83–110. [DOI] [PubMed] [Google Scholar]
- Callaway J. (1988) A proposed mechanism for the visions of dream sleep. Med Hypotheses 26: 119–124. [DOI] [PubMed] [Google Scholar]
- Ciprian-Ollivier J., Cetkovich-Bakmas M. (1997) Altered consciousness states and endogenous psychoses: a common molecular pathway? Schizophr Res 28: 257–265. [DOI] [PubMed] [Google Scholar]
- Cohen S. (1960) Lysergic acid diethylamide: side effects and complications. J Nerv Ment Dis 130: 30–40. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ditman K. (1962) Complications associated with lysergic acid diethylamide (LSD-25). JAMA 181: 161–162. [DOI] [PubMed] [Google Scholar]
- Daumann J., Heekeren K., Neukirch A., Thiel C., Möller-Hartmann W., Gouzoulis-Mayfrank E. (2008) Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology 200: 573–583. [DOI] [PubMed] [Google Scholar]
- Daumann J., Wagner D., Heekeren K., Neukirch A., Thiel C., Gouzoulis-Mayfrank E. (2010) Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol 24: 1515–1524. [DOI] [PubMed] [Google Scholar]
- De Araujo D., Ribeiro S., Cecchi G., Carvalho F., Sanchez T., Pinto J., et al. (2012) Seeing with the eyes shut: neural basis of enhanced imagery following ayahuasca ingestion. Hum Brain Mapp 33: 2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos R. (2011) Possible risks and interactions of the consumption of ayahuasca and cannabis in humans. In: dos Santos R. (ed.) The ethnopharmacology of ayahuasca, Transworld Research Network: Kerala. Available at: https://issuu.com/researchsignpost/docs/rafael (accessed 16 August 2016).
- Dos Santos R., Balthazar F., Bouso J., Hallak J. (2016. a) The current state of research on ayahuasca: a systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J Psychopharmacol in press. doi: 10.1177/0269881116652578 [DOI] [PubMed] [Google Scholar]
- Dos Santos R., Osório F., Crippa J., Riba J., Zuardi A., Hallak J. (2016. b) Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol 6: 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos R., Strassman R. (2008) Ayahuasca and psychosis. Br J Psychiatry (eLetter), 3 December Available at: http://bjp.rcpsych.org/content/190/1/81.2.e-letters#ayahuasca-and-psychosis (accessed 16 August 2016).
- Frecska E., Bokor P., Winkelman M. (2016) The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecska E., Szabo A., Winkelman M., Luna L., McKenna D. (2013) A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J Neural Transm 120: 1295–1303. [DOI] [PubMed] [Google Scholar]
- Gable R. (2007) Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 102: 24–34. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A., Kersgaard B., Addy P. (2016) Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol 24: 229–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin J., Stoff D., Wyatt R. (1978) Transmethylation hypothesis: a review of progress. In: Lipton M., DiMascio A., Killam K. (eds) Psychopharmacology: a generation of progress. New York, NY: Raven Press. [Google Scholar]
- Gonzalez-Maeso J., Ang R., Yuen T., Chan P., Weisstaub N., López-Giménez J., et al. (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammenos D., Barker S. (2015) On the transmethylation hypothesis: stress, N,N-dimethyltryptamine, and positive symptoms of psychosis. J Neural Transm 122: 733–739. [DOI] [PubMed] [Google Scholar]
- Hendricks P., Clark C., Johnson M., Fontaine K., Cropsey K. (2014) Hallucinogen use predicts reduced recidivism among substance-involved offenders under community corrections supervision. J Psychopharmacol 28: 62–66. [DOI] [PubMed] [Google Scholar]
- Hendricks P., Thorne C., Clark C., Coombs D., Johnson M. (2015) Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol 29: 280–288. [DOI] [PubMed] [Google Scholar]
- Jacob M., Presti D. (2005) Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Med Hypotheses 64: 930–937. [DOI] [PubMed] [Google Scholar]
- Johansen P., Krebs T. (2015) Psychedelics not linked to mental health problems or suicidal behavior: a population study. J Psychopharmacol 29: 270–279. [DOI] [PubMed] [Google Scholar]
- Johnson M., Richards W., Griffiths R. (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol 22: 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock J., Boerner U., Becker C. (1974) Coma, hyperthermia and bleeding associated with massive LSD overdose: a report of eight cases. Western J Med 120: 183–188. [PMC free article] [PubMed] [Google Scholar]
- Kometer M., Schmidt A., Jäncke L., Vollenweider F. (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33: 10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs T., Johansen P. (2013) Psychedelics and mental health: a population study. PLoS One 8: e63972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate B., Feeney K. (2012) Ayahuasca and the process of regulation in Brazil and internationally: implications and challenges. Int J Drug Policy 23: 154–161. [DOI] [PubMed] [Google Scholar]
- Labate B., Rose I., dos Santos R. (2009) Ayahuasca religions: a comprehensive bibliography and critical essays. Santa Cruz, CA: Multidisciplinary Association for Psychedelic Studies. [Google Scholar]
- Lima F. (1996. –97) The ritual use of hoasca: comments and advice. MAPS Newsletter 7: 25–26. [Google Scholar]
- Lima F., Naves M., Motta J., Migueli J., Brito G., et al. (2002) Sistema de monitoramento psiquiátrico em usuários do chá hoasca. Rev Bras Psiquiatr 24 (Suppl. 2): 120–141. [Google Scholar]
- Lima F., Tófoli L. (2011) An epidemiological surveillance system by the UDV: mental health recommendations concerning the religious use of hoasca. In Labate B., Jungaberle H. (eds) The internationalization of ayahuasca. Zurich/Berlin: Lit Verlag. [Google Scholar]
- Malleson N. (1971) Acute adverse reactions to LSD in clinical and experimental use in the United Kingdom. Br J Psychiatry 118: 229–230. [DOI] [PubMed] [Google Scholar]
- McKenna D., Riba J. (2015) New World tryptamine hallucinogens and the neuroscience of ayahuasca. Curr Top Behav Neurosci in press. doi: 10.1007/78542015368. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. and The PRISMA Group. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J., Holloway T., Albizu L., Sealfon S., González-Maeso J. (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493: 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller F., Lenz C., Steiner M., Dolder P., Walter M., Lang U., et al. (2016) Neuroimaging in moderate MDMA use: a systematic review. Neurosci Biobehav Rev 62: 21–34. [DOI] [PubMed] [Google Scholar]
- Naranjo P. (1959) Estudio comparativo de la harmina, la dietilamida del ácido lisérgico (LSD-25) y la mescalina [Comparative study of harmine, lysergic acid diethylamide (LSD-25) and mescaline]. Revista de la Confederación Médica Panamericana 6: 1–8. [Google Scholar]
- Nichols D. (2016) Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes A., dos Santos R., Osório F., Sanches R., Crippa J., Hallak J. (2016) Effects of ayahuasca and its alkaloids on drug dependence: a systematic literature review of quantitative studies in animals and humans. J Psychoactive Drugs 48: 195–205. [DOI] [PubMed] [Google Scholar]
- Nutt D., King L., Phillips L. and Independent Scientific Committee on Drugs. (2010) Drug harms in the UK: a multicriteria decision analysis. Lancet 376: 1558–1565. [DOI] [PubMed] [Google Scholar]
- Osório F., Sanches R., Macedo L., dos Santos R., Maia-de-Oliveira J., Wichert-Ana L., et al. (2015) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Rev Bras Psiquiatr 37: 13–20. [DOI] [PubMed] [Google Scholar]
- Ott J. (1994) Ayahuasca analogues: pangaean entheogens. Kennewick, WA: Natural Books Co. [Google Scholar]
- Ott J. (1999) Pharmahuasca: human pharmacology of oral DMT plus harmine. J Psychoactive Drugs 31: 171–177. [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F., Andrade K., Tofoli L., Santos A., Crippa J., Hallak J., et al. (2015) The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS One 10: e0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson N., Darby W., Sandhu P. (2015) N,N-Dimethyltryptamine-induced psychosis. Clin Neuropharmacol 38:141–143. [DOI] [PubMed] [Google Scholar]
- Pennes H., Hoch P. (1957) Psychotomimetics, clinical and theoretical considerations: harmine, WIN-299 and nalline. Am Journal Psychiatry 113: 887–892. [DOI] [PubMed] [Google Scholar]
- Pomilio A., Vitale A., Ciprian-Ollivier J., Cetkovich-Bakmas M., Gómez R., Vázquez G. (1999) Ayahoasca: an experimental psychosis that mirrors the transmethylation hypothesis of schizophrenia. J Ethnopharmacol 65: 29–51. [DOI] [PubMed] [Google Scholar]
- Riba J., Barbanoj M. (2006) Ayahuasca. In: Peris J., Zurián J., Martínez G., Valladolid G. (eds) Tratado SET de Transtornos Adictivos, Ed. Madrid, Spain: Médica Panamericana. [Google Scholar]
- Riba J., McIlhenny E., Bouso J., Barker S. (2015) Metabolism and urinary disposition of N,N-dimethyltryptamine after oral and smoked administration: a comparative study. Drug Test Anal 7: 40140–40146. [DOI] [PubMed] [Google Scholar]
- Riba J., Rodríguez-Fornells A., Urbano G., Morte A., Antonijoan R., Montero M., et al. (2001) Subjective effects and tolerability of the South American psychoactive beverage ayahuasca in healthy volunteers. Psychopharmacology 154: 85–95. [DOI] [PubMed] [Google Scholar]
- Riba J., Romero S., Grasa E., Mena E., Carrió I., Barbanoj M. (2006) Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology 186: 93–98. [DOI] [PubMed] [Google Scholar]
- Sanches R., de Lima Osório F., dos Santos R., Macedo L., Maia-de-Oliveira J., Wichert-Ana L., et al. (2016) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol 36: 77–81. [DOI] [PubMed] [Google Scholar]
- Schenberg E., Alexandre J., Filev R., Cravo A., Sato J., Muthukumaraswamy S., et al. (2015) Acute biphasic effects of ayahuasca. PLoS One 10: e0137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes R., Hofmann A. (1992) Plants of the Gods: their sacred, healing, and hallucinogenic powers. Rochester, VT: Healing Arts Press. [Google Scholar]
- Shulgin A., Shulgin A. (1997) TIHKAL: the continuation. Berkeley: Transform Press. [Google Scholar]
- Smart R., Bateman K. (1967) Unfavourable reactions to LSD: a review and analysis of the available case reports. Can Med Assoc J 97: 1214–1221. [PMC free article] [PubMed] [Google Scholar]
- Smith D., Raswyck G., Davidson L. (2014) From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs 46: 3–10. [DOI] [PubMed] [Google Scholar]
- Slotkin T., Distefano V., Au W. (1970) Blood levels and urinary excretion of harmine and its metabolites in man and rats. J Pharmacol Exp Ther 173: 26–30. [PubMed] [Google Scholar]
- Stilo S., Murray R. (2010) The epidemiology of schizophrenia: replacing dogma with knowledge. Dialogues Clin Neurosci 12: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman R. (1984) Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis 172: 577–595. [DOI] [PubMed] [Google Scholar]
- Strassman R. (2001). DMT: the spirit molecule: a doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press. [Google Scholar]
- Strassman R., Qualls C., Berg L. (1996) Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry 39: 784–795. [DOI] [PubMed] [Google Scholar]
- Strassman R., Qualls C., Uhlenhuth E., Kellner R. (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51: 98–108. [DOI] [PubMed] [Google Scholar]
- Studerus E., Kometer M., Hasler F., Vollenweider F. (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol 25: 1434–1452. [DOI] [PubMed] [Google Scholar]
- Supreme Court of the United States (2005) Gonzales Alberto R., General Attorney, et al. (Petitioners), v. O Centro Espírita Beneficiente União do Vegetal, et al. (Respondents), no 04-1084. On Writ of Certiorari to the US Court of Appeals for the Tenth Circuit. Brief of Robert Gable, Ed.D., Ph.D., Harriet de Wit, Ph.D., Wayne Hall, Ph.D., Chris-Ellyn Johanson, Ph.D., William A. McKim, Ph.D., Daniel M. Perrine, Ph.D., and Manuel Tancer, M.D., as Amici Curiae in support of respondents. New Mexico, September 9. [Google Scholar]
- Szabo A., Kovacs A., Frecska E., Rajnavolgyi E. (2014) Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS One 9: e106533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmulewicz A., Valerio M., Smith J. (2015) Switch to mania after ayahuasca consumption in a man with bipolar disorder: a case report. Int J Bipolar Disord 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittarelli R., Mannocchi G., Pantano F., Romolo F. (2015) Recreational use, analysis and toxicity of tryptamines. Curr Neuropharmacol 13: 26–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umut G., Küçükparlak I., Özgen G., Türkcan A. (2011) A mood disorder episode with an onset under chronic cannabis consumption and accompanied with psychotic features immediately after N,N-dimethyltryptamine (DMT) use: a case report. Düşünen Adam 24: 246–250. [Google Scholar]
- Valle M., Maqueda A., Rabella M., Rodríguez-Pujadas A., Antonijoan R., Romero S., et al. (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26: 1161–1175. [DOI] [PubMed] [Google Scholar]
- Vallersnes O., Dines A., Wood D., Yates C., Heyerdahl F., Hovda K., et al. (2016) Psychosis associated with acute recreational drug toxicity: a European case series. BMC Psychiatry 16: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Amsterdam J., Opperhuizen A., van den Brink W. (2011) Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol 59: 423–429. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam J., Pennings E., Brunt T., van den Brink W. (2013) Physical harm due to chronic substance use. Regul Toxicol Pharmacol 66: 83–87. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam J., Nutt D., Phillips L., van den Brink W. (2015) European rating of drug harms. J Psychopharmacol 29: 655–660. [DOI] [PubMed] [Google Scholar]
- Vitale A., Ollivier J., Vitale V., Romero E., Pomillo A. (2010) Estudio clínico de marcadores de hipermetilación indólica en las alteraciones de la percepción. Acta Bioquím Clín Latinoam 44: 627–642. [Google Scholar]
- Vitale A., Pomilio A., Cañellas C., Vitale M., Putz E., Ciprian-Ollivier J. (2011) In vivo long-term kinetics of radiolabeled N,N-dimethyltryptamine and tryptamine. J Nucl Med 52: 970–977. [DOI] [PubMed] [Google Scholar]
- Vollenweider F., Kometer M. (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11: 642–651. [DOI] [PubMed] [Google Scholar]
- Vollenweider F., Vollenweider-Scherpenhuyzen M., Bäbler A., Vogel H., Hell D. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9:3897–3902. [DOI] [PubMed] [Google Scholar]
- Walsh Z., Hendricks P., Smith S., Kosson D., Thiessen M., Lucas P., et al. (2016) Hallucinogen use and intimate partner violence: prospective evidence consistent with protective effects among men with histories of problematic substance use. J Psychopharmacol 30: 601–607. [DOI] [PubMed] [Google Scholar]
- Warren J., Dham-Nayyar P., Alexander J. (2013) Recreational use of naturally occurring dimethyltryptamine - contributing to psychosis? Aust N Z J Psychiatry 47: 398–399. [DOI] [PubMed] [Google Scholar]