Abstract
Angiogenesis, the formation of new blood vessel, plays an important role for the growth and metastasis of malignant tumors. The recent identification of specific growth factors for lymphatic vessels and of new lymphatic-specific markers provided evidence for an active role of the lymphatic system during the tumor growth and metastasis processes. Tumor lymphangiogenesis has been shown to play a role in promoting tumor growth and metastasis of tumor cells to distant sites.
Integrins play keys roles in the regulation of angiogenesis and lymphangiogenesis during normal development and several diseases. Indeed, integrins control vascular and lymphatic endothelial cell adhesion, migration and survival. Importantly, integrin inhibitors can block angiogenesis and lymphangiogenesis.
In this chapter, we will highlight the role of integrins during angiogenesis and lymphangiogenesis as well as the function of individual integrins during vascular development, postnatal angiogenesis and lymphangiogenesis. We will discuss the role of integrins as potential therapeutic targets for the control of tumor angiogenesis, lymphangiogenesis and metastatic spread in the treatment of cancer. We will also describe methods to analyze expression and function of integrins during angiogenesis and lymphangiogenesis.
Keywords: Cancer, integrins, angiogenesis, lymphangiogenesis, migration, adhesion, immunohistochemistry
1. Introduction
Angiogenesis and lymphangiogenesis (the development of new blood vessels and lymphatic) play critical roles during embryonic development, physiological processes such as wound healing and reproduction and numerous diseases, including inflammation, tumor progression and metastasis.
1.1. Angiogenesis
Angiogenesis is the process by which new blood vessels develop from the existing vasculature (1). Angiogenesis is not only a critical physiological mechanism for embryonic development and tissue repair, but it also promotes diseases such as tumor growth, diabetic retinopathy and arthritis.
The principal cells promoting angiogenesis are endothelial cells, which line all blood vessels. To achieve new blood vessel formation, endothelial cells need to escape from their quiescent and stable location by degrading the basement membrane. Then, endothelial cells migrate toward a gradient of angiogenic factor such as VEGF-A (Vascular Endothelial Growth Factor-A) or bFGF (basic Fibroblast Growth Factor), released by activated cells. These cells may include platelets, tumor cells, tumor-associated macrophages and fibroblasts. Furthermore, endothelial cells also proliferate, thereby providing enough new cells, which will be can organize into the tubular structures that form blood vessels.
All of these steps (basement membrane disruption, cell migration, proliferation and tube formation) are regulated by members of the integrin family and which can consequently serve as targets to control the development of new vessels (2).
1.2. Lymphangiogenesis
The formation of new lymphatic vessels, or lymphangiogenesis, provides one of the main routes for tumor metastasis, especially for tumors of the breast, lung and gastrointestinal tract, which frequently colonize draining regional lymph nodes. Compared to the blood vasculature, little is known about the biology of the lymphatic vessels in tumors, the regulation of tumor lymphangiogenesis or the mechanisms that determine the interactions of tumor cells with the lymphatic vessels (3). Recently, specific growth factors inducing the development of lymphatic endothelial cells have been characterized. These factors, VEGF-C and VEGF-D, bind the endothelial cell-specific tyrosine kinase receptors VEGF-R2 and VEGF-R3 (3). VEGF-R2 is a crucial mediator of angiogenesis whereas VEGF-R3 regulates growth of lymphatic vessels. Many human tumors express VEGF-C, and increased VEGF-C expression correlates with lymph node metastasis in, for example, thyroid, prostate, gastric, colorectal and lung cancer. In breast cancer, VEGF-C expression correlates with lymph node positive tumors, whereas VEGF-D showed expression predominantly in inflammatory breast carcinoma (3).
Studies using various rodent models have provided evidence that tumor lymphangiogenesis facilitates lymphatic metastasis. In a transgenic mouse model, overexpression of VEGF-C in the β-cells of the pancreatic islets increased lymphangiogenesis around the primary tumor and enhanced tumor-cell spread to the draining lymph nodes (4). More importantly, tumor growth, lymphangiogenesis and lymph node metastasis is inhibited by a blocking antibody VEGFR-3 (5). Finally, new findings indicate that select integrins can modulate lymphangiogenesis and consequently may affect tumor metastasis (6).
1.2. The integrin family of adhesion receptors
Integrins are a family of heterodimeric transmembrane glycoproteins mediating cell-cell and cell-Extra Cellular Matrix (ECM) interactions. The integrin family consists of 8 α and 18 β subunits that can associate to form 24 unique integrin heterodimers (7). Each integrin receptor heterodimer binds a specific set of endogenous ligands, which may include ligands in the ECM (collagen, fibronectin, vitronectin for example), soluble ligands and ligands on other cells surfaces such as VCAM-1 (Vascular Cell Adhesion Molecule-1) or ICAM-1 (InterCellular Adhesion Molecule-1) (Figure 1). Each integrin subunit consists of an extracellular domain, a single transmembrane region and a short cytoplasmic region. Upon ligand binding, as series of intracellular signaling events is initiated. Integrin ligation to its ligand promotes integrin clustering and subsequent integrin-mediated intracellular signal transduction. This integrin signaling promotes endothelial and lymphatic endothelial cell migration, proliferation and survival (8).
Figure 1.
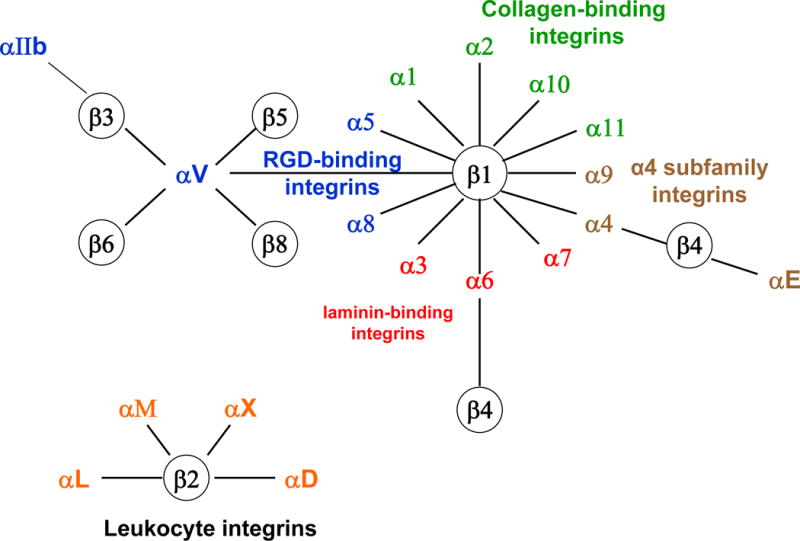
The integrin family of adhesion of adhesion receptors and their ligands. There are 18 α and 8 β subunits which assemble to form 24 different heterodimers. Heterodimer composition confers ligand specificity. The main ligands for integrins in the extracellular space are extracellular matrix proteins, such as laminin, collagen, vitronectin and fibronectin. Moreover, integrins can also bind cellular counter-receptors (VCAM-1 or ICAM-1) and soluble molecules (fibrinogen).
1.4. Role of integrins in angiogenesis
Angiogenesis depends on a timely and spatially interaction between vascular cells, ECM, growth factors and proteases. Integrins can mediate cell adhesion to the components of the extracellular matrix and to other cells, as well as make transmembrane connections to the cytoskeleton and activate many intracellular signaling pathways (9). Endothelial cells are anchorage-dependent cells. Integrins facilitate endothelial cell binding to the ECM. Thus, the up-regulation of endothelial cell integrins by pro-angiogenic factors sustains cell viability, increases cell sensitivity to growth factors and is required for migration. Endothelial cells have been reported to express up to ten different integrins (Table 1); α1β1, α2β1, α3β1, α4β1, α5β1, α6β1, α6β4, αvβ3, αvβ5 and αvβ8 (10).
Table 1.
Role of integrins in angiogenesis and lymphangiogenesis. This table summarizes the effect of genetic ablation of different integrin subunits on the vascular and lymphatic development in mouse embryo and during postnatal life.
| Integrin | Major ligands | Mouse Phenotype |
|---|---|---|
| α1β1 | Collagen, laminin | α1−/− : normal vascular development; reduced adult angiogenesis |
| α2β1 | Collagen, laminin | α2−/− : normal vascular development; enhanced tumor angiogenesis |
| α4β1 | CS1 fibronectin, VCAM-1 | α4−/−: embryonic lethal; 50% die at E9.5–10.5, failure of chorion-allantois fusion; 50% die at E11.5 owing to cardiovascular defects |
| α5β1 | Fibronectin, L1-CAM | α5−/− : embryonic lethal E10–11; yolk sac and embryonic vessel defects |
| α6β1 | Laminin | α6−/− : embryonic lethal; lethal skin defects; no vascular defect |
| α9β1 | Tensacin, fibronectin, thrombospondin, VCAM-1, collagen, laminin | α9−/− : post-natal lethality P8–P12; chylothrorax (accumulation of lymph in the pleural cavity) |
| αMβ2 | ICAM-1, fibrinogen | αM−/− : normal development |
| αvβ3 | Fibronectin, vitronectin, von Willebrand factor, tensacin, DEL-1, osteopontin | αv−/− : 80% embryonic lethality E9.5; 20% die P20 with brain hemorrhage β3−/− : 50% embryonic and early post-natal lethality; enhanced angiogenesis in surviving adults |
| αvβ5 | Vitronectin, osteopontin, DEL-1 | αv−/− : 80% embryonic lethality E9.5; 20% die P20 with brain hemorrhage β5−/− : normal development; reduced adult angiogenesis in response to certain angiogenic factors. |
| αvβ8 | Collagen, laminin, fibronectin | β8−/− : disrupted brain blood vessel formation |
| α6β4 | Laminin 5 | β4−/−: normal vascular development but lethal skin defects |
During angiogenesis, integrins α1β1 and α6β4 are often down-regulated, and integrins α5β1 and αvβ3 are up-regulated or expressed de novo (8). Pathological angiogenesis is often associated with up-regulation of the expression of certain integrins, including integrin α5β1 and αvβ3 (11). Integrins α2β1 and α1β1 are known to promote cell migration, proliferation and matrix reorganization, and thus they are important in non-quiescent cells during dynamic situations, such as angiogenesis. VEGF significantly induces their expression on the endothelial cell surface. Inhibiting the function of integrins α1β1 and α2β1 by antibodies leads to selective inhibition of VEGF-driven angiogenesis in vivo without any effects on the pre-existing vasculature. Therefore, it has been suggested that integrins α1β1 and α2β1 play roles in pathological angiogenesis (12). Interestingly, it has been shown that tumor angiogenesis is markedly reduced in α1-null mice. This reduction may be caused by overexpression of metalloproteases (MMPs) and consequent generation of angiostatin, an inhibitor of angiogenesis proteolytically derived from plasminogen (13).
The αv integrins play important roles in angiogenesis. Integrin αvβ3 is selectively expressed on growing blood vessels. Importantly, in vivo angiogenesis in corneal or chorioallantoic membrane model induced by bFGF depends on αvβ3, whereas angiogenesis initiated by VEGF-A depends on αvβ5 (14). While results from studies of integrin antagonists indicate that αv integrins promote angiogenesis, genetic deletion studies indicate that αv integrins are not required for angiogenesis. Integrin αvβ3 deficient mice show normal developmental angiogenesis, but increased pathological angiogenesis (15). In contrast with these genetic studies, blockade of integrin αvβ3 as well as αvβ5 function using integrin antagonists disrupts tumor angiogenesis (16). Both integrins αvβ3 and α5β1 mediate pro-apoptotic signals when they are unligated or occupied by a soluble ligand (17). One hypothesis to explain this conflict is that αv integrins act as negative regulators of angiogenesis; once deleted in development, angiogenesis occurs at an accelerated rate. An alternative hypothesis is that animals lacking αv integrins develop compensatory changes in VEGF signaling that permit angiogenesis to occur during embryogenesis. In fact, β3 null mice exhibit enhanced tumor angiogenesis compared with normal mice, with strongly upregulated VEGF-R2 expression and signaling (18). Taken together, these studies suggest that compensatory VEGF-R2 signaling changes may play a role in the survival of β3 deficient animals.
Additional approaches have clarified the much-disputed role of αv integrins in angiogenesis. Animals expressing the point mutations Y747F and Y759F in the integrin β3 cytoplasmic tail develop normally, but exhibit reduced growth factor and tumor induced angiogenesis in vivo (19). Mutant endothelial cells exhibit impaired adhesion, spreading, migration and tube formation, as well as impaired complex formation between VEGF receptor-2 and β3 integrin. These results provide genetic evidence that integrin β3 plays an important role in promoting angiogenesis. Together, these diverse results can be interpreted to indicate that integrin αvβ3 plays an important role in angiogenesis and that loss of expression of this integrin in development can be partially compensated for by upregulation of other angiogenesis signaling pathways. Recently, it has been discovered that αvβ3 integrin binds to MMP-2 and thus this co-operation may regulate endothelial cell migration and other functions necessary for angiogenesis (20). Similar to the integrin β3, integrin β4 plays an important role in angiogenesis. The loss of integrin β4 significantly inhibits tumor angiogenesis suggesting a role for integrin α6β4, although its expression is usually down-regulated during angiogenesis (21).
The important role of integrins during in tumor angiogenesis has led to the development of antagonists of integrins as a therapeutic tool for controlling tumor progression. Preclinical studies have suggested that antagonists of several integrins might be useful to suppress tumor angiogenesis and growth either alone or in combination with current cancer therapy (22).
1.5. Role of integrins in lymphangiogenesis
Although the role of integrins in angiogenesis is well documented, little is known about the expression and functional relevance of integrins during lymphangiogenesis. The first evidence of the role of integrins in lymphangiogenesis has been provided by Huang et al. Indeed, this study suggests that the α9 integrin is required for the normal development of the lymphatic system, including the thoracic duct, and that α9 deficiency could be one cause of congenital chylothorax (accumulation of lymph in the pleural cavity) (23). Moreover, in murine embryos, expression of VEGF-R3 and integrin α9 is increased in Prox1-expressing lymphatic endothelial cells (LECs). Knockdown of Prox1 expression in human LECs led to decrease in the expression of integrin α9 and VEGF-R3, resulting in the decreased chemotaxis toward VEGF-C, suggesting integrin α9 may function as a key regulator of lymphangiogenesis acting downstream of Prox1 (24). Importantly, α9β1 integrin can bind VEGF-C and VEGF-D then promotes cell adhesion and migration (25).
Moreover, it has been shown that VEGF-A enhances expression of both integrin α1β1 and α2β1 in lymphatic endothelial cells, promoting their capacity to form cords and their migration. Interestingly, systemic blockade of these integrins potently inhibits wound-associated lymphangiogenesis in vivo (26). More recently, the integrin α1 has been found to be expressed in lymphatic endothelial cells isolated from patients with lymphangioma (27). Several lines of evidence are consistent with a role of α5β1 integrin in lymphangiogenesis mediated through VEGF-R3 signaling. It has been shown that integrin α5 is expressed by human lymphatic endothelial cells in culture (28) and by a subpopulation of resting and proliferating lymphatic vessels in mouse cornea (29). Selective inhibition of α5β1 integrin reduces lymphangiogenesis in a mouse model of suture-induced corneal inflammation (29). More recently, it has been shown that α5β1 integrin blockade reduces lymphatic sprouting and growth in airway inflammation after M. pulmonis infection but does not reduce blood vessel remodeling or macrophage recruitment (6). Furthermore, endostatin, which can inhibit endothelial cell migration by binding to α5β1 integrin (30) reduces lymphangiogenesis in skin tumors (31). Our studies reported that the integrin α4 is expressed on tumor lymphatic endothelium. Selective blockade of this integrin can block lymphangiogenesis and tumor metastasis (32). However, it seems that αv integrins do not play a role in tumor lymphangiogenesis (32). Therefore, several integrins can regulate lymphangiogenesis in physiological and pathological conditions (Table 1). Importantly, antagonists of these integrins may be useful to prevent tumor lymphangiogenesis and metastasis.
1.6. Integrins as therapeutic agents in oncology
Blockade of integrin/ECM-ligand interactions inhibits tumor metastasis and angiogenesis and can be achieved by function-blocking antibodies, small organic molecules, and peptidomimetics. Antagonists of proangiogenic integrins, such as, αvβ3, αvβ5 and α5β1 are under clinical evaluation (Table 2).
Table 2.
Integrin antagonists tested in clinical trials for cancer therapy. This table summarizes the effect of different integrin antagonists on angiogenesis and tumor progression in cancer patients
| Drug Name | Target | Drug Type | Tumor type (Trial Phase) |
|---|---|---|---|
| Abegrin (MEDI-522) | αvβ3 | Humanized antibody | Colorectal, melanoma, renal (Phase II) |
| CNTO 95 | αvβ3 and αvβ5 | Human antibody | Advanced refractory cancers (Phase I) |
| Cilengitide | αvβ3 and αvβ5 | Peptide | Brain, head and neck, glioblastoma, leukemia, melanoma, prostate (Phase II/III) |
| Volociximab (M200) | α5β1 | Chimeric mouse-human antibody | Non-small lung, melanoma, pancreatic, renal (Phase II) |
| ATN-161 | α5β1 | peptide | Malignant glioma (Phase I/II) |
Integrins αvβ3 and αvβ5 are involved in angiogenesis and expressed in malignancies such as melanoma, gliomas, and cancers of the breast, prostate, and colon. Abegrin, (Medi-522), a humanized anti-αvβ3 antibody, was the first anti-integrin therapeutic agent to be tested in clinical trials for cancer (33). A recent study in patients with solid tumors showed that Abegrin had functional efficacy by reducing focal adhesion kinase activity in blood vessels (34). Based on these results, phase III cancer clinical trials are being to be evaluated.
On the basis of preclinical studies showing that both integrins αvβ3 and αvβ5 regulate angiogenesis, a human monoclonal antibody directed against both αvβ3 and αvβ5 integrins, CNTO 95 has been developed. CNTO 95 reduced angiogenesis and tumor growth in human melanoma xenografts in nude mice and rats (35). CNTO 95 is now under evaluation in a phase I/II clinical trial for the treatment of patients with melanoma (36). As CNTO 95 inhibits both integrins αvβ3and αvβ5, two of the integrins that promote tumour angiogenesis, it might have widespread clinical utility. Additionally, most carcinoma cells express integrin αvβ5, which has been shown to promote tumour cell invasion (37). Targeting the αv integrins might thus block both tumor cell invasion and metastasis and tumor angiogenesis.
For these reasons, Cilengitide (EMD-121974), a synthetic cyclic penta-peptide small-molecule inhibitor of αvβ3 and αvβ5 integrins has been developed (38). The peptide has demonstrated anti-angiogenic and antitumor activities in vitro and in vivo. In a phase 1 trial in patients with advanced solid tumors, Cilengitide was administered twice weekly every 28 days and was well tolerated with no dose-limiting toxicities observed at the tested dose levels (39). This agent is currently evaluated in phase II and III trials for glioblastoma, non-small cell lung cancer, melanoma and pancreatic and prostate cancer (40).
Integrin α5β1 is expressed mainly on vascular endothelial cells and up-regulated together with fibronectin in tumor neovasculature. Volociximab is a chimeric human IgG4 against α5β1 that inhibits angiogenesis independent of VEGF/VEGF-R and induces apoptosis in proliferating, but not quiescent, endothelial cells in preclinical experiments (41). Volociximab was evaluated in phase II clinical trials for metastatic melanoma, renal cell carcinoma and non-small cell lung cancer (42). Volociximab is being tested in advanced ovarian cancer and in combination with gemcitabine in metastatic pancreatic cancer (43). Another inhibitor of integrin α5β1, the peptide ATN-161, is also developed in clinical trials. In animal models of colon cancer, ATN-161 reduced metastases and improved survival when combined with chemotherapy (44). Thus, these two integrins α5β1-inhibiting agents might offer future benefit to cancer patients.
Nevertheless, as many integrins can promote tumor angiogenesis and metastasis, it is not yet clear whether targeting one or more than one will have the most significant effect on tumor progression. Moreover, it is also likely that integrins antagonists may be combined with radio-chemotherapy or with other angiogenesis inhibitors such as VEGF inhibitors (Avastin).
2. Materials
2.1. Cell Culture
Endothelial Growth Medium (EGM) (Cambrex) supplemented with 10% fetal bovine serum (FBS), bFGF and VEGF.
Solution of trypsin (0.25%) and ethylenediamine tetraacetic acid (EDTA) (1mM) (Invitrogen)
Incubator 37°C, 5% CO2
2.2. Flow cytometry
Phosphate Buffered Saline pH7.4 containing 2% FBS (wash buffer)
Primary antibodies diluted in PBS 2% FBS
Fluorochrome-conjugated secondary antibody diluted in PBS 2% FBS
Paraformaldehyde 0.5% in PBS
FACS Calibur flow cytometer (BD Biosciences)
2.3. Cell Adhesion
Non tissue 48-well plate (Corning)
Extracellular Matrix Proteins (ECM): vitronectin, fibronectin, CS-1 fibronectin diluted in PBS (5μg/ml)
Blocking solution: PBS containing 2% heat-denatured bovine serum albumin (BSA)
Adhesion Buffer: Hanks Balanced Salt Solution, 10mM Hepes pH7.4, 2mM MgCl2, 2mM CaCl2, 0.2mM MnCl2, 1% BSA)
Paraformaldehyde 3.7%
Crystal violet 2% in sodium borate
Acid Acetic 10%
Plate reader to measure absorbance at 560nm
2.4. Cell Migration
24-well plate (Corning)
8μm Costar Transwells (Corning)
Blocking solution: PBS containing 3% BSA
Migration Buffer: DMEM, 10mM Hepes pH7.4, 1.8mM MgCl2, 1.8mM CaCl2, 0.2mM MnCl2, 1% BSA)
Paraformaldehyde 3.7%
Crystal violet 2% in sodium borate
2.5 Immunohistochemistry on frozen mouse tissue sections
Humid chamber
Coplin jars
Cold acetone 100%
PBS 1× pH7.4
Hydrophobic pen (Dako)
Normal Goat Serum
Polyclonal Rabbit anti-mouse LYVE-1 (RDI)
Monoclonal Rat anti-Mouse CD31 (MEC 13.3, BD Biosciences)
DAPI
Fluorescent mounting media (Dako)
Clear nail varnish
3. Methods to study integrins during angiogenesis and lymphangiogenesis
3.1 Integrin Expression on Human Umbilical Vein Endothelial Cells (HUVEC) and Lymphatic Endothelial Cells (LEC)
HUVEC and LEC are grown in endothelial growth medium (EGM-2) containing 10% fetal bovine serum (FBS), bFGF and VEGF (Cambrex).
Expression levels of human integrin α4β1, α5β1, αvβ3 and αvβ5 on HUVEC and LEC are determined by flow cytometry.
HUVEC and LEC are washed in PBS then detached with by trypsin treatment for 5min (see Note 1).
Cells are resuspended in EGM-2 containing serum and washed twice in cold PBS containing 2% FBS.
Cells are then incubated for 1 hour on ice with the following antibodies (1–10μg antibody diluted in PBS containing 2% FBS): mouse anti-human α4β1 (HP1/2), mouse anti-human α5β1 (JBS5), mouse anti-human αvβ3 (LM609) and mouse anti-human αvβ5 (P1F6). Cells are also incubated with isotype control antibodies IgG2a and IgG2b as negative control.
Cells are washed twice with PBS containing 2% FBS and incubated for 1 hour with fluorochrome conjugated goat anti-mouse secondary antibody on ice, in the dark.
Cells are washed twice by centrifugation with PBS containing 2% FBS, fixed with 0.5% paraformaldehyde and then analyzed by flow cytometry.
3.2 In vitro adhesion assay
To determine whether specific integrins regulate HUVEC and LEC adhesion, 48-well plates are coated with different ECM proteins.
Wells are coated overnight at 4°C with 5μg/ml of vitronectin, fibronectin or CS-1 fibronectin diluted in PBS. Wells that are not coated are used as negative controls. Perform triplicate samples per group
The next day, plates are blocked with PBS 5%BSA for 2 hours at 37°C.
After detachment, HUVEC and LEC (250,000 cells/well) are resuspended in adhesion buffer in the presence or not of blocking antibodies (25μg/ml): anti-human α4β1 (HP1/2), anti-human α5β1 (JBS5), anti-human αvβ3 (LM609) and anti-human αvβ5 (P1F6). These blocking antibodies are used as competitive inhibitors of cells adhesion to ECM proteins.
Cells are incubated at 37°C, 5% CO2 for 10 to 30 min.
Plates are carefully washed three times with warm adhesion buffer, and nonadherent cells are removed by aspiration.
Adherent remaining cells were then fixed by incubation in 3.7% paraformaldehyde for 1 hour.
Cells are then stained with 1% crystal violet in sodium borate for 1h
Plates are well washed with distilled water to remove excess crystal violet, air-dried overnight and extracted by incubation in 200μl acid acetic.
100μl of each of these extracts is measured at 560nm using a plate reader.
3.2 In vitro migration assay
To determine whether specific integrins regulate HUVEC and LEC migration, cell migration assays are performed using Costar Transwells.
Inserts are coated overnight at 4°C with 5μg/ml of vitronectin, fibronectin or CS-1 fibronectin diluted in PBS. Inserts that are not coated are used as negative controls. Perform triplicate samples per group.
Non specific binding sites are blocked by incubation with 3% BSA in PBS for 1 hour at 37°C.
Resuspend cells in migration buffer
Add 50,000 cells in the presence or not of integrins blocking antibodies to the upper chamber and incubate at 37°C, 5% CO2.
Cells are allowed to migrate from the upper to lower chamber for 4 hours at 37°C, 5% CO2.
Remove non migrating cells from the upper chamber by wiping the upper surface with a cotton swab.
Cells that had migrated to the lower surface of the Transwell insert are fixed for 15 minutes with 3.7% paraformaldehyde and incubated in a 2% crystal violet in sodium borate.
Wash extensively with distilled water to remove excess crystal violet
Count the number of cells that had migrated to the bottom of the insert in 5 random 200× fields per replicate.
3.3 Identification of blood and lymphatic vessels in tumor tissue
To identify blood and lymphatic vessels in tissue, mouse tumor cryosections (from Lewis Lung Carcinoma, B16 melanoma models for example) are immunostained to detect CD31 or Platelet-Endothelial Cell Adhesion Molecule (PECAM), a specific marker for vascular endothelial cells and LYVE-1, a marker of the lymphatic endothelium.
Allow slides of sections 5μm thick to equilibrate at room temperature.
Label slides with a pencil, noting specimen
Place slides in a glass coplin jar containing 100% cold acetone (pre-cooled at −20°C) for 2 minutes to fix
Carefully dry the slides using tissue and draw a box around each specimen with a hydrophobic pen to retain the antibody volumes on the section.
Wash slides twice for 5 minutes in PBS (see Note 2).
Create a humidified chamber by placing a damp paper towel in the bottom of a plastic box with a sealing lid.
Places slides flat on the staining tray and block non-specific antibody-bindibg sites by applying approximately 100μl of 8% Normal Goat Serum (NGS) in PBS on each encircled section.
Incubate 2h at room temperature overnight at 4°C.
Apply primary antibody (anti-CD31 or anti-LYVE1) at 5μg/ml in 2% NGS in PBS. Apply only block buffer to some section that serves as a negative control (see Note 3).
Incubate for 2 hours at room temperature
Place slides in a coplin jar containing PBS and wash slides 3 times for 5 minutes with agitation.
Carefully dry the slides and apply 100μl secondary antibody (488-conjugated goat anti-rat IgG for CD31 and 488-conjugated goat anti-rabbit for LYVE1) diluted 1/1000 in 2% NGS in PBS (see Note 4).
Incubate for 1 hour at room temperature.
Place slides in a coplin jar containing PBS and wash slides 3 times for 5 minutes with agitation.
Carefully dry slides and apply 100μl DAPI to stain the nuclei.
Incubate for 5 minutes
Place slides in a coplin jar containing PBS and wash slides 3 times for 5 minutes with agitation.
Carefully dry slides and apply fluorescent mounting media
Place a coverslip gently on the section.
Seal each slide by painting around the edge of the coverslip with clear nail varnish.
Store slides in the refrigerator in the dark.
5. Conclusion
Angiogenesis and lymphangiogenesis have crucial roles in promoting tumor growth and metastasis. A substantial body of experimental evidence indicates that integrins regulate endothelial cell migration and survival during tumor angiogenesis and lymphangiogenesis. Indeed, changes in integrin expression and/or function are directly involved in angiogenesis, inflammation, tumor growth and metastasis. Therefore, the development of integrin antagonists might be useful in blocking tumor metastasis in cancer patients. Preclinical evidence indicates that integrins are valuable targets for the design of novel cancer therapeutics (Figure 2).
Figure 2.
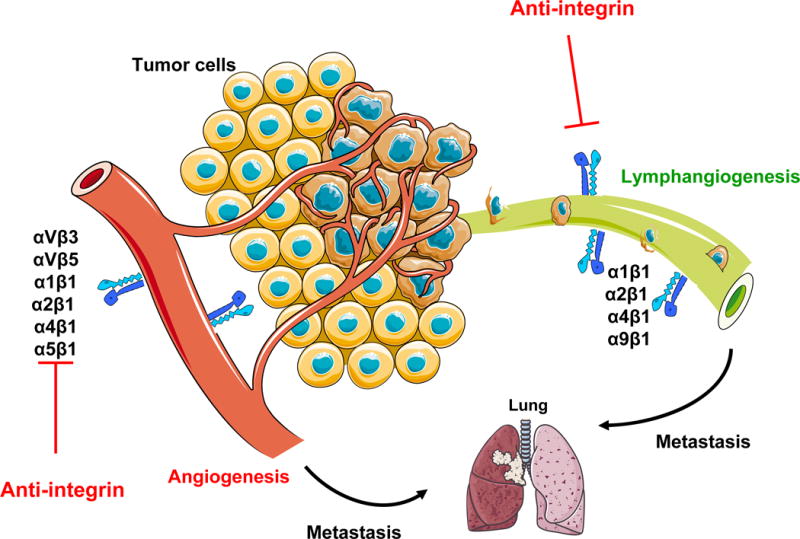
Role of integrins in tumor angiogenesis and lymphangiogenesis. The tumor microenvironment activates or upregulates expression of integrins such as α1β1, α2β1, α4β1, α5β1 and αvβ3 on blood vessels and α1β1, α2β1, α4β1 and α9β1 on lymphatic vessels. Then, these integrins promote endothelial and lymphatic cells migration and survival during invasion of tumor tissue. Angiogenesis and lymphangiogenesis promote metastasis to local and distant organ such as lung.
Footnotes
Cell confluency could influence integrin expression. Therefore, always use cells at the same confluence (70–80%) to analyze integrin expression.
It is beneficial from this stage onward to ensure that the sections do no dry out. Therefore, the slides should be dried in small batches (2–4 slides) before adding the next solution.
The use of a negative control antibody is necessary to confirm the validity of the staining. This should either be an isotype-matched antibody or serum from the relevant species.
LYVE1, while a widely used marker for lymphatic endothelium, is expressed by other cell types, including macrophages which are abundant in the tumor microenvironment. Therefore, LYVE1 staining should be identified with care. Moreover, other lymphatic markers such as podoplanin, Prox1 or VEGFR3 should be tested to confirm the presence of lymphatic vessels in tumor.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–21. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–53. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 4.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. Embo J. 2001;20:672–82. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–25. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Ni A, Ayeni OA, Baluk P, Yao LC, Vossmeyer D, Zischinsky G, Zahn G, Knolle J, Christner C, McDonald DM. alpha5beta1 Integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174:2378–87. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 8.Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene. 2008;27:6285–98. doi: 10.1038/onc.2008.304. [DOI] [PubMed] [Google Scholar]
- 9.Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8:918–21. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- 10.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–8. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A, Kalluri R. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci U S A. 2005;102:2934–9. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94:13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:22027. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 16.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 17.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 19.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–28. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 21.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–83. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–13. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 23.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–15. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, Niwa H, Kubo H, Suda T, Miyazono K. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–9. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–96. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 26.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J. 2004;18:1111–3. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 27.Norgall S, Papoutsi M, Rossler J, Schweigerer L, Wilting J, Weich HA. Elevated expression of VEGFR-3 in lymphatic endothelial cells from lymphangiomas. BMC Cancer. 2007;7:105. doi: 10.1186/1471-2407-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202:205–14. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–72. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 31.Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67:11528–35. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- 32.Garmy-Susini B, Makale M, Fuster M, Varner JA. Methods to study lymphatic vessel integrins. Methods Enzymol. 2007;426:415–38. doi: 10.1016/S0076-6879(07)26018-5. [DOI] [PubMed] [Google Scholar]
- 33.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93:9764–9. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Pier T, McNeel DG, Wilding G, Friedl A. Effects of a monoclonal anti-alphavbeta3 integrin antibody on blood vessels - a pharmacodynamic study. Invest New Drugs. 2007;25:49–55. doi: 10.1007/s10637-006-9013-8. [DOI] [PubMed] [Google Scholar]
- 35.Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada MT. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–35. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 36.Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, Hope L, Valle JW, Radford JA, Lawrance J, Saunders MP, Munteanu MC, Nakada MT, Nemeth JA, Davis HM, Jiao Q, Prabhakar U, Lang Z, Corringham RE, Beckman RA, Jayson GC. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–35. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 37.Brooks PC, Klemke RL, Schon S, Lewis JM, Schwartz MA, Cheresh DA. Insulin-like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo. J Clin Invest. 1997;99:1390–8. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stupp R, Ruegg C. Integrin inhibitors reaching the clinic. J Clin Oncol. 2007;25:1637–8. doi: 10.1200/JCO.2006.09.8376. [DOI] [PubMed] [Google Scholar]
- 39.Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O’Bryant CL, Baron A, Gallemann D, Colevas D, Eckhardt SG. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007;18:1400–7. doi: 10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- 40.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, Hossfeld DK, Stoger H, Neyns B, Herzog P, Piedbois P, Dobrowolski F, Scheithauer W, Hawkins R, Katz F, Balcke P, Vermorken J, van Belle S, Davidson N, Esteve AA, Castellano D, Kleeff J, Tempia-Caliera AA, Kovar A, Nippgen J. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramakrishnan V, Bhaskar V, Law DA, Wong MH, DuBridge RB, Breinberg D, O’Hara C, Powers DB, Liu G, Grove J, Hevezi P, Cass KM, Watson S, Evangelista F, Powers RA, Finck B, Wills M, Caras I, Fang Y, McDonald D, Johnson D, Murray R, Jeffry U. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–86. [PubMed] [Google Scholar]
- 42.Kuwada SK. Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr Opin Mol Ther. 2007;9:92–98. [PubMed] [Google Scholar]
- 43.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–37. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 44.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, McCarty MF, Bucana CD, Mazar AP, Ellis LM. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]


