Abstract
Loss of CD28 expression by CD8+ T cells occurs with age and during chronic inflammatory conditions. CD8+CD28− T cells are a heterogeneous cell subpopulation whose function ranges from immunosuppressive to effector. Here we analyzed the role of CD8+CD28− T cells in the pathogenesis of systemic sclerosis (SSc), a connective tissue disorder characterized by autoimmunity, vasculopathy and extensive cutaneous and visceral fibrosis. We show that the frequency of CD8+CD28− T cells is increased in the blood and affected skin of SSc patients, independent of patient age, and correlates with the extent of skin fibrosis. We found that the majority of skin-tropic CD8+CD28− T cells are resident in the skin lesions of patients in the early stage of the disease, exhibit an effector memory phenotype and present a strong cytolytic activity ex vivo. Skin-resident and circulating SSc CD8+CD28− T cells produce high levels of the pro-fibrotic cytokine IL-13, which induces collagen production by normal and SSc dermal fibroblasts. Thus, our findings indicate that CD8+CD28− T cells represent a pathogenic T-cell subset in SSc and likely play a critical role in the early stage of SSc skin disease.
INTRODUCTION
Lifetime immune stimulation to common antigens leads to gradual loss of CD28 expression by human T cells, particularly within the CD8+ T-cell compartment (Strioga et al., 2011). However, age-independent CD8+CD28− T-cell accumulation has also been observed during chronic viral infections and in some autoimmune conditions, likely resulting from persistent antigenic stimulation (Strioga et al., 2011, Weng et al., 2009).
Human CD8+CD28− T cells are generally defined as antigen-specific, oligoclonally expanded, terminally differentiated, senescent T cells (Valenzuela and Effros, 2002). Nevertheless, they are functionally active and some of their effector functions result from costimulatory receptors other than CD28 (Abedin et al., 2005). Human CD8+CD28− T cells are functionally heterogeneous, exhibiting either cytotoxic or immunosuppressive functions as well as the ability to produce cytokines, including IFNγ, TNFα, and IL-13 (Fann et al., 2005, Strioga et al., 2011).
Changes of CD8+CD28− T-cell numbers have been observed in several autoimmune diseases (Dvergsten et al., 2013, Mikulkova et al., 2010, Schirmer et al., 2002, Sun et al., 2008). In most of them CD8+CD28− T cells show highly cytotoxic activity and are associated with more severe disease manifestations, suggesting that they play an active role in the autoimmune response (Schirmer et al., 2002, Sun et al., 2008). However, in other autoimmune conditions CD8+CD28− lymphocytes are associated with suppressive activity (Mikulkova et al., 2010, Tulunay et al., 2008).
Systemic sclerosis (SSc) is an autoimmune disease characterized by inflammation, vasculopathy and fibrosis (Gabrielli et al., 2009). Although SSc is a clinically heterogeneous disorder, skin fibrosis is the prominent pathological manifestation (Gabrielli et al., 2009). Multiple studies have shown that inflammatory cells infiltrating affected skin produce cytokines and growth factors that activate fibroblasts, resulting in excessive fibrosis (Varga and Abraham, 2007). We previously showed that more severe skin thickening is associated with over-production of the profibrotic cytokine IL-13 by peripheral blood CD8+ T cells (Fuschiotti et al., 2009) and defects in the molecular control of IL-13 production (Medsger et al., 2011). Significantly, we found high numbers of CD8+ T cells and IL-13+ cells in the fibrotic SSc skin, particularly in the early stage of the disease (Fuschiotti et al., 2013).
Previous studies have shown that CD8+CD28− T cells are expanded in the blood of SSc patients (Fenoglio et al., 2011). However, their contribution to disease pathogenesis has not been characterized in detail. Based on these data and on recent findings showing that skin-resident T cells can contribute to autoimmune and inflammatory skin diseases (Clark, 2015), we seek to investigate whether CD8+CD28− lymphocytes are directly implicated in SSc skin disease.
RESULTS
The frequency of circulating and skin-resident CD8+CD28− T cells is increased in SSc patients independent of age
We determined CD28 expression by circulating CD8+ T cells from SSc patients and age-matched NDs using flow cytometry (Figure 1a–b). SSc patients exhibited a higher frequency of CD8+CD28− T cells with a median of 45.1% compared to controls (median 19.5%; p< 0.0001). Furthermore, we found higher frequencies of CD8+CD28− T cells in dcSSc (median 52.1%) compared to lcSSc (median 28.4%; p< 0.0001; Figure 1c). As expected, there was a correlation between the frequency of CD8+CD28− T cells and age in both SSc patients (r=0.51, p< 0.0001) and NDs (r=0.43, p<0.0001). In regression analysis the frequency of CD28+CD28− T cells was a significant independent predictor of SSc presence (p=0.05) after adjustment for age (p=0.04) and the interaction of age and CD28+/CD28− positivity.
Figure 1. Age-independent accumulation of CD8+CD28− T cells in the blood and skin of SSc patients correlates with the extent of skin fibrosis.
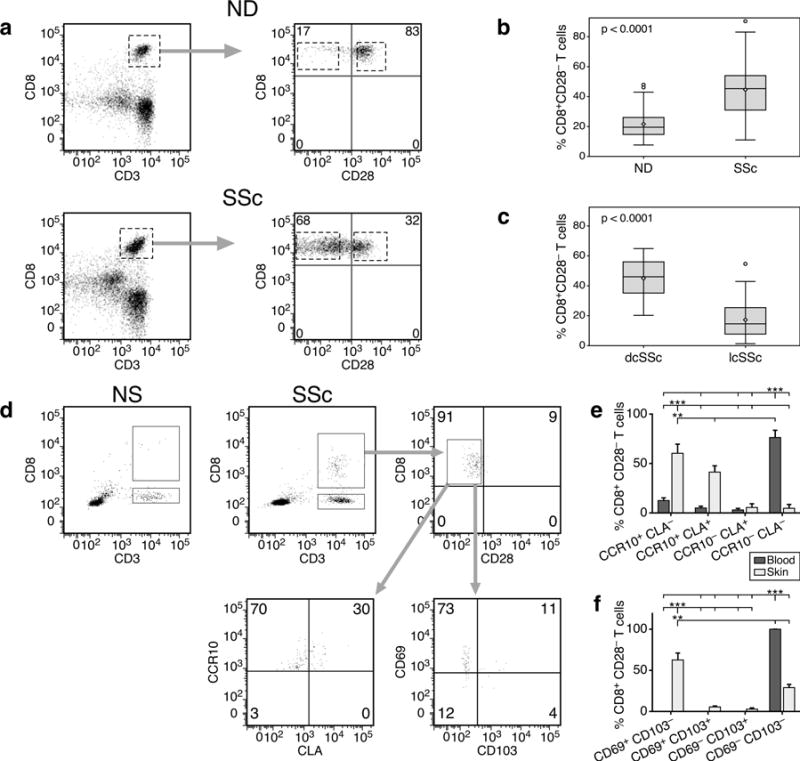
Representative CD28 expression profiles by peripheral blood CD8+ T cells in controls or patients (a). Lymphocyte population was gated according to light scatter characteristics (FSC/SSC). CD8+ T cells were identified as CD8+CD3+ cells. Proportion of circulating CD8+CD28− T cells in patients (n=65) and controls (n=32) (b) or in dcSSc (n=28) and lcSSc (n=37) patients (c). Groups in (b) and (c) are shown as boxplots with the median indicated as horizontal line and whiskers from minimum to maximum. P values were determined by Wilcoxon rank sum test. (d) Representative cell surface phenotype of skin-resident CD8+ T cells in normal (NS, n=5) or SSc (n=5) skin. Freshly isolated skin cell suspensions were gated on lymphocyte scatter and CD8 positivity. Comparison of skin-homing receptor expression (E) and skin-resident marker expression (f) by CD8+CD28− T cells in blood and skin of SSc patients. Statistics by ANOVA followed by post hoc Tukey’s test.
We next investigated the relationship between SSc CD8+CD28− T cells and skin thickness measured by mRSS, and found they correlated moderately (r=0.66, p< 0.0001). After adjusting for age, CD8+CD28− T cell frequency continued to highly correlate with mRSS (r=0.72; p<0.001) in SSc patients. We found no significant association between CD8+CD28− T-cell percentages and disease duration in the patients analyzed (data not shown). Moreover, the frequency of CD4+CD28− T cells also increased with age in patients and controls but without significant accumulations in patients (data not shown), suggesting that loss of CD28 expression in SSc is more a feature of CD8+ rather than CD4+ T lymphocytes.
We next investigated whether CD8+CD28− cells were present in the sclerotic skin of patients. Cell suspensions obtained from 3 mm skin biopsies from patients with early dcSSc were analyzed by flow cytometry. Gating strategies are depicted in Figure 1d. We identified both CD8+ and CD4+ T cells from patient samples (2.9±0.9% and 5.0±0.9%, respectively). However, in the same size biopsy of normal skin from age-matched donors we found CD4+ T cells (6.3±0.7%) but only scant numbers of CD8+ T cells (0.6±0.9%), insufficient for further analyses. This finding is in line with our current (Figures 3d, 4d, S1) and previous work (Fuschiotti et al., 2013) in which we could not detect any CD8+ T cells by IHC and IF in normal skin. By multicolor flow cytometry, we demonstrated that the majority of CD8+ T cells in the skin of patients lacks expression of CD28 (72.3±13.8%, Figure 1d). This was confirmed by IHC analyses, in which we detected high numbers of CD8+ T cells in early dcSSc patients but were unable to detect any CD28 expression (Figure S1). Further analyses showed that skin SSc CD8+CD28− T cells expressed skin-homing receptors such as CCR10 and CLA (Figure 1d–e) and exhibited markers of skin-resident T cells (Watanabe et al., 2015) such as expression of the early acute activation marker CD69 (Figure 1d and f), although only few cells expressed the αE integrin CD103.
Figure 3. SSc CD8+CD28− T cells in blood and skin express markers of cytotoxicity.
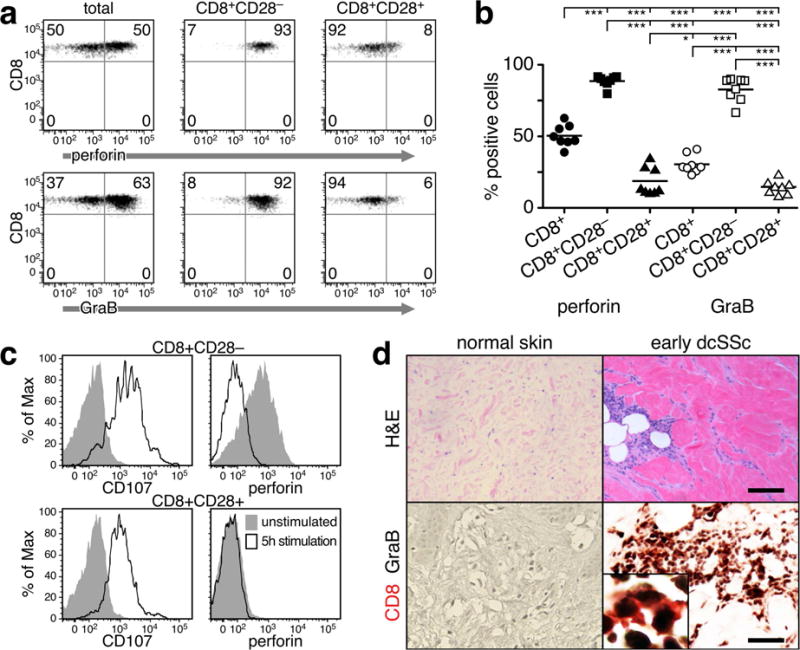
(a) Perforin and GraB expression in total CD8+ and CD8+CD28+/− circulating T cells in a representative SSc patient. (b) Frequency of cells expressing perforin and GraB in each CD8 subset from 8 SSc patients. Each symbol represents one patient or a control. Statistics by ANOVA and post-hoc Tukey’s test. (c) Surface expression of CD107a and intracellular content of perforin were analyzed by flow cytometry in stimulated and unstimulated SSc CD8+CD28+/− T cells. A representative example is shown. (d) Expression of GraB by CD8+ T cells in the affected skin of patients with early dcSSc. Representative examples of H&E staining of NS and SSc skin (upper panel – scale bar = 40μm). Double-color immunohistochemistry for CD8 (red) and GraB (black) in normal (NS, n=4) and SSc skin (n=5), (200×, Inset 500×) (lower panel – scale bar = 20μm). A representative example is shown.
Figure 4. SSc CD8+CD28− T cells produce high levels of IL-13.
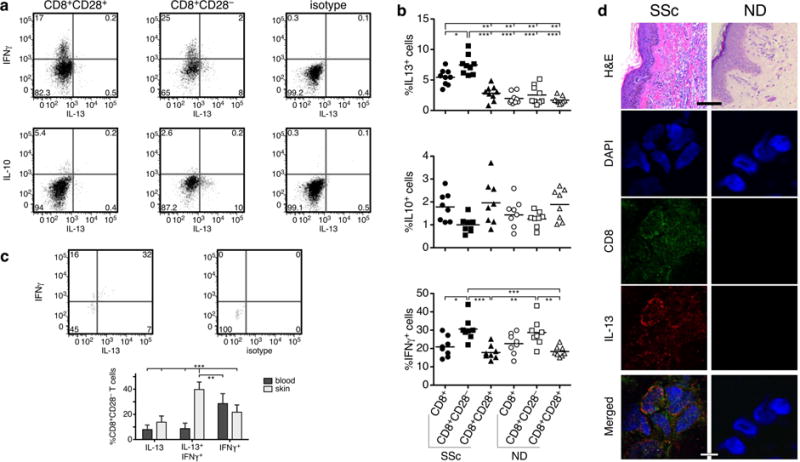
Purified CD8+CD28+ and CD8+CD28− T-cell subsets from SSc patients and NDs were cultured in vitro for 5 days and then stimulated for 6 hours with PMA and ionomycin. Intracellular cytokine staining was used to determine the proportion of IL-13+ (n=9), IL-10+ (n=8), and IFNγ+ (n=8) cells. (a) A representative example from one SSc patient is shown. (b) Comparison of the mean percentage of cytokine-positive cells in each CD8+ T-cell subset from SSc patients and age-matched NDs. Each symbol represents one patient or a control. Statistics by ANOVA followed by post hoc Tukey’s test. (c) Cytokine production by skin-resident CD8+CD28− T cells was determined as indicated above. A representative example is shown (upper panels). Comparison of cytokine production by CD8+CD28− T cells in the blood and skin of (n=5) (lower panel). Statistics by ANOVA followed by post hoc Tukey’s test. (d) Representative examples of H&E staining of NS and SSc skin (upper panel – scale bar = 40μm). Representative example of double color immunofluorescence staining for CD8 and IL-13, 1000× (lower panels – scale bar = 1μm). Skin samples from 5 early dcSSc patients and 4 NDs were analyzed giving similar results. DAPI stains nuclei.
A comparison of the expression of these markers between skin-resident and circulating CD8+CD28− T cells is shown in Figure 1e–f. Expression of skin-homing receptors was limited to only a small fraction of circulating CD8+CD28− T cells, while most of skinresident CD8+CD28− lymphocytes expressed one or both of these receptors (Figure 1e). As expected, expression of CD69 was only detected on skin-resident cells, while circulating CD28− lymphocytes were uniformly negative (Figure 1f).
Circulating and skin-resident SSc CD8+CD28− T cells exhibit effector and effector/memory phenotypes and express markers of cytotoxicity
To characterize the functional phenotype of SSc CD8+CD28− T cells we assessed the relationship between expression of CD45RA/CD27 and CD28 by flow cytometry (Hamann et al., 1997, Takata and Takiguchi, 2006). Our analysis revealed that SSc CD8+CD28− T cells were uniformly present in the effector and effector/memory subpopulations in all patients tested (Figure 2a–b, p<0.001). In comparison, we found that only a few age-matched NDs exhibited high numbers of CD8+CD28− T cells in the effector and effector/memory pools (Figure 2b, p<0.001 vs. patients) and none in the other subsets. Skin-resident CD8+CD28−T cells uniformly exhibited an effector/memory phenotype (Figure 2c).
Figure 2. Blood and skin-resident SSc CD8+CD28− T cells exhibit effector and effector/memory phenotypes.
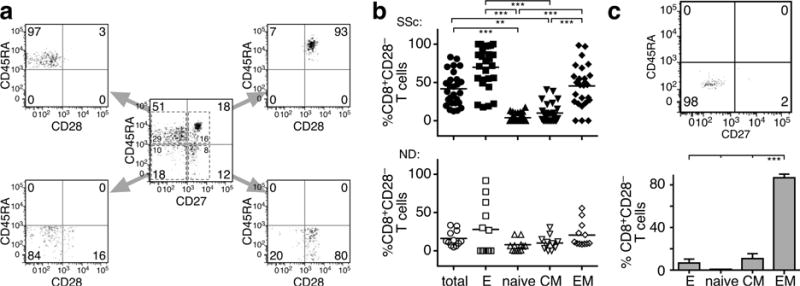
(a–b) Cell surface CD28 expression by circulating CD8+ T-cell subsets. (a) Data are gated on lymphocyte scatter and CD8 positivity. CD8+-gated cells were separated into naïve (CD45RA+CD27+), CM (CM, CD45RA−CD27+), effector/memory (EM, CD45RA− CD27−), and effector (E, CD45RA+CD27−) based on CD45RA and CD27 expression. Each of these subsets was analyzed for CD28 co-expression. A representative example is depicted. (b) Frequency of CD8+CD28− T cells in total, effector, naïve, CM and EM CD8+ T cells from SSc patients (n=29) and NDs (n=18). Each symbol represents one patient or a control. The mean response is shown as a horizontal line. (c) Expression of CD45RA and CD27 by SSc skin-resident CD8+CD28− T cells. CD8+CD28− T cells were gated as described in Figure 1d. A representative example is shown (upper panel). Phenotype of skin-resident CD8+CD28− T cells from 5 patients. In panels (b and d), statistics by ANOVA followed by post-hoc Tukey’s test.
Cytolytic effector molecules, such as perforin and GraB, are used as markers for effector CD8+ lymphocytes (Appay et al., 2002, Takata and Takiguchi, 2006, Wolint et al., 2004). Figure 3a–b shows that the majority of circulating SSc CD8+CD28− T cells expressed high levels of perforin and GraB molecules, supporting previous studies showing that CD8+CD27−CD28−CD45RA+/− cells have potent cytotoxic activity (Takata and Takiguchi, 2006). Cell surface expression of the granular membrane protein CD107a has been widely used as a marker for degranulating cytotoxic lymphocytes (Aktas et al., 2009, Betts et al., 2003). In Figure 3c we show that CD107a expression was low in unstimulated cells and was induced on the cell surface of SSc CD8+CD28− T cells following activation-induced degranulation. In parallel, intracellular perforin levels were high in unstimulated cells but declined following activation and acquisition of CD107a cell surface expression (Figure 3c, upper panel). In contrast, CD8+CD28+ cells exhibited up-regulated CD107a expression after activation, but perforin was not detected before or after stimulation (Figure 3c, lower panel), consistent with previous studies showing that memory CD8+ T lymphocytes are unable to induce immediate cytotoxic activity (Wolint et al., 2004). On immunohistochemical analysis, double staining of CD8 and GraB demonstrated the presence of CD8+GraB+ cells in the affected skin of patients with early active disease (Figure 3d). Double positive cells were found predominantly in perivascular areas as well as in the deep dermis and the subdermal fat layer, whereas NS was negative for CD8 and GraB expression (Figure 3d).
SSc CD8+CD28− T cells in the blood and skin of patients produce high levels of IL-13 and IFNγ
We have shown previously that peripheral blood CD8+ T cells from SSc patients produce high levels of pro-fibrotic IL-13 (Fuschiotti et al., 2009). Here we sought to determine the CD8+ T-cell subset responsible for excessive IL-13 production. We purified CD8+CD28+ and CD8+CD28− subsets from the blood of patients and controls, and determined IL-13 production by intracellular staining. We found that IL-13 produced by SSc CD8+ T cells is entirely from the CD8+CD28− T cells in all patients tested (Figure 4a–b). No IL-13 production was detected in CD28+ lymphocytes (p<0.001, Figure 4a–b). We also observed a small but not statistically significant increase in IL-13 in blood CD8+CD28− cells from a few normal controls, in line with previous observations (Fann et al., 2005).
We next determined production of IFNγ and IL-10 by circulating CD8+CD28− T cells. We found high level production of IFNγ by CD8+CD28− T cells compared to CD8+CD28+ cells, both in patients and controls (Figure 4a–b), likely related to the effector phenotype of these cells. We found no IL-10 producers in the CD28− subsets of either patients or controls (Figure 4a–b) and no TGFβ production in all samples tested (data not shown).
Comparison of cytokine production between skin and blood SSc CD8+CD28− T cells from the same patient in each case revealed a substantial increase in the frequency of IL-13+IFNγ+ in the skin (Figure 4c, p<0.001). Moreover, by immunofluorescence staining we observed a significant accumulation of CD8+IL-13+ cells in the dermis, particularly in areas with increased matrix deposition in the skin of 5 early dcSSc patients (Figure 4d). As shown earlier (Fuschiotti et al., 2013), no CD8+ T cells or expression of IL-13 were detected in normal skin matched for anatomical site (Fig. 4d).
SSc CD8+CD28− T cells induces a profibrotic phenotype in SSc and normal dermal fibroblasts in vitro and are cytotoxic
We co-cultured ND and SSc dermal fibroblasts with CD8+CD28− T-cell supernatants to establish whether IL-13 produced by SSc CD8+CD28− T cells has a pro-fibrotic effect on skin fibroblasts. Culture supernatants from 5-day in vitro activated SSc and ND CD8+CD28+/− T cells were added to dermal fibroblasts. After a 24-hour incubation, we assessed COL1A1 production by western blot in fibroblasts lysates and culture supernatant. We found that SSc CD8+CD28− T-cell supernatants induce a significant increase of COL1A1 production in ND and SSc dermal fibroblasts compared to those from normal controls or CD8+CD28+ subsets. Similar results were obtained for fibronectin production (data not shown). Furthermore, pre-treatment of SSc CD8+CD28− T-cell supernatants with a neutralizing anti-IL-13 antibody reduced COL1A1 production by ND and SSc dermal fibroblasts (Figure 5a–d), suggesting that IL-13 produced by SSc CD8+CD28− T cells is playing a primary role in inducing extracellular matrix production by fibroblasts.
Figure 5. SSc CD8+CD28− T cells are pro-fibrotic and cytotoxic to dermal fibroblasts in vitro.
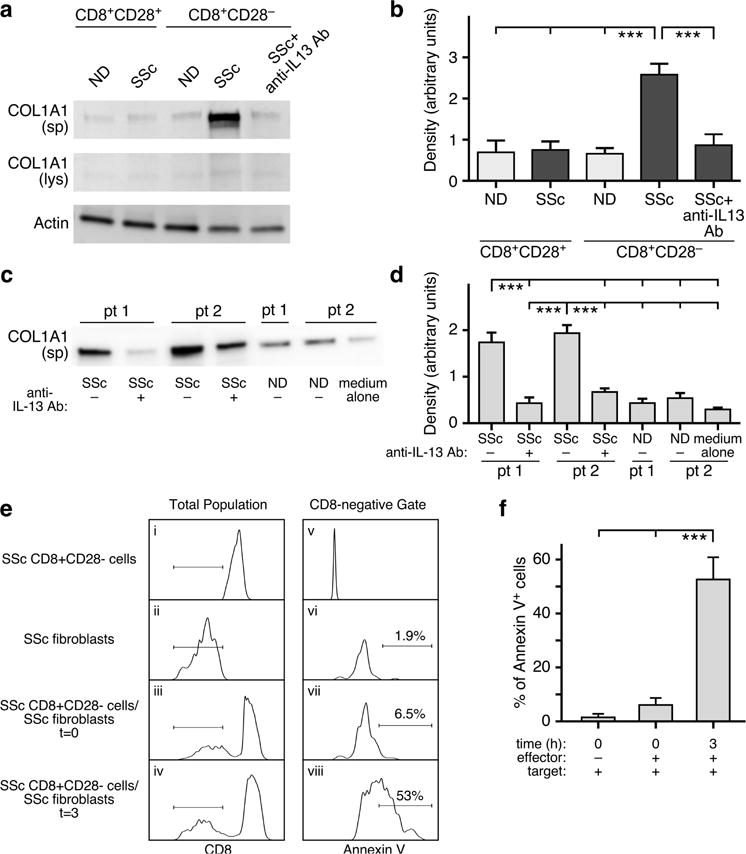
(a–b) Normal dermal fibroblasts were stimulated for 24 hours with CD8+CD28− and CD8+CD28− T-cell subset supernatants from ND (n=7) or SSc patients (n=8) with or without pre-incubation with a neutralizing anti-IL-13 antibody. COL1A1 production was determined in culture supernatants (sp) or cell lysates (lys) by Western blot. A representative experiment out of seven independent experiments from different SSc patients is shown. SSc dermal fibroblasts were obtained from two early dcSSc patients (pt 1 and 2) and were cultured with ND (n=8) and SSc (n=10) CD8+CD28− T-cell supernatants. (c) Representative Western blot for COL1A1 protein expression in fibroblast culture media (sp) and densitometric quantification of secreted COL1A1 protein (d). SSc CD8+CD28− cells (ei) or fibroblasts (eii) alone as well as mixed at a 5:1 ratio (eiii, eiv) were incubated in media for 0 or 3 hours. Lymphocyte and fibroblast populations were gated according to light scatter characteristics (FSC/SSC). The horizontal lines in panels ei–eiv designate the CD8-negative target cell gate used for analysis of Annexin V positivity in panels ev–eviii. This experiment is representative of three similar experiments. Percent Annexin V-positive cells was determined by population gating (f). Statistics in b,d, and f by ANOVA followed by post hoc Tukey’s test.
Using the Annexin V-based cytotoxicity assay (Goldberg et al., 1999), we next assessed the cytotoxic function of freshly isolated SSc CD8+CD28− T cells on allogeneic SSc dermal fibroblasts. In this assay system, APC-conjugated CD8 mAb was used to differentiate effector (SSc CD8+CD28− lymphocytes) from target (fibroblast) cell populations. SSc CD8+CD28− cells are CD8high while fibroblasts are CD8-negative (Figure 5ei and eii). The Annexin V-FITC staining pattern of the gated CD8-negative population is shown in Figure 5ev–eviii. Fibroblast targets are almost entirely Annexin V-negative before exposure to SSc CD8+CD28− lymphocytes (Figure 5evi and f). At t=0 (Figure 5evii and f), only 6.8±2.3% of the fibroblasts are Annexin V-positive using population gating analysis, while at 3 hours co-culture, 54.3±7.9% of fibroblasts are Annexin V-positive (Figure 5eviii and f). Thus, our findings indicate that SSc CD8+CD28− T cells possess cytolytic activity against allogeneic dermal fibroblasts.
DISCUSSION
CD8+CD28− T cells are expanded in the peripheral blood of patients with chronic inflammatory diseases. Although some studies have analyzed these cells in SSc (Fenoglio et al., 2011), their functional role in the pathogenesis of the disease is not well characterized. In this study, we found an age-independent accumulation of CD8+CD28− T cells in the blood and affected skin of SSc patients that correlated with the extent of skin fibrosis. Most CD8+CD28− T cells were skin-tropic or skin-resident and exhibited pro-fibrotic and cytotoxic functions. Thus, our results indicate that CD8+CD28− T cells represent a pathogenic subset in SSc and likely play a critical role in SSc skin disease.
Skin-resident memory T cells provide rapid in situ protection against most common pathogens (Clark et al., 2006, Thome and Farber, 2015), however, their dysregulation can contribute to autoimmune and inflammatory skin diseases (Clark, 2015). We found that most CD8+ T cells in the thick skin of SSc patients have lost expression of CD28 and express skin-homing receptors such as CLA and CCR10. Expression analysis of tissue-resident markers CD69 and CD103 revealed that the majority of skin SSc CD8+CD28− T cells are CD69+CD103− as found in skin-resident T cells confined to normal dermis (Watanabe et al., 2015). This phenotype was further confirmed by immunohistochemical analysis of affected skin of dcSSc patients, showing that CD8+CD28− T cells were restricted to the lower dermis and subdermal fat. Upregulation of CD69 and CD103 expression by skin-resident CD8+CD28− T cells may result from distinct signals received by these cells in situ, such as TCR activation (Ziegler et al., 1994) or in response to specific cytokines (Shiow et al., 2006). Significantly, tissue-resident memory T cells downregulate CD28 in response to TCR-triggered activation/proliferation (Lo et al., 2011). CD69 also retains cells in tissue by suppressing the activity of the sphingosine-1-phosphate receptor (Matloubian et al., 2004, Skon et al., 2013).
Phenotypic characterization of skin-resident SSc CD8+CD28− cells indicates that they all have an effector-memory phenotype, while circulating SSc CD8+CD28− cells represent a subset of highly differentiated effector T cells that express perforin and GraB at high levels, produce IFNγ and possess immediate strong cytolytic activity ex vivo. A previous gene expression study showed a 4-fold higher expression of GraB in SSc skin compared to NS (Rice et al., 2015). Significantly, we found numerous CD8+CD28−IFNγ+ and CD8+CD28−GraB+ T cells in the affected skin of early dcSSc patients, where they could be directly involved in disease pathogenesis. Autoantigens in systemic autoimmune diseases may be generated by GraB produced from cytotoxic T lymphocytes (Casciola-Rosen et al., 1999) and, interestingly, autoantibodies from a subset of SSc patients recognize self-protein fragments generated by GraB (Kahaleh and Fan, 1997). Furthermore, GraB and perforin inhibit endothelial cell growth in SSc after vascular injury (Kahaleh and Fan, 1997). Thus, SSc CD8+CD28− T cells may be inducing vascular damage and initiating and propagating specific autoimmune responses.
A recent study showed that peripheral blood SSc CD8+CD28− T cells have suppressor activity in vitro (Fenoglio et al., 2011). While the mechanism was not identified, we were unable to detect any production of immunosuppressive cytokines such as IL-10 and TGFβ by ex vivo isolated SSc CD8+CD28− cells. Both cytotoxic and suppressor subsets may coexist, in line with the knowledge that CD8+CD28− lymphocytes are characterized by multiple functional subsets (Strioga et al., 2011). However, the existence and relevance of such an inhibitory subset has yet to be examined in vivo.
IL-13 is a prominent cytokine participating in the pathogenesis of fibrosis in many diseases (Wynn, 2004). The importance of IL-13 in SSc is supported by recent work by us (Fuschiotti et al., 2013, Fuschiotti et al., 2009) and others (Aliprantis et al., 2007, Greenblatt et al., 2012, Hasegawa et al., 1997). We first described IL-13 production by CD8+ T cells with SSc (Fuschiotti, 2011, Fuschiotti et al., 2013, Fuschiotti et al., 2009). Here we show both in skin and blood that CD8+CD28− T cells are the CD8+ T-cell subset responsible for excessive IL-13 production. Moreover, IL-13 produced by SSc CD8+CD28− T-cell supernatants induced extracellular matrix production in normal and SSc dermal fibroblasts, supporting the notion that SSc CD8+CD28−IL-13+ T cells are profibrotic. Strikingly, same patient analyses showed that the proportion of skin-resident CD8+CD28− T cells producing IL-13 is greatly increased compared to their circulating counterparts, and that these cells also produce IFNγ. Furthermore, we provide direct evidence that CD8+CD28−IL-13+ T cells are present in the affected skin of SSc patients in the early inflammatory stage of the disease, and are therefore directly implicated in mediating dermal fibrosis in SSc.
In conclusion, we identified a pathogenic CD8+ T-cell subset that resides in the affected skin of SSc patients in the early stage of disease and which has direct cellular cytotoxicity and pro-fibrotic function. This finding sheds light on the pathogenesis of SSc skin disease.
MATERIAL AND METHODS
Blood and skin samples
Research protocols involving human subjects were approved by the Institutional Review Board of the University of Pittsburgh. All participants gave their written informed consent.
We obtained blood samples from 65 SSc patients seen in the Scleroderma Clinic, University of Pittsburgh Medical Center (UPMC). The patients were well-characterized in terms of disease subtype, clinical features and therapy and fulfilled either the classification criteria for SSc proposed by the American College of Rheumatology (Masi, 1980) or the early SSc diagnostic criteria of LeRoy and Medsger (LeRoy and Medsger, 2001). Disease subtype, internal organ involvement (presence and severity) were assessed according to established criteria (LeRoy et al., 1988, Medsger et al., 2003). Patients were classified into two subsets on the basis of the extent of skin involvement: diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc). Age range was 22– 80 years with a mean±SD of 49.1±15.3. The female-to-male ratio was 4:1. Thirty-seven patients had lcSSc and 28 dcSSc. Disease duration in these two groups was 7.1±4.7 and 5.3±3.9 years (mean±SD), respectively. Control blood samples were obtained from 35 normal donors (NDs) of the Central Blood Bank of Pittsburgh. Age range was 20–72 years and the female-to-male ratio was 3:5.
Full-thickness skin biopsy specimens (3 mm) were obtained from clinically involved skin of 15 early dcSSc patients (disease duration < 2 years), 13 from the UPMC and 2 from the NIAMS Scleroderma Center of Research Translation Repository (Boston University). Site-matched normal skin samples (NS) were obtained from 13 age- and sex-matched healthy donors with no history of connective tissue diseases, recruited at the UPMC (n=8) and from the Cooperative Human Tissue Network (n=5).
Blood and skin samples were collected only from SSc patients not treated with immunosuppressive drugs or treated with low dose prednisone (30%, dose: 5–10 mg/day).
Cell isolation and culture
PBMCs purification from human blood and CD8+ T-cell isolation were performed as previously described (Fuschiotti et al., 2009). CD8+CD28− T cells were purified by negative cell sorting from the enriched CD8+ T-cell subset (Miltenyi Biotech). The purity of CD8+CD28+/− T cells (>90%) was determined by flow cytometry.
The 3 mm skin biopsies from 5 dcSSc patients and from 5 age-matched NDs were digested using the Whole Dissociation Skin kit (Miltenyi), following the manufacturer’s instructions. In preliminary experiments we compared this method with collagenase digestion (Schaerli et al., 2004) and obtained similar results in terms of yield and phenotype of isolated T cells.
In some experiments cells were cultured in complete RPMI-1640 medium (GIBCO, Invitrogen) and activated with plate-immobilized anti-CD3 (UCHT1, 5μg/ml, BD Biosciences) mAb and rhIL-2 (20 U/ml, PeproTech).
Dermal fibroblast isolation and culture were performed as previously described (Fuschiotti et al., 2013). Briefly, normal and SSc skin fibroblasts were grown to near confluence in complete DMEM medium and were serum starved overnight prior to adding aliquots of supernatant from control or SSc CD8+ T-cell subsets (1:2 dilution). For IL-13 inhibition experiments, conditioned SSc supernatants were pre-incubated for 1 hour at 37°C with an anti-IL-13 neutralizing antibo dy (0.1 μg/ml, Peprotech) before being added to fibroblasts. After 24 hours, fibroblasts and culture supernatants were harvested and Western blot was performed to measure protein levels of COL1A1. Band intensities were quantified using ImageJ (http://rsbweb.nih.gov/ij/).
Annexin V-based killing assay
Annexin V-based cytolysis assay was performed as previously described (Goldberg et al., 1999). Briefly, effector (SSc CD8+CD28− T cells, n=5) and target cells (SSc fibroblasts, n=2) were co-cultured at 5:1 ratio for 0 or 3 hours at 37°C. Cells were then pelleted by centrifugation, washed, and stained for CD8 and Annexin V expression (ebioscience), followed the manufacturer’s indications. Stained cells were analyzed by flow cytometry.
Analysis of cell surface and intracellular protein expression
Cell surface phenotype, cytokines, perforin, and granzyme B (GraB) production of purified blood and skin T-cell subsets were determined by multicolor flow cytometry as described previously (Fuschiotti et al., 2009). Degranulation assay was performed by determining cell surface expression of CD107a in cells activated for 5 hours with plate-immobilized anti-CD3 mAb as previously described (Betts et al., 2003).
Immunohistochemistry (IHC) and immunofluorescence (IF) microscopy
Paraffin-embedded skin biopsies (5mm) were stained with anti-human mouse anti-CD8 and rabbit anti-GraB (Abcam) Abs, as previously described (Fuschiotti et al., 2013). Skin samples were imaged on an Axiovert PLUS microscope (Zeiss) equipped with an Axiocam (MRC) and analyzed using the Axiovision software.
Frozen samples cryo-sectioned at 7μm were stained for double-color immunofluorescence (Geskin et al., 2015) using mouse anti-CD8 and rat anti-IL-13 antibodies (Abcam). Images were collected on an Olympus Fluoview 1000 confocal microscope equipped with an oil immersion 100× objective.
Statistical analysis
Correlation analyses were performed by calculating the Spearman rank correlation coefficient with partial correlation analysis performed to adjust for age (SAS 9.3 Software). Wilcoxon rank sum test was used to compare the modified Rodnan skin scores (mRSS) (Clements et al., 1993) with blood CD8+CD28− frequencies. Multiple group comparisons were done by one-way ANOVA followed by a post-hoc Tukey’s test (Prism, GraphPad Software). We considered p<0.05 (indicated * in Figures) as significant, p<0.01 (**) as very significant, and p<0.001 (***) as highly significant.
Supplementary Material
Acknowledgments
We thank Mary Lucas, BSN, MPH, for providing clinical details of patients, and Dana Ivanco, EMT-I, CCMA, CCRC, for recruiting eligible patients to this study (Division of Rheumatology and Clinical Immunology). We also thank Drs. Penelope Morel and John McKolanis (Department of Immunology) for providing some of the blood samples from healthy controls and Jason Devlin (Department of Cell Biology) for immunofluorescence confocal microscopy. We are grateful to Dr. Binfeng Lu (Department of Immunology) for critical reading of the manuscript.
Funding: This work was supported by National Institutes of Health /National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R03 AR065755, University of Pittsburgh and Centocor Biotech Inc. to PF. The Scleroderma Research Fund, Taub Fund (Chicago, IL), Zale Foundation (Dallas, TX), and Shoemaker Endowment (Pittsburgh, PA) to TAM; and NIH R01AR068219 to ATL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
The authors declare that no competing financial interests or conflicts exist.
References
- Abedin S, Michel JJ, Lemster B, Vallejo AN. Diversity of NKR expression in aging T cells and in T cells of the aged: the new frontier into the exploration of protective immunity in the elderly. Exp Gerontol. 2005;40:537–48. doi: 10.1016/j.exger.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254:149–54. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Wang J, Fathman JW, Lemaire R, Dorfman DM, Lafyatis R, et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc Natl Acad Sci U S A. 2007;104:2827–30. doi: 10.1073/pnas.0700021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7:269rv1. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–70. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- Dvergsten JA, Mueller RG, Griffin P, Abedin S, Pishko A, Michel JJ, et al. Premature cell senescence and T cell receptor-independent activation of CD8+ T cells in juvenile idiopathic arthritis. Arthritis Rheum. 2013;65:2201–10. doi: 10.1002/art.38015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann M, Chiu WK, Wood WH, 3rd, Levine BL, Becker KG, Weng NP. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol Rev. 2005;205:190–206. doi: 10.1111/j.0105-2896.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Fenoglio D, Battaglia F, Parodi A, Stringara S, Negrini S, Panico N, et al. Alteration of Th17 and Treg cell subpopulations co-exist in patients affected with systemic sclerosis. Clin Immunol. 2011;139:249–57. doi: 10.1016/j.clim.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Fuschiotti P. CD8(+) T cells in systemic sclerosis. Immunol Res. 2011;50:188–94. doi: 10.1007/s12026-011-8222-1. [DOI] [PubMed] [Google Scholar]
- Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA., Jr Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013;65:236–46. doi: 10.1002/art.37706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuschiotti P, Medsger TA, Jr, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009;60:1119–28. doi: 10.1002/art.24432. [DOI] [PubMed] [Google Scholar]
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. The New England journal of medicine. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- Geskin LJ, Viragova S, Stolz DB, Fuschiotti P. Interleukin-13 is over-expressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood. 2015 doi: 10.1182/blood-2014-07-590398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JE, Sherwood SW, Clayberger C. A novel method for measuring CTL and NK cell-mediated cytotoxicity using annexin V and two-color flow cytometry. J Immunol Methods. 1999;224:1–9. doi: 10.1016/s0022-1759(98)00038-6. [DOI] [PubMed] [Google Scholar]
- Greenblatt MB, Sargent JL, Farina G, Tsang K, Lafyatis R, Glimcher LH, et al. Interspecies Comparison of Human and Murine Scleroderma Reveals IL-13 and CCL2 as Disease Subset-Specific Targets. The American journal of pathology. 2012;180:1080–94. doi: 10.1016/j.ajpath.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328–32. [PubMed] [Google Scholar]
- Kahaleh MB, Fan PS. Mechanism of serum-mediated endothelial injury in scleroderma: identification of a granular enzyme in scleroderma skin and sera. Clin Immunol Immunopathol. 1997;83:32–40. doi: 10.1006/clin.1996.4322. [DOI] [PubMed] [Google Scholar]
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Medsger TA, Jr, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21:S42–6. [PubMed] [Google Scholar]
- Medsger TA, Jr, Ivanco DE, Kardava L, Morel PA, Lucas MR, Fuschiotti P. GATA-3 upregulation in CD8+ T cells is a biomarker of immune dysfunction in systemic sclerosis, resulting in excess IL-13 production. Arthritis Rheum. 2011;63:1738–47. doi: 10.1002/art.30489. [DOI] [PubMed] [Google Scholar]
- Mikulkova Z, Praksova P, Stourac P, Bednarik J, Strajtova L, Pacasova R, et al. Numerical defects in CD8+CD28− T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis. Cell Immunol. 2010;262:75–9. doi: 10.1016/j.cellimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Rice LM, Ziemek J, Stratton EA, McLaughlin SR, Padilla CM, Mathes AL, et al. A Longitudinal Biomarker for the Extent of Skin Disease in Patients with Diffuse Cutaneous Systemic Sclerosis. Arthritis & rheumatology. 2015 doi: 10.1002/art.39287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, et al. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199:1265–75. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Goldberger C, Wurzner R, Duftner C, Pfeiffer KP, Clausen J, et al. Circulating cytotoxic CD8(+) CD28(−) T cells in ankylosing spondylitis. Arthritis Res. 2002;4:71–6. doi: 10.1186/ar386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhong W, Lu X, Shi B, Zhu Y, Chen L, et al. Association of Graves’ disease and prevalence of circulating IFN-gamma-producing CD28(−) T cells. J Clin Immunol. 2008;28:464–72. doi: 10.1007/s10875-008-9213-4. [DOI] [PubMed] [Google Scholar]
- Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–40. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–35. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulunay A, Yavuz S, Direskeneli H, Eksioglu-Demiralp E. CD8+CD28−, suppressive T cells in systemic lupus erythematosus. Lupus. 2008;17:630–7. doi: 10.1177/0961203308089400. [DOI] [PubMed] [Google Scholar]
- Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–25. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7:279ra39. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–36. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem cells. 1994;12:456–65. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


