Abstract
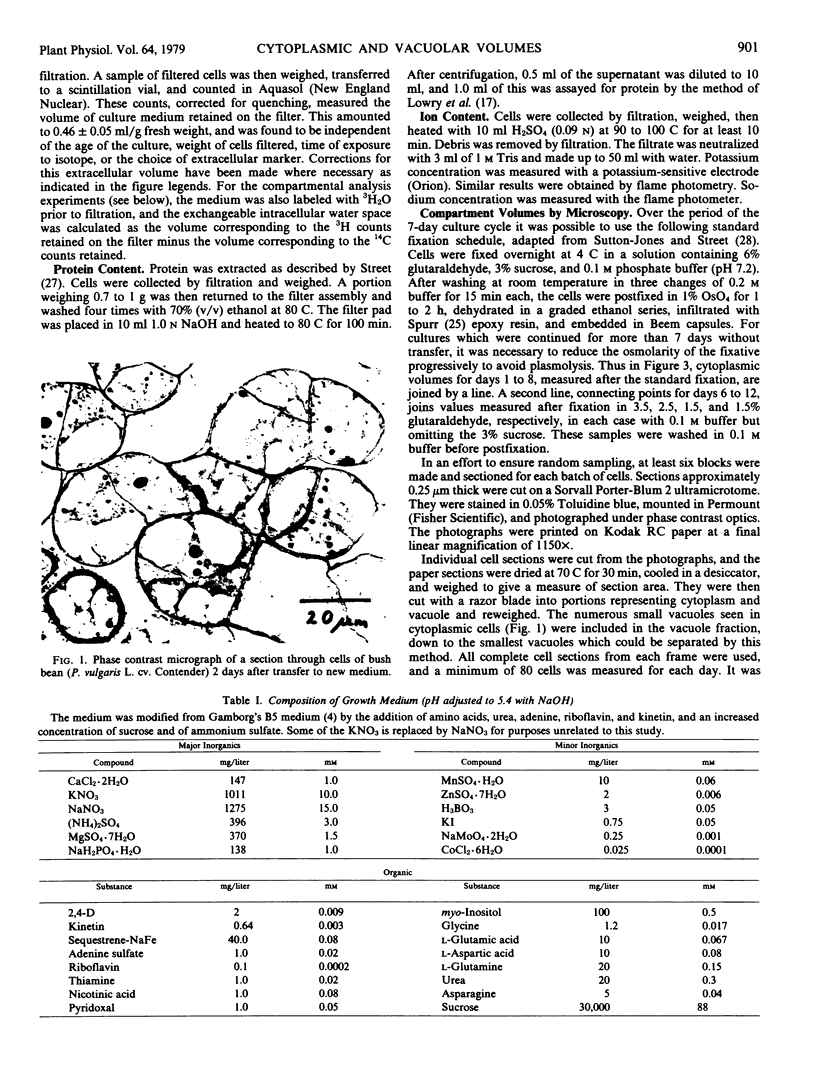
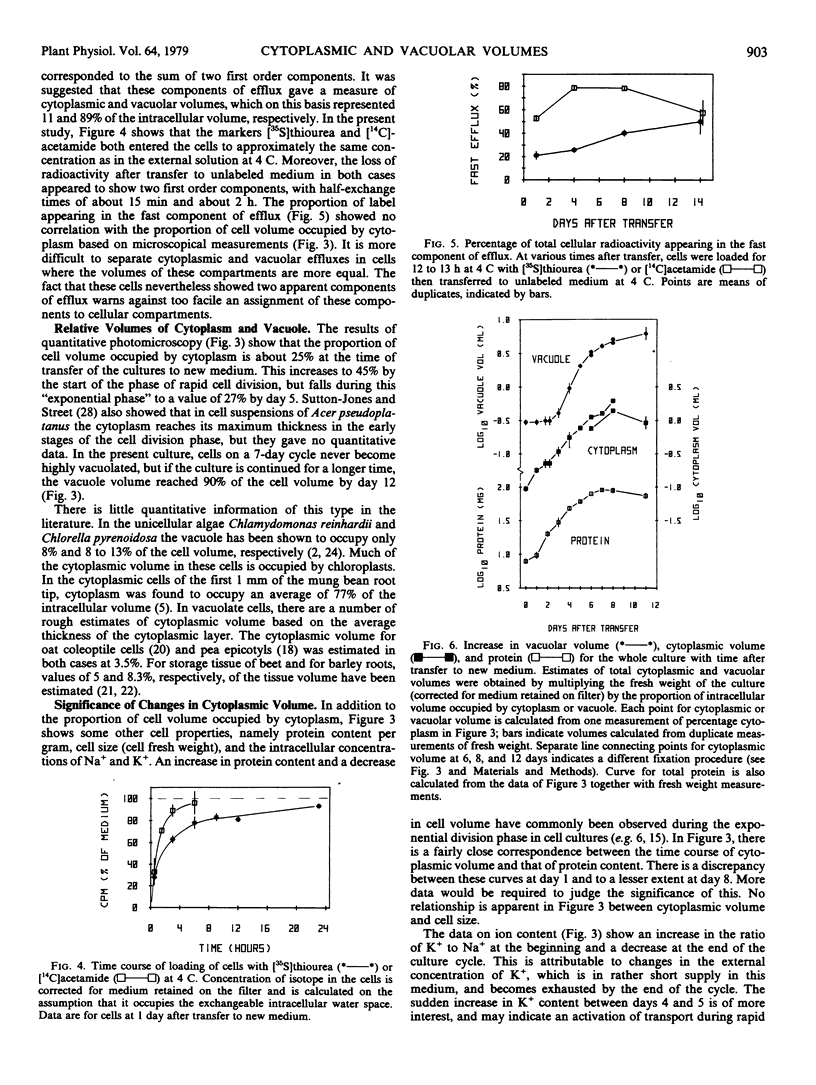
Quantitative microscopical measurements have been made of the proportion of cell volume occupied by cytoplasm in a cell suspension culture derived from cotyledons of bush bean (cv. Contender). On a 7-day culture cycle, the content of cytoplasm varies from 25% at the time of transfer to 45% at the start of the phase of rapid cell division. If the culture is continued beyond 7 days, the vacuole volume reaches 90% of cell volume by day 12.
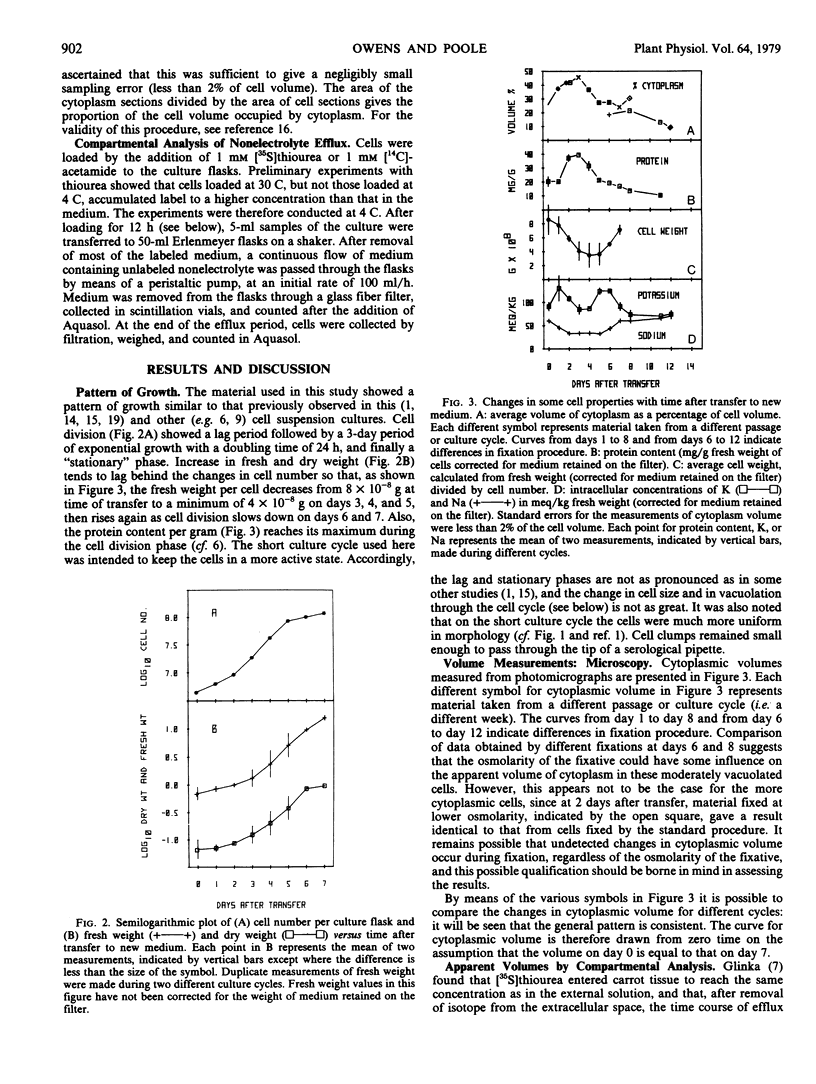
Attempts to measure relative cytoplasmic volumes by compartmental analysis of nonelectrolyte efflux were unsuccessful. The proportion of cell volume occupied by cytoplasm is roughly correlated with protein content, but shows no correlation with cell size or with intracellular concentrations of K or Na. The most striking observation is that the growth of cytoplasmic volume for the culture as a whole appears to be constant throughout the culture cycle, despite changes in the rate of cell division, cell size, rate of increase in fresh or dry weight, amount of cytoplasm per cell, the cellular concentration or fluxes of Na or K, and the rate of vacuolation. It is suggested that cytoplasmic volume is under the control of its own regulatory mechanism, which operates to give a constant exponential increase in cytoplasmic volume independently of most other observed cellular properties.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson A. W., Jr, Gunning B. E., John P. C., McCullough W. Dual mechanisms of ion absorption. Science. 1972 May 12;176(4035):694–695. doi: 10.1126/science.176.4035.694. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gerson D. F., Poole R. J. Chloride accumulation by mung bean root tips: a low affinity active transport system at the plasmalemma. Plant Physiol. 1972 Nov;50(5):603–607. doi: 10.1104/pp.50.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z. Fluxes of a nonelectrolyte and compartmentation in cells of carrot root tissue. Plant Physiol. 1974 Feb;53(2):307–311. doi: 10.1104/pp.53.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to hypertonic media. Further evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):396–412. doi: 10.1085/jgp.58.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOUD A. V., BARANY W. C., PACK B. A. QUANTITATIVE EVALUATION OF CYTOPLASMIC STRUCTURES IN ELECTRON MICROGRAPHS. Lab Invest. 1965 Jun;14:996–1008. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Active and passive transport of potassium in cells of excised pea epicotyls. Plant Physiol. 1970 Feb;45(2):133–138. doi: 10.1104/pp.45.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Simulation of Cl Uptake by Low-salt Barley Roots as a Test of Models of Salt Uptake. Plant Physiol. 1969 Oct;44(10):1417–1427. doi: 10.1104/pp.44.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schötz F., Bathelt H., Arnold C. G., Schimmer O. Die Architektur und Organisation der Chlamydomonas-Zelle. Ergebnisse der Elektronenmikroskopie von Serienschnitten und der daraus resultierenden dreidimensionalen Rekonstruktion. Protoplasma. 1972;75(3):229–254. doi: 10.1007/BF01279818. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]