Abstract
The protein tyrosine phosphatase PTP1B is a negative regulator of insulin signaling and a therapeutic target for type 2 diabetes. Our previous studies have shown that the closely related tyrosine phosphatase TCPTP might also contribute to the regulation of insulin receptor (IR) signaling in vivo (S. Galic, M. Klingler-Hoffmann, M. T. Fodero-Tavoletti, M. A. Puryer, T. C. Meng, N. K. Tonks, and T. Tiganis, Mol. Cell. Biol. 23:2096-2108, 2003). Here we show that PTP1B and TCPTP function in a coordinated and temporally distinct manner to achieve an overall regulation of IR phosphorylation and signaling. Whereas insulin-induced phosphatidylinositol 3-kinase/Akt signaling was prolonged in both TCPTP−/− and PTP1B−/− immortalized mouse embryo fibroblasts (MEFs), mitogen-activated protein kinase ERK1/2 signaling was elevated only in PTP1B-null MEFs. By using phosphorylation-specific antibodies, we demonstrate that both IR β-subunit Y1162/Y1163 and Y972 phosphorylation are elevated in PTP1B−/− MEFs, whereas Y972 phosphorylation was elevated and Y1162/Y1163 phosphorylation was sustained in TCPTP−/− MEFs, indicating that PTP1B and TCPTP differentially contribute to the regulation of IR phosphorylation and signaling. Consistent with this, suppression of TCPTP protein levels by RNA interference in PTP1B−/− MEFs resulted in no change in ERK1/2 signaling but caused prolonged Akt activation and Y1162/Y1163 phosphorylation. These results demonstrate that PTP1B and TCPTP are not redundant in insulin signaling and that they act to control both common as well as distinct insulin signaling pathways in the same cell.
The insulin receptor (IR) is a transmembrane protein tyrosine kinase (PTK) that upon binding insulin phosphorylates itself as well as target substrates, such as the IR substrate 1 (IRS-1), Cbl, and p52Shc (3, 45, 57, 58). These phosphorylation events allow for the recruitment and activation of signaling pathways, including the Ras/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways that mediate the metabolic, transcriptional, and mitogenic actions of insulin. Insulin signaling is integral to the regulation of glucose homeostasis acting in the liver, striated muscle, and adipose tissue to promote glucose uptake and glycogen synthesis as well as to inhibit glycogenolysis and gluconeogenesis (3, 45, 57, 58). Insulin resistance in liver, muscle, and fat is the underlying pathogenic feature of type 2 diabetes and is attributable to defects in insulin receptor signaling (45). It is important to note that the IR is also expressed in most other tissues of the human body, including red blood cells (17, 42), endothelial cells (28, 32), and neuronal tissue (5), and it may serve to control varied biological processes, including testes determination (39), ageing (50), body weight, and reproduction (5). Indeed, dysfunctional insulin signaling in endothelial cells may contribute to the vascular complications associated with diabetes (4, 28, 32), whereas insulin resistance in neuronal tissue may predispose individuals to the development of neurodegenerative disorders (46). Given the important role of insulin signaling in various biological responses, it is necessary that insulin signaling be tightly controlled. Protein tyrosine phosphatases (PTPs) catalyze the dephosphorylation of tyrosyl-phosphorylated proteins (56) and are known to be important negative regulators of insulin receptor signaling (8, 19).
The endoplasmic reticulum-targeted protein tyrosine phosphatase PTP1B is particularly important in IR regulation and is a physiological regulator of glucose homeostasis (12, 18, 29, 30). Mice lacking PTP1B exhibit enhanced insulin sensitivity attributable to increased IR phosphorylation in liver and muscle (12, 30). Moreover, antisense oligonucleotides that suppress PTP1B expression in mouse and rat animal models of insulin resistance can enhance insulin sensitivity and normalize blood glucose (22, 43, 63). Although substantial data indicate that PTP1B dephosphorylates the IR and possibly IRS-1 (18, 20, 29), the detailed mechanism by which PTP1B regulates IR activation and signaling and the relative contribution of other PTPs to IR inactivation remain unclear.
TCPTP is a ubiquitous tyrosine-specific phosphatase in which the catalytic domain has a high degree of primary (72% identity, 86% similarity; TCPTP residues 43 to 288) and tertiary structure similarity to that of PTP1B (2, 25, 27). Two splice variants of TCPTP are expressed: a 48-kDa form (TC48) which, like PTP1B, is targeted to the endoplasmic reticulum, and a shorter 45-kDa form (TC45) that has access to both nuclear and cytoplasmic substrates (16, 25, 35, 48, 54). Both forms are expressed in humans, whereas only TC45 is expressed in mice (16, 26, 48, 52). Previously we have shown that TCPTP can recognize the IR as a cellular substrate and that IR activation and signaling are enhanced in cells that lack TCPTP (16). In response to insulin, TCPTP-D182A substrate-trapping mutants formed stable complexes with tyrosine-phosphorylated IR, and both IR phosphorylation and PI3K/Akt signaling were elevated or prolonged in TCPTP−/− mouse embryo fibroblast (MEFs) compared to phosphorylation and signaling of TCPTP+/+ or TCPTP (TC45 or TC48)-reconstituted MEFs. In addition, the suppression of TCPTP protein levels in human hepatoma HepG2 cells results in increased insulin-induced Akt signaling (38), and TC45 has been shown to be inactivated by reactive oxygen species that are produced in response to insulin (38), as has been shown for PTP1B (36-38, 55). Although these studies affirm that TCPTP has an integral role in IR regulation in vivo, it remains unclear whether TCPTP acts concurrently with PTP1B to regulate insulin signaling.
In this study, we have examined the relative contribution of PTP1B and TCPTP to IR activation and signaling. We have compared insulin signaling in PTP1B−/− versus TCPTP−/− cells and used RNA interference to knock down TCPTP expression in PTP1B−/− cells. Our studies indicate that despite their high degree of catalytic domain sequence identity, PTP1B and TCPTP are not redundant phosphatases but instead act in a temporally distinct manner and cooperatively regulate IR phosphorylation and signaling in the same cell.
MATERIALS AND METHODS
Materials.
Insulin from bovine pancreas was purchased from Sigma (St. Louis, Mo.), and hygromycin B, Opti-MEM I, and Lipofectamine 2000 were from Invitrogen (Carlsbad, Calif.). Monoclonal IR β-subunit (IRβ; clone CT-3l) and IRS-1 (clone 8-63) antibodies used for immunoblotting and IR β-subunit (clone CT-l) antibody used for immunoprecipitation were purchased from NeoMarkers (Fremont, Calif.), polyclonal IR β-subunit phosphospecific pYpY1162/1163 and pY972 antibodies were from BioSource International (Camarillo, Calif.), and goat antiactin (SC-1616) was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Polyclonal phospho-Akt (Ser473) and Akt antibodies and monoclonal anti-phospho-ERK1/2 were from Cell Signaling (Beverly, Mass.). Anti-PTP1B FG6 was supplied by D. Hill (Calbiochem Oncogene Research Products, Cambridge, Mass.), the monoclonal phosphotyrosine (pTyr) G98 antibody has been described previously (54), and the monoclonal pTyr 4G10 was from Upstate Biotechnology (Lake Placid, N.Y.). The monoclonal TCPTP 3E2 antibody and the TCPTP−/− (EFM14) and TCPTP+/+ (EFM11) mouse embryo fibroblast cell lines (26) were provided by M. L. Tremblay (McGill University, Quebec, Canada).
Preparation of siRNA oligonucleotide.
To design specific small interfering RNA (siRNA) duplexes, we scanned the coding sequence of TCPTP and selected sequences of 5′AA(N19)3′ (where N is any nucleotide). We performed BLAST searches against human and mouse genome databases to ensure specificity for TCPTP. Oligonucleotide 5′AACAGATACAGAGATGTAAGC3′ and oligonucleotide 5′AAGATTGACAGACACCTAAAT3′ for mouse and 5′AAGATTGACAGACAACTAATA3′ for human were chosen for generation of siRNA1, siRNA2m, and siRNA2h, respectively. The oligonucleotide 5′AACGTACGCGGAATACTTCGA3′, targeting the coding region of the firefly luciferase gene, was chosen for the generation of an siRNA control. Twenty-one-nucleotide siRNA duplexes were generated by utilizing the Silencer siRNA Construction kit (Ambion) according to the manufacturer's instructions; reagents for the generation of the glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) siRNA control were provided in the kit.
Cell culture.
Spontaneously immortalized TCPTP−/− (EFM14) and TCPTP+/+ (EFM11) MEFs (26) and immortalized PTP1B−/− MEFs (23, 60) were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum (FBS), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. PTP1B−/− cells reconstituted with PTP1B (+PTP1B) have been described previously (23, 60). In these cells, wild-type human PTP1B had been reconstituted by retroviral means, and pools of infected cells rather than individual clones were selected to avoid potential clone-to-clone variations. +PTP1B cells were cultured in the same medium plus 100 μg of hygromycin B/ml, but all experiments were performed in the absence of hygromycin B. Where indicated, cells were serum starved for 4 h in medium containing 0.1% (vol/vol) FBS.
Transient transfection with siRNAs.
Prior to transfection with siRNA, PTP1B−/− cells were incubated in antibiotic-free DMEM containing 5% FBS for 16 to 24 h. siRNA1 was used routinely at 100 nM, whereas siRNA2 (siRNA2m and siRNA2h) was used at 40 nM; luciferase or GAPDH control siRNAs were used at the corresponding concentrations. Transfections were performed overnight with Opti-MEM I without serum and antibiotics by using Lipofectamine 2000 (Invitrogen) and the indicated siRNAs according to the manufacturer's instructions. At 24 h posttransfection, cells were seeded equally into 6-well plates in DMEM containing 5% FBS, and at 48 h they were serum starved for 2 h and then stimulated with insulin.
Immunoblotting.
Cells were lysed in NP-40 buffer (50 mM Tris [pH 7.5], 1% [wt/vol] NP-40, 150 mM NaCl, 50 mM NaF, 1 mM sodium orthovanadate, leupeptin [5 μg/ml], pepstatin [1 μg/ml], 1 mM benzamidine, 2 mM phenylmethylsulfonyl fluoride) and clarified by centrifugation (12,000 × g, 5 min, 4°C). Equal amounts of proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with the indicated antibodies. When comparing either TCPTP−/− to TCPTP+/+ cells, PTP1B−/− to +PTP1B cells, or cells treated with or without siRNAs, insulin stimulations were undertaken at the same time and proteins from the respective cell lines were resolved by SDS-PAGE and transferred onto the same Immobilon-P membrane (Millipore, Bedford, Mass.) for immunoblot analysis.
Immunoprecipitations.
TCPTP−/− and TCPTP+/+ or PTP1B−/− and +PTP1B cells were serum starved and stimulated with 10 nM insulin for the indicated times. Cells were lysed in NP-40 buffer, and the lysates were precleared with Pansorbin (Calbiochem, Cambridge, Mass.) for 30 min at 4°C. The precleared lysates were clarified by centrifugation (12,000 × g for 5 min at 4°C) and supernatants were incubated with 1 to 2 μg of IR β-subunit antibody to precipitate the IR for 16 h at 4°C under constant mixing. Immune complexes were collected on protein G-Sepharose 4 Fast Flow (Pharmacia, Uppsala, Sweden) for 60 min at 4°C with agitation, washed four times with NP-40 buffer and once with 50 mM Tris (pH 7.5), and then resolved by SDS-PAGE and immunoblotted as indicated.
RESULTS
Insulin signaling in PTP1B−/− and TCPTP−/− cells.
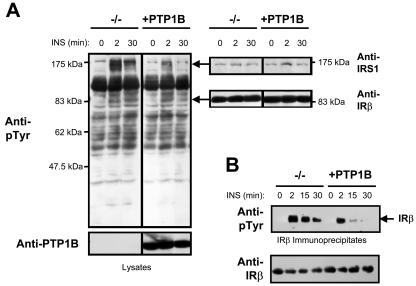
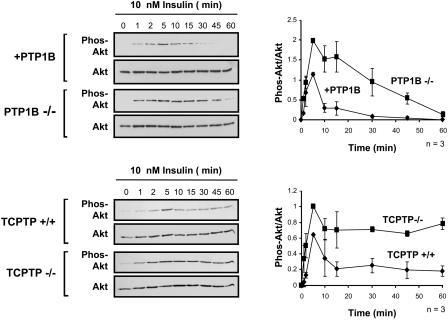
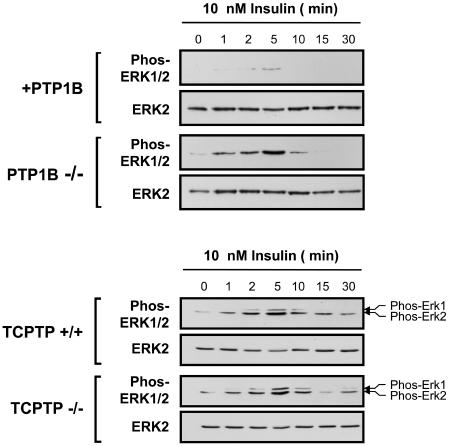
As a first step towards elucidating the relative contributions of PTP1B and TCPTP to insulin signaling, we examined the insulin-induced tyrosine phosphorylation status of proteins in PTP1B−/− MEFs compared to those in which PTP1B had been reconstituted stably (23, 60) (Fig. 1). These cell lines expressed similar levels of insulin-signaling proteins, including the IR β-subunit, IRS-1, PI3K, protein kinase Akt, and the MAPK ERK1/2 (Fig. 1). In response to insulin, the phosphorylation state of several proteins was enhanced in cells lacking PTP1B (Fig. 2A). In particular, tyrosine phosphorylation of a protein comigrating with IRS-1, as detected by immunoblot analysis using phosphotyrosine-specific antibodies, was enhanced dramatically in PTP1B−/− MEFs (Fig. 2A). In addition, in response to insulin, IRβ pTyr was enhanced significantly in PTP1B-null cells (Fig. 2A and B). Hence, these results are consistent with those of previous studies demonstrating that IRβ and IRS-1 phosphorylation are enhanced by approximately 1.75- to 2.5-fold in the livers and skeletal muscle of PTP1B knockout mice following bolus insulin administration (12). In contrast to PTP1B-null cells, our previous studies have shown that although IRβ pTyr was enhanced and/or prolonged in TCPTP−/− cells in response to insulin, elevated IRS-1 pTyr could not be detected (16). Hence, we reasoned that PTP1B and TCPTP may differentially regulate insulin signaling in vivo. To explore this possibility, we compared the activation of the PI3K/Akt pathway, which mediates many of the metabolic actions of insulin, to the Ras/MAPK pathway, which promotes transcription and mitogenesis (45), in TCPTP−/− versus TCPTP+/+ MEFs and PTP1B−/− versus PTP1B-reconstituted MEFs. PI3K/Akt and Ras/MAPK activation were monitored by immunoblot analysis utilizing antibodies specific for the phosphorylated and activated forms of Akt and ERK1/2, respectively (Fig. 3 and 4). Whereas Akt phosphorylation was elevated and/or prolonged in cells that lacked either TCPTP or PTP1B compared to that of their wild-type or reconstituted counterparts (Fig. 3), ERK1/2 phosphorylation was enhanced only in PTP1B-null cells (Fig. 4), indicating that TCPTP and PTP1B may differentially regulate insulin-induced MAPK signaling in vivo. Consistent with results for TCPTP−/− versus TCPTP+/+ MEFs (Fig. 4), no significant difference in ERK1/2 signaling was observed between TCPTP−/− and TC45-reconstituted cells (data not shown), whereas Akt phosphorylation is prolonged in TCPTP-null versus TCPTP-reconstituted cells (16). Notably, no difference in epidermal growth factor (EGF)-induced PI3K/Akt signaling was observed in TCPTP−/− versus TCPTP+/+ cells (data not shown), whereas others have reported no difference in platelet-derived growth factor (PDGF)-induced MAPK or Akt signaling in TCPTP−/− cells (26, 40). Furthermore, EGF- or PDGF-induced Akt signaling is not altered in PTP1B-null cells (23) and ERK1/2 signaling is either minimally enhanced or otherwise diminished in response to PDGF, EGF, or insulin-like growth factor-1 in the same PTP1B−/− background (6, 7, 9, 23). Hence, signaling is not enhanced in general in cells lacking either TCPTP or PTP1B, and the contribution of the two phosphatases to the regulation of cellular signaling can vary depending on the stimulus.
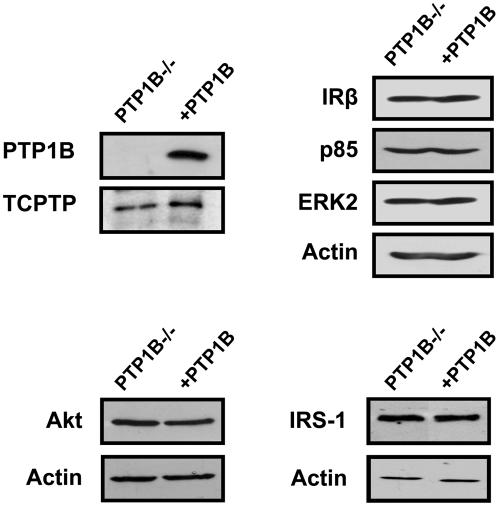
FIG. 1.
Expression of signaling proteins mediating insulin action in PTP1B−/− and PTP1B-reconstituted fibroblasts. Equal amounts of protein from PTP1B−/− and PTP1B-reconstituted (+PTP1B) fibroblasts were resolved by SDS-PAGE and immunoblotted with antibodies specific for PTP1B, TCPTP, IRβ, IRS-1, the p85 regulatory subunit of PI3K, the protein kinase Akt, the MAPK ERK2, and actin.
FIG. 2.
IRβ and IRS-1 tyrosine phosphorylation are enhanced in PTP1B-null cells. (A) PTP1B−/− and PTP1B-reconstituted (+PTP1B) fibroblasts were deprived of serum for 4 h and stimulated with 10 nM insulin (INS) for the indicated times. Cells were lysed in NP-40 buffer and clarified by centrifugation, and proteins were resolved by SDS-PAGE and immunoblotted with phosphotyrosine (pTyr)-specific antibodies. They were then stripped and reprobed with IRβ- or IRS-1-specific antibodies. Molecular mass standards (Prestained; New England BioLabs) are indicated. (B) PTP1B−/− and +PTP1B fibroblasts were serum starved for 4 h and stimulated with 10 nM insulin for the indicated times. IRβ immunoprecipitates were resolved by SDS-PAGE and immunoblotted as indicated.
FIG. 3.
Insulin-induced PI3K/Akt signaling is prolonged in PTP1B−/− and TCPTP−/− cells. Either PTP1B−/− and +PTP1B fibroblasts or TCPTP−/− and TCPTP+/+ fibroblasts were deprived of serum and stimulated with 10 nM insulin for the indicated times. Total lysates were prepared in 3× Laemmli buffer, resolved by SDS-PAGE, immunoblotted with antibodies to the phosphorylated and activated Akt (Phos-Akt), and then stripped and reprobed for total Akt. Representative immunoblots are shown. The graphs on the right-hand side show the Phos-Akt immunoblots quantitated by densitometric analysis and normalized for total Akt protein in the corresponding Akt immunoblots, with the Phos-Akt/Akt ratio in the absence of insulin being set at zero. Units shown are arbitrary and are the means ± standard errors of four independent experiments.
FIG. 4.
Insulin-induced MAPK signaling is enhanced in PTP1B−/− but not TCPTP−/− cells. Either PTP1B−/− and +PTP1B fibroblasts or TCPTP−/− and TCPTP+/+ fibroblasts were deprived of serum and stimulated with 10 nM insulin for the indicated times. Total lysates were prepared in 3× Laemmli buffer, resolved by SDS-PAGE and immunoblotted with antibodies to the phosphorylated and activated ERK1/2 (Phos-ERK1/2), and then stripped and reprobed for total ERK2. The immunoblots shown are representative of at least three independent experiments.
RNAi-mediated suppression of TCPTP.
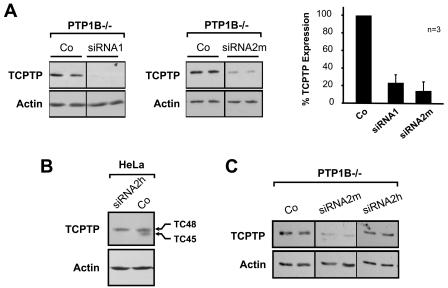
To examine whether PTP1B and TCPTP might act concurrently to regulate insulin signaling, we established RNA interference (RNAi) (10) for the suppression of TCPTP expression in PTP1B−/− MEFs. We utilized two TCPTP-specific small interfering RNAs, designated siRNA1 (targeting human and murine TCPTP) and siRNA2m (targeting murine TC45) as well as siRNAs targeting the firefly luciferase gene or the glyceraldehyde 3-phosphate dehydrogenase gene as negative controls. Both TCPTP-specific siRNAs suppressed TCPTP expression by roughly 80 to 90% (Fig. 5A) compared to that of PTP1B−/− cells treated with transfection reagent alone (data not shown) or cells transfected with either luciferase (Fig. 5A) or GAPDH control siRNA (data not shown). siRNA1 targets both TCPTP variants, TC45 and TC48 (data not shown), whereas siRNA2m targets the C-terminal PRLTDT sequence that is present in TC45 but not TC48, the endoplasmic reticulum-targeted TCPTP variant (25, 53). TC48 protein is not detectable in mouse fibroblasts (16, 26, 48, 52), but it is present in human cells. siRNA2h targeting human TC45 (the coding sequence for PRLTDT in human TC45 differs by three nucleotides from that in murine TC45) suppressed the expression of TC45 but not TC48 in human cell lines, including HeLa (Fig. 5B) and U2OS (data not shown) cells, but had no effect on the expression of endogenous TCPTP in the murine PTP1B−/− cells (Fig. 5C). In addition, neither siRNA1 nor siRNA2m had any effect on the expression of actin, Akt, ERK2 (Fig. 6), or IRβ (see Fig. 9), indicating that the TCPTP siRNAs act specifically and do not suppress the expression of heterologous proteins.
FIG. 5.
siRNA-mediated suppression of TCPTP expression. (A) PTP1B−/− cells were transfected with TCPTP-specific siRNAs (siRNA1 targets TC45 and TC48, and siRNA2m targets murine TC45). Cells were lysed in NP-40 buffer and clarified by centrifugation, and proteins were resolved by SDS-PAGE and immunoblotted with TCPTP or actin antibodies. Representative immunoblots are shown. TCPTP suppression from three independent experiments was quantified and normalized for actin. (B) HeLa cells were transfected with the human TC45-specific siRNA (siRNA2h, which differs from siRNA2m by three nucleotides), and cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies specific for TCPTP or actin. (C) PTP1B−/− murine fibroblasts were transfected with mouse (siRNA2m) or human (siRNA2h) TC45-specific siRNA, and cell lysates were resolved by SDS-PAGE and immunoblotted for TCPTP and actin. Co, control.
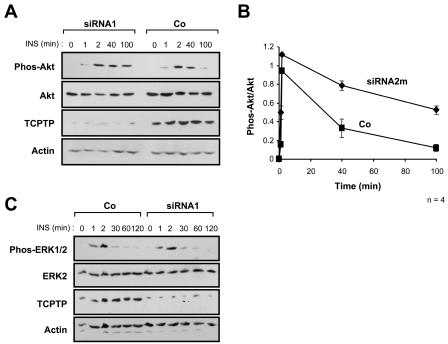
FIG. 6.
Suppression of TCPTP expression in PTP1B−/− cells prolongs PI3K/Akt but not MAPK signaling. PTP1B−/− cells were transfected with TCPTP-specific siRNA1 (A and C), siRNA2m (B), or luciferase control siRNA (Co). At 24 h posttransfection, cells were serum starved and stimulated with 10 nM insulin (INS) as indicated. Cells were lysed in NP-40 buffer and clarified by centrifugation, and proteins were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. In panels A and C the immunoblots are representative of at least three independent experiments. (B) Phosphorylated Akt (Phos-Akt) immunoblots from siRNA2m versus control-transfected cells were quantitated by densitometric analysis and normalized for total Akt protein in the corresponding Akt immunoblots, with the Phos-Akt/Akt ratio in the absence of insulin being set at zero. Units shown are arbitrary and are the means ± standard errors of four independent experiments.
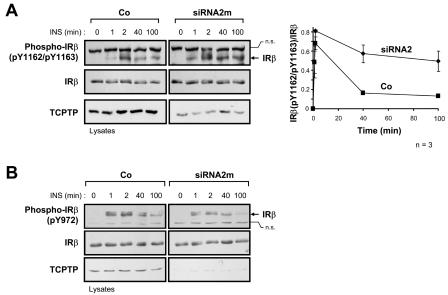
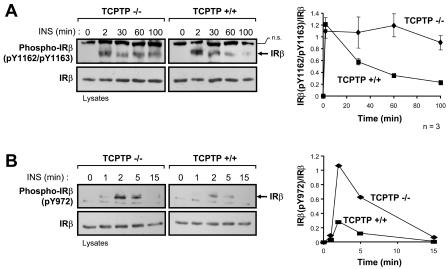
FIG. 9.
Suppression of TCPTP expression in PTP1B−/− cells prolongs IRβ Y1162/Y1163 phosphorylation. PTP1B−/− cells were transfected with TCPTP-specific siRNA2m or luciferase control siRNA (Co). At 24 h posttransfection, cells were serum starved and stimulated with 10 nM insulin (INS) as indicated. Cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies to IRβ phosphorylated on (A) Y1162/Y1163 [Phospho-IRβ(pY1162/pY1163)] or (B) Y972 [Phospho-IRβ(pY972)] and then stripped and reprobed for IRβ. IRβ is indicated by an arrow in the phosphorylation immunoblots. Nonspecific proteins (n.s.) recognized by either the Phospho-IRβ(pY1162/pY1163) antibody or the Phospho-IRβ(pY972) antibody are also indicated and confirm equal protein loading. The immunoblots shown are representative of three independent experiments. The graph on the right-hand side shows Phospho-IRβ(pY1162/pY1163) immunoblots quantitated by densitometric analysis and normalized for total IRβ protein in the corresponding IRβ immunoblots, with the Phospho-IRβ(pY1162/pY1163)/IRβ ratio in the absence of insulin being set at zero. Units shown are arbitrary and are the means ± standard errors of three independent experiments.
Effect of TCPTP suppression on insulin signaling in PTP1B-null cells.
Next we sought to suppress TCPTP expression in PTP1B-null cells by using RNA interference (10) to examine whether insulin signaling was enhanced further. We found that the suppression of TCPTP expression with either siRNA1 or siRNA2m resulted in prolonged PI3K/Akt signaling as monitored by the phosphorylation status of Akt (Fig. 6A and B). While Akt phosphorylation declined at 40 to 60 min in both PTP1B−/− untransfected cells (Fig. 3) and control siRNA-transfected cells (Fig. 6A and B), phosphorylation of Akt declined more slowly and was elevated for at least 100 min in PTP1B−/− cells in which TCPTP expression was suppressed with TCPTP-specific siRNAs (Fig. 6A and B). Importantly, siRNA2h targeting of human TC45 (Fig. 5B), which did not suppress TCPTP expression in PTP1B−/− cells (Fig. 5C), had no effect on Akt phosphorylation (data not shown), demonstrating that the suppression of TCPTP expression was essential for prolonged PI3K/Akt signaling. Moreover, we found that suppression of TCPTP in PTP1B−/− cells with either siRNA1 (Fig. 6C) or siRNA2m (data not shown) had no effect on ERK1/2 activation, demonstrating that signaling in general is not enhanced in cells treated with TCPTP-specific siRNAs. Taken together with the results shown in Fig. 4, these data confirm that TCPTP does not regulate insulin-induced ERK1/2 signaling. Importantly, these results demonstrate that TCPTP and PTP1B together contribute to the regulation of insulin-induced PI3K signaling. Hence, despite their high degree of sequence identity, this study affirms that at least in a cellular context, PTP1B and TCPTP are not redundant but instead can cooperate to regulate both common and distinct PTK-mediated signaling events in the same cell.
Regulation of IR phosphorylation by PTP1B and TCPTP.
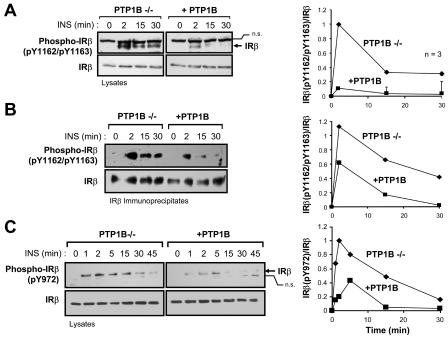
To provide insight into the molecular mechanisms by which TCPTP and PTP1B differentially regulate insulin signaling, we next examined their role in IR dephosphorylation. Upon binding insulin, the IR autophosphorylates on Y1162/Y1163 in the activation loop of the IR PTK catalytic domain; Y1162/Y1163 phosphorylation is required for IR activation and subsequent phosphorylation of other IR sites, such as the juxtamembrane Y972 site that contributes to IRS-1 recruitment (11). The substrate-binding surfaces of the TCPTP and PTP1B catalytic domains allow for the recognition of proteins phosphorylated on tandem tyrosines (44). In vitro, both PTP1B and TCPTP can dephosphorylate the tandem Y1162/Y1163 phosphorylation site (41, 44), and overexpression studies utilizing TCPTP substrate-trapping mutants (14, 54) have shown that TCPTP has the capacity to recognize this site in a cellular context (16). We utilized phosphorylation-specific antibodies to compare IRβ Y1162/Y1163 and Y972 phosphorylation in PTP1B−/− and TCPTP−/− cells (Fig. 7 and 8). Consistent with the above-mentioned in vitro studies, phosphorylation of IRβ on Y1162/Y1163 was elevated in PTP1B-null cells compared to that of PTP1B-reconstituted cells (Fig. 7A and B), demonstrating for the first time that in vivo, the phosphorylation state of the Y1162/Y1163 site is regulated by PTP1B. This is consistent with preliminary data demonstrating that phosphorylation of the Y1162/Y1163 site is enhanced in the livers of PTP1B-null mice (F. G. Haj and B. G. Neel, unpublished data). As expected, the enhanced Y1162/Y1163 phosphorylation in PTP1B-null cells coincided with enhanced Y972 phosphorylation (Fig. 7C) and enhanced phosphorylation of a protein comigrating with IRS-1 (Fig. 2A), both of which might have occurred as a consequence of elevated IRβ Y1162/Y1163 phosphorylation and IR activation. However, although IRβ phosphorylation was elevated in PTP1B-null cells, the kinetics of phosphorylation were similar to those observed in PTP1B-reconstituted cells (Fig. 7), peaking at ∼2 min and declining thereafter, indicating that additional PTPs must dephosphorylate these sites in vivo. In contrast to PTP1B-null cells, phosphorylation of the tandem Y1162/Y1163 site was not increased at early time points of insulin stimulation in TCPTP−/− cells compared to TCPTP+/+ cells (Fig. 8A). Instead, Y1162/Y1163 phosphorylation in TCPTP−/− cells was sustained relative to TCPTP+/+ cells (Fig. 8A). Hence, these results indicate that TCPTP and PTP1B may differentially contribute to Y1162/Y1163 dephosphorylation. The elevated Y1162/Y1163 phosphorylation in PTP1B-null cells versus the sustained Y1162/Y1163 phosphorylation in TCPTP-null cells might be indicative of temporal differences in PTP1B and TCPTP action that may arise from spatial limitations. Whereas PTP1B is targeted to the endoplasmic reticulum (15) and might have restricted access to the IR following its endocytosis, TC45 has access to proteins in different subcellular compartments (25). In addition to sustained Y1162/Y1163 phosphorylation, we found that Y972 phosphorylation was enhanced in TCPTP−/− cells relative to that of TCPTP+/+ cells at time points where Y1162/Y1163 phosphorylation was not significantly altered (Fig. 8), indicating that TCPTP might independently dephosphorylate the IRβ on Y972. Hence, these results indicate that TCPTP can regulate both the Y1162/Y1163 and Y972 IRβ phosphorylation sites in vivo. In accordance with this, we found that Y1162/Y1163 phosphorylation was prolonged when TCPTP expression in PTP1B−/− cells was suppressed with TCPTP-specific siRNAs (Fig. 9A). In contrast, suppression of TCPTP in PTP1B-null cells did not result in any significant further enhancement of Y972 phosphorylation (Fig. 9B). These results indicate that PTP1B and TCPTP can act in a coordinated manner to regulate IR phosphorylation in vivo and that their concordant actions control the intensity and duration of insulin signaling, respectively.
FIG. 7.
IRβ Y1162/Y1163 and Y972 phosphorylation are enhanced in cells that lack PTP1B. PTP1B−/− and +PTP1B fibroblasts were deprived of serum and stimulated with 10 nM insulin (INS) as indicated. Cells were lysed in NP-40 buffer and clarified by centrifugation. (A) Clarified cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies specific for IRβ phosphorylated on Y1162/Y1163 [Phospho-IRβ(pY1162/pY1163)], and then they were stripped and reprobed with IRβ antibodies. IRβ in the Phospho-IRβ immunoblot is indicated by an arrow. A nonspecific protein (n.s.) recognized by the phosphorylation antibody is also shown and confirms equal protein loading. The graph on the right shows Phospho-IRβ(pY1162/pY1163) immunoblots quantitated by densitometric analysis and normalized for total IRβ protein in the corresponding IRβ immunoblots, with the Phospho-IRβ(pY1162/pY1163)/IRβ ratio in the absence of insulin being set at zero. Units shown are arbitrary and are the means ± standard errors of three independent experiments. (B) IRβ immunoprecipitates were resolved by SDS-PAGE, immunoblotted with Phospho-IRβ(pY1162/pY1163) antibodies, and then stripped and reprobed for IRβ. The immunoblot shown is representative of two independent experiments. (C) Proteins from cell lysates were resolved by SDS-PAGE, immunoblotted with antibodies specific for IRβ phosphorylated on Y972 [Phospho-IRβ(pY972)], and then stripped and reprobed for IRβ. The immunoblot shown is representative of at least three independent experiments. IRβ in the Phospho-IRβ(pY972) immunoblot is indicated by an arrow. A nonspecific protein (n.s.) that is recognized variably by the phosphorylation antibody is also indicated. The graphs at the right-hand side of panels B and C show the Phospho-IRβ immunoblots quantitated by densitometric analysis and normalized for total IRβ protein in the corresponding IRβ immunoblots, with the Phospho-IRβ/IRβ ratio in the absence of insulin being set at zero. Units are arbitrary.
FIG. 8.
Phosphorylation of the IRβ on Y972 is enhanced, whereas Y1162/Y1163 phosphorylation is prolonged in TCPTP-null cells. TCPTP−/− and TCPTP+/+ fibroblasts were deprived of serum and stimulated with 10 nM insulin (INS) as indicated. Cells were lysed in NP-40 buffer and clarified by centrifugation, and proteins were resolved by SDS-PAGE and immunoblotted with antibodies to the phosphorylated IRβ. (A) Immunoblots of cell lysates were probed with antibodies specific for the IRβ Y1162/1163 phosphorylation site [Phospho-IRβ(pY1162/pY1163)] and then stripped and reprobed for IRβ. IRβ is indicated by an arrow in the phosphorylation immunoblot. A nonspecific protein (n.s.) recognized by the phosphorylation antibody is also shown and confirms equal protein loading. The graph on the right-hand side shows Phospho-IRβ(pY1162/pY1163) immunoblots quantitated by densitometric analysis and normalized for total IRβ protein in the corresponding IRβ immunoblots, with the Phospho-IRβ(pY1162/pY1163)/IRβ ratio in the absence of insulin being set at zero. Units shown are arbitrary and are the means ± standard errors of three independent experiments. (B) Immunoblots were probed with antibodies specific for the IRβ Y972 phosphorylation site [Phospho-IRβ(pY972)] and then stripped and reprobed with IRβ antibodies. Similar results were observed in two independent experiments. IRβ in the phosphorylation immunoblot is indicated by an arrow. A nonspecific protein (n.s.) recognized by the phosphorylation antibody is also indicated and confirms equal protein loading. The graph at the right-hand side shows the Phospho-IRβ(pY972) immunoblot quantitated by densitometric analysis and normalized for total IRβ protein, with the Phospho-IRβ(pY972)/IRβ ratio in the absence of insulin being set at zero. Units shown are arbitrary.
DISCUSSION
In this study we have shown that PTP1B and TCPTP, two closely related enzymes in the PTP family, act to regulate IR phosphorylation within the same cell. Our results demonstrate that PTP1B and TCPTP are not redundant phosphatases but instead that they cooperate to control the duration and intensity of insulin signaling.
We found that the loss of either PTP1B or TCPTP resulted in prolonged PI3K/Akt signaling and that the combined loss of the two phosphatases, through the suppression of TCPTP expression in PTP1B-null cells, further prolonged signaling. Whereas Akt signaling in PTP1B-null cells persisted for 40 to 60 min, loss of TCPTP in these cells resulted in Akt activation being maintained for at least 100 min. This coincided with the distinct IR Y1162/Y1163 phosphorylation kinetics in TCPTP−/− versus PTP1B−/− MEFs. Although Y1162/Y1163 phosphorylation was elevated significantly in PTP1B−/− cells relative to PTP1B-reconstituted cells, Y1162/Y1163 phosphorylation declined after 2 min with kinetics similar to those observed for PTP1B-reconstituted cells. In contrast, Y1162/Y1163 phosphorylation was not elevated in TCPTP-null cells but was sustained for at least 100 min, analogous to the sustained activation of Akt. In a previous study, we also noted sustained total IR pTyr in TCPTP−/− versus TCPTP+/+ cells (16). These results suggest that although both PTPs may act on the Y1162/Y1163 site and may thus regulate IR activation and signaling, the mechanisms by which PTP1B and TCPTP act on Y1162/Y1163 may differ. Although we cannot exclude the possibility that PTP1B and TCPTP exhibit differences in their affinities for the Y1162/Y1163 site, the high degree of active-site identity and similarity for the PTP1B and TCPTP catalytic domains (2, 25, 27, 41) suggests that this may not be a major factor. Instead, our results indicate that PTP1B and TCPTP act in a temporally distinct manner to regulate phosphorylation; whereas PTP1B acts on this site early in IR signaling to control the intensity of IR activation and signaling, TCPTP serves primarily to modulate the duration of IR signaling by acting on Y1162/Y1163 at later time points. This supposition was borne out by the prolonged Y1162/Y1163 phosphorylation in PTP1B-null cells upon RNAi-mediated suppression of TCPTP. The differential kinetics of dephosphorylation of the IR by PTP1B and TCPTP might be attributable to their distinct subcellular localizations, but further studies are needed to address this issue.
In PTP1B−/− cells, Y972 and IRS phosphorylation were also elevated in response to insulin, and this coincided with elevated Y1162/Y1163 phosphorylation. Although we cannot exclude the possibility that PTP1B might act on the Y972 site and IRS proteins directly, it is probable that their enhanced phosphorylation was attributable to enhanced Y1162/Y1163 phosphorylation and IR activation. In contrast to PTP1B-null cells, Y972 phosphorylation in TCPTP−/− cells was elevated at early time points of insulin stimulation where no significant difference was noted for the Y1162/Y1163 site, indicating that TC45 acts directly on Y972 in vivo. These results indicate that TCPTP differentially regulates IR phosphorylation sites in vivo and that TCPTP might act in distinct subcellular compartments to mediate its effects on insulin signaling.
Finally, in PTP1B-null cells, insulin-induced ERK1/2 signaling was elevated, consistent with results of other studies demonstrating that overexpressed PTP1B can suppress insulin-induced ERK1/2 signaling (47). However, no difference was observed in ERK1/2 signaling in TCPTP−/− cells and no further increase in ERK1/2 signaling was observed in PTP1B−/− cells after RNAi-mediated TCPTP suppression. These results indicate that TCPTP and PTP1B differentially regulate insulin-induced mitogen-activated protein kinase signaling in vivo. The activation of ERK1/2 in response to insulin can occur by various means including the direct phosphorylation of the adaptor protein Shc and recruitment of Grb2 or via IRS-1 phosphorylation and Grb2 recruitment (3). It is unlikely that the elevated ERK1/2 signaling in PTP1B−/− cells was attributable to enhanced Y972 phosphorylation, because this site was hyperphosphorylated in both PTP1B−/− and TCPTP−/− MEFs. Instead, we speculate that the elevated ERK1/2 activation in PTP1B-null cells may have been attributable to the elevated Y1162/Y1163 phosphorylation and IR activation, because Y1162/Y1163 phosphorylation was not elevated in TCPTP-null cells. The elevated IRS-1 pTyr in PTP1B−/− cells might have also contributed to the enhanced ERK1/2 signaling, but whether this occurred as a consequence of the increased Y1162/Y1163 phosphorylation and IR activation or whether PTP1B acts directly on IRS-1, as has been proposed previously (20), remains unknown.
Whereas our studies indicate that PTP1B and TCPTP might be the primary phosphatases regulating IR Y1162/Y1163 phosphorylation in vivo, our results also demonstrate that additional PTPs must act on the IR, because Y972 continued to be dephosphorylated in TCPTP−/− and PTP1B−/− cells and in PTP1B−/− cells following TCPTP suppression, at time points where IR Y1162/Y1163 phosphorylation was either elevated or sustained. Previous studies have reported that the tyrosine phosphatase LAR can also regulate insulin signaling (1, 24, 33, 34, 61, 62), but whether it acts on Y972 directly is unknown. Further studies are clearly warranted to identify the PTP(s) involved in the dephosphorylation of the Y972 site as well as other sites on the IR, such as those in the C terminus which also contribute to signaling (13, 21, 31, 49, 51).
A major hallmark in the development of type 2 diabetes is the resistance of peripheral tissues to insulin action. Insulin resistance is thought to be attributable to defects or aberrations in signaling events downstream of the IR, with mutations in the IR itself being rare (45). One approach for enhancing insulin action and alleviating insulin resistance could involve the inhibition of PTPs otherwise involved in the suppression of insulin signaling (18, 29). Indeed, the ability of PTP1B antisense oligonucleotides to normalize blood glucose levels in diabetic animal models has seen such oligonucleotides move into phase II clinical trials (ISIS113715; ISIS Pharmaceuticals, Carlsbad, Calif.), whereas PTP1B inhibitory drugs are in preclinical development (18, 29, 55). Although TCPTP is expressed abundantly in liver and skeletal muscle (T. Tiganis, unpublished observations), it is not clear whether TCPTP regulates glucose homeostasis in vivo, because TCPTP−/− mice have severe hematopoietic defects and die soon after birth (59), precluding metabolic analyses. Moreover, it is possible that TCPTP may otherwise play a more prominent role in IR regulation elsewhere, such as the brain, where the IR controls body weight and reproduction (5). Ultimately, the generation of mice with targeted inactivation of TCPTP in insulin-responsive tissues may provide a definitive assessment of its contribution to IR function in vivo.
Our studies show for the first time that multiple PTPs can act to regulate IR activation and signaling in the same cell. We demonstrate that PTP1B and TCPTP can act in unison to control IR phosphorylation to regulate both common and distinct insulin signaling pathways. Our results attest to the specific, nonredundant nature of even closely related phosphatases, such as PTP1B and TCPTP, and have general implications for the PTP family, emphasizing their exquisite specificity and their capacity to act in a highly coordinated manner in vivo.
Acknowledgments
We thank Karen Lee and Wong Wen Seen for technical assistance and Catherine van Vliet for critical reading of the manuscript.
This work was supported by the NHMRC of Australia (T.T.) and the National Institutes of Health (CA53840 to N.K.T., DK60838 to B.G.N., and DK60839 to B.B.K.). T.T. is a Monash University Logan Fellow.
REFERENCES
- 1.Ahmad, F., and B. J. Goldstein. 1997. Functional association between the insulin receptor and the transmembrane protein-tyrosine phosphatase LAR in intact cells. J. Biol. Chem. 272:448-457. [PubMed] [Google Scholar]
- 2.Andersen, J. N., O. H. Mortensen, G. H. Peters, P. G. Drake, L. F. Iversen, O. H. Olsen, P. G. Jansen, H. S. Andersen, N. K. Tonks, and N. P. Moller. 2001. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 21:7117-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan, P. 2001. Insulin signalling. J. Cell Sci. 114:1429-1430. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee, M. 2001. Biochemistry and molecular cell biology of diabetic complications. Nature 414:813-820. [DOI] [PubMed] [Google Scholar]
- 5.Bruning, J. C., D. Gautam, D. J. Burks, J. Gillette, M. Schubert, P. C. Orban, R. Klein, W. Krone, D. Muller-Wieland, and C. R. Kahn. 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122-2125. [DOI] [PubMed] [Google Scholar]
- 6.Buckley, D. A., A. Cheng, P. A. Kiely, M. L. Tremblay, and R. O'Connor. 2002. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol. Cell. Biol. 22:1998-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, A., G. S. Bal, B. P. Kennedy, and M. L. Tremblay. 2001. Attenuation of adhesion-dependent signaling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J. Biol. Chem. 276:25848-25855. [DOI] [PubMed] [Google Scholar]
- 8.Drake, P. G., and B. I. Posner. 1998. Insulin receptor-associated protein tyrosine phosphatase(s): role in insulin action. Mol. Cell. Biochem. 182:79-89. [PubMed] [Google Scholar]
- 9.Dube, N., A. Cheng, and M. L. Tremblay. 2004. The role of protein tyrosine phosphatase 1B in Ras signaling. Proc. Natl. Acad. Sci. USA 101:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dykxhoorn, D. M., C. D. Novina, and P. A. Sharp. 2003. Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 4:457-467. [DOI] [PubMed] [Google Scholar]
- 11.Eck, M. J., S. Dhe-Paganon, T. Trub, R. T. Nolte, and S. E. Shoelson. 1996. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell 85:695-705. [DOI] [PubMed] [Google Scholar]
- 12.Elchebly, M., P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C. C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay, and B. P. Kennedy. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544-1548. [DOI] [PubMed] [Google Scholar]
- 13.Faria, T. N., V. A. Blakesley, H. Kato, B. Stannard, D. LeRoith, and C. T. Roberts, Jr. 1994. Role of the carboxyl-terminal domains of the insulin and insulin-like growth factor I receptors in receptor function. J. Biol. Chem. 269:13922-13928. [PubMed] [Google Scholar]
- 14.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangioni, J. V., P. H. Beahm, V. Shifrin, C. A. Jost, and B. G. Neel. 1992. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell 68:545-560. [DOI] [PubMed] [Google Scholar]
- 16.Galic, S., M. Klingler-Hoffmann, M. T. Fodero-Tavoletti, M. A. Puryer, T. C. Meng, N. K. Tonks, and T. Tiganis. 2003. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol. Cell. Biol. 23:2096-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambhir, K. K., J. A. Archer, and C. J. Bradley. 1978. Characteristics of human erythrocyte insulin receptors. Diabetes 27:701-708. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein, B. J. 2002. Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J. Clin. Endocrinol. Metab. 87:2474-2480. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein, B. J., F. Ahmad, W. Ding, P. M. Li, and W. R. Zhang. 1998. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol. Cell. Biochem. 182:91-99. [PubMed] [Google Scholar]
- 20.Goldstein, B. J., A. Bittner-Kowalczyk, M. F. White, and M. Harbeck. 2000. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 275:4283-4289. [DOI] [PubMed] [Google Scholar]
- 21.Gottschalk, W. K., R. Lammers, and A. Ullrich. 1992. The carboxy terminal 110 amino acid portion of the insulin receptor is important for insulin signalling to pyruvate dehydrogenase. Biochem. Biophys. Res. Commun. 189:906-911. [DOI] [PubMed] [Google Scholar]
- 22.Gum, R. J., L. L. Gaede, S. L. Koterski, M. Heindel, J. E. Clampit, B. A. Zinker, J. M. Trevillyan, R. G. Ulrich, M. R. Jirousek, and C. M. Rondinone. 2003. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes 52:21-28. [DOI] [PubMed] [Google Scholar]
- 23.Haj, F. G., B. Markova, L. D. Klaman, F. D. Bohmer, and B. G. Neel. 2003. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J. Biol. Chem. 278:739-744. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto, N., E. P. Feener, W. R. Zhang, and B. J. Goldstein. 1992. Insulin receptor protein-tyrosine phosphatases. Leukocyte common antigen-related phosphatase rapidly deactivates the insulin receptor kinase by preferential dephosphorylation of the receptor regulatory domain. J. Biol. Chem. 267:13811-13814. [PubMed] [Google Scholar]
- 25.Ibarra-Sanchez, M., P. D. Simoncic, F. R. Nestel, P. Duplay, W. S. Lapp, and M. L. Tremblay. 2000. The T-cell protein tyrosine phosphatase. Semin. Immunol. 12:379-386. [DOI] [PubMed] [Google Scholar]
- 26.Ibarra-Sanchez, M. J., J. Wagner, M. T. Ong, C. Lampron, and M. L. Tremblay. 2001. Murine embryonic fibroblasts lacking TC-PTP display delayed G1 phase through defective NF-κB activation. Oncogene 20:4728-4739. [DOI] [PubMed] [Google Scholar]
- 27.Iversen, L. F., K. B. Moller, A. K. Pedersen, G. H. Peters, A. S. Petersen, H. S. Andersen, S. Branner, S. B. Mortensen, and N. P. Moller. 2002. Structure determination of T cell protein tyrosine phosphatase. J. Biol. Chem. 20:20. [DOI] [PubMed] [Google Scholar]
- 28.Jiang, Z. Y., Y. W. Lin, A. Clemont, E. P. Feener, K. D. Hein, M. Igarashi, T. Yamauchi, M. F. White, and G. L. King. 1999. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Investig. 104:447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, T. O., J. Ermolieff, and M. R. Jirousek. 2002. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug. Discov. 1:696-709. [DOI] [PubMed] [Google Scholar]
- 30.Klaman, L. D., O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker-Krongrad, G. I. Shulman, B. G. Neel, and B. B. Kahn. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20:5479-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohanski, R. A. 1993. Insulin receptor autophosphorylation. II. Determination of autophosphorylation sites by chemical sequence analysis and identification of the juxtamembrane sites. Biochemistry 32:5773-5780. [DOI] [PubMed] [Google Scholar]
- 32.Kuboki, K., Z. Y. Jiang, N. Takahara, S. W. Ha, M. Igarashi, T. Yamauchi, E. P. Feener, T. P. Herbert, C. J. Rhodes, and G. L. King. 2000. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 101:676-681. [DOI] [PubMed] [Google Scholar]
- 33.Kulas, D. T., B. J. Goldstein, and R. A. Mooney. 1996. The transmembrane protein-tyrosine phosphatase LAR modulates signaling by multiple receptor tyrosine kinases. J. Biol. Chem. 271:748-754. [DOI] [PubMed] [Google Scholar]
- 34.Kulas, D. T., W. R. Zhang, B. J. Goldstein, R. W. Furlanetto, and R. A. Mooney. 1995. Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J. Biol. Chem. 270:2435-2438. [DOI] [PubMed] [Google Scholar]
- 35.Lam, M. H., B. J. Michell, M. T. Fodero-Tavoletti, B. E. Kemp, N. K. Tonks, and T. Tiganis. 2001. Cellular stress regulates the nucleocytoplasmic distribution of the protein tyrosine phosphatase TCPTP. J. Biol. Chem. 276:37700-37707. [DOI] [PubMed] [Google Scholar]
- 36.Mahadev, K., H. Motoshima, X. Wu, J. M. Ruddy, R. S. Arnold, G. Cheng, J. D. Lambeth, and B. J. Goldstein. 2004. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 24:1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahadev, K., A. Zilbering, L. Zhu, and B. J. Goldstein. 2001. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J. Biol. Chem. 276:21938-21942. [DOI] [PubMed] [Google Scholar]
- 38.Meng, T. C., D. A. Buckley, S. Galic, T. Tiganis, and N. K. Tonks. 2004. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 279:37716-37725. [DOI] [PubMed] [Google Scholar]
- 39.Nef, S., S. Verma-Kurvari, J. Merenmies, J. D. Vassalli, A. Efstratiadis, D. Accili, and L. F. Parada. 2003. Testis determination requires insulin receptor family function in mice. Nature 426:291-295. [DOI] [PubMed] [Google Scholar]
- 40.Persson, C., C. Savenhed, A. Bourdeau, M. L. Tremblay, B. Markova, F. D. Bohmer, F. G. Haj, B. G. Neel, A. Elson, C. H. Heldin, L. Ronnstrand, A. Ostman, and C. Hellberg. 2004. Site-selective regulation of platelet-derived growth factor beta receptor tyrosine phosphorylation by T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 24:2190-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran, C., R. Aebersold, N. K. Tonks, and D. A. Pot. 1992. Sequential dephosphorylation of a multiply phosphorylated insulin receptor peptide by protein tyrosine phosphatases. Biochemistry 31:4232-4238. [DOI] [PubMed] [Google Scholar]
- 42.Robinson, T. J., J. A. Archer, K. K. Gambhir, V. W. Hollis, Jr., L. Carter, C. Bradley, and C. J. Bradley. 1979. Erythrocytes: a new cell type for the evaluation of insulin receptor defects in diabetic humans. Characteristics of human erythrocyte insulin receptors. Science 205:200-202. [DOI] [PubMed] [Google Scholar]
- 43.Rondinone, C. M., J. M. Trevillyan, J. Clampit, R. J. Gum, C. Berg, P. Kroeger, L. Frost, B. A. Zinker, R. Reilly, R. Ulrich, M. Butler, B. P. Monia, M. R. Jirousek, and J. F. Waring. 2002. Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes 51:2405-2411. [DOI] [PubMed] [Google Scholar]
- 44.Salmeen, A., J. N. Andersen, M. P. Myers, N. K. Tonks, and D. Barford. 2000. Molecular basis for recognition and dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol. Cell 6:1401-1412. [DOI] [PubMed] [Google Scholar]
- 45.Saltiel, A. R., and C. R. Kahn. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799-806. [DOI] [PubMed] [Google Scholar]
- 46.Schubert, M., D. Gautam, D. Surjo, K. Ueki, S. Baudler, D. Schubert, T. Kondo, J. Alber, N. Galldiks, E. Kustermann, S. Arndt, A. H. Jacobs, W. Krone, C. R. Kahn, and J. C. Bruning. 2004. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 101:3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu, S., H. Maegawa, K. Egawa, K. Shi, M. Bryer-Ash, and A. Kashiwagi. 2002. Mechanism for differential effect of protein-tyrosine phosphatase 1B on Akt versus mitogen-activated protein kinase in 3T3-L1 adipocytes. Endocrinology 143:4563-4569. [DOI] [PubMed] [Google Scholar]
- 48.Simoncic, P. D., A. Lee-Loy, D. L. Barber, M. L. Tremblay, and C. J. McGlade. 2002. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 12:446-453. [DOI] [PubMed] [Google Scholar]
- 49.Tartare, S., I. Mothe, A. Kowalski-Chauvel, J. P. Breittmayer, R. Ballotti, and E. Van Obberghen. 1994. Signal transduction by a chimeric insulin-like growth factor-1 (IGF-1) receptor having the carboxyl-terminal domain of the insulin receptor. J. Biol. Chem. 269:11449-11455. [PubMed] [Google Scholar]
- 50.Tatar, M., A. Bartke, and A. Antebi. 2003. The endocrine regulation of aging by insulin-like signals. Science 299:1346-1351. [DOI] [PubMed] [Google Scholar]
- 51.Tavare, J. M., R. M. O'Brien, K. Siddle, and R. M. Denton. 1988. Analysis of insulin-receptor phosphorylation sites in intact cells by two-dimensional phosphopeptide mapping. Biochem. J. 253:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ten Hoeve, J., M. J. Ibarra-Sanchez, Y. Fu, W. Zhu, M. Tremblay, M. David, and K. Shuai. 2002. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell. Biol. 22:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiganis, T. 2002. Protein tyrosine phosphatases: dephosphorylating the epidermal growth factor receptor. IUBMB Life 53:3-14. [DOI] [PubMed] [Google Scholar]
- 54.Tiganis, T., A. M. Bennett, K. S. Ravichandran, and N. K. Tonks. 1998. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 18:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonks, N. K. 2003. PTP1B: from the sidelines to the front lines! FEBS Lett. 546:140-148. [DOI] [PubMed] [Google Scholar]
- 56.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 57.White, M. F. 1997. The insulin signalling system and the IRS proteins. Diabetologia 2(Suppl. 40):S2-S17. [DOI] [PubMed] [Google Scholar]
- 58.White, M. F. 1998. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182:3-11. [PubMed] [Google Scholar]
- 59.You-Ten, K. E., E. S. Muise, A. Itie, E. Michaliszyn, J. Wagner, S. Jothy, W. S. Lapp, and M. L. Tremblay. 1997. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zabolotny, J. M., K. K. Bence-Hanulec, A. Stricker-Krongrad, F. Haj, Y. Wang, Y. Minokoshi, Y. B. Kim, J. K. Elmquist, L. A. Tartaglia, B. B. Kahn, and B. G. Neel. 2002. PTP1B regulates leptin signal transduction in vivo. Dev. Cell. 2:489-495. [DOI] [PubMed] [Google Scholar]
- 61.Zabolotny, J. M., Y. B. Kim, O. D. Peroni, J. K. Kim, M. A. Pani, O. Boss, L. D. Klaman, S. Kamatkar, G. I. Shulman, B. B. Kahn, and B. G. Neel. 2001. Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc. Natl. Acad. Sci. USA 98:5187-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, W. R., P. M. Li, M. A. Oswald, and B. J. Goldstein. 1996. Modulation of insulin signal transduction by ectopic overexpression of the receptor-type protein-tyrosine phosphatase LAR. Mol. Endocrinol. 10:575-584. [DOI] [PubMed] [Google Scholar]
- 63.Zinker, B. A., C. M. Rondinone, J. M. Trevillyan, R. J. Gum, J. E. Clampit, J. F. Waring, N. Xie, D. Wilcox, P. Jacobson, L. Frost, P. E. Kroeger, R. M. Reilly, S. Koterski, T. J. Opgenorth, R. G. Ulrich, S. Crosby, M. Butler, S. F. Murray, R. A. McKay, S. Bhanot, B. P. Monia, and M. R. Jirousek. 2002. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc. Natl. Acad. Sci. USA 99:11357-11362. [DOI] [PMC free article] [PubMed] [Google Scholar]