Abstract
During gestation, fetal nutrition is entirely dependent on maternal diet. Maternal consumption of excess fat during pregnancy has been linked to an increased risk of neurologic disorders in offspring, including attention deficit/hyperactivity disorder, autism, and schizophrenia. In a mouse model, high-fat diet (HFD)–fed offspring have cognitive and executive function deficits as well as whole-genome DNA and promoter-specific hypomethylation in multiple brain regions. Dietary methyl donor supplementation during pregnancy or adulthood has been used to alter DNA methylation and behavior. Given that extensive brain development occurs during early postnatal life—particularly within the prefrontal cortex (PFC), a brain region critical for executive function—we examined whether early life methyl donor supplementation (e.g., during adolescence) could ameliorate executive function deficits observed in offspring that were exposed to maternal HFD. By using operant testing, progressive ratio, and the PFC-dependent 5-choice serial reaction timed task (5-CSRTT), we determined that F1 female offspring (B6D2F1/J) from HFD-fed dams have decreased motivation (decreased progressive ratio breakpoint) and require a longer stimulus length to complete the 5-CSRTT task successfully, whereas early life methyl donor supplementation increased motivation and shortened the minimum stimulus length required for a correct response in the 5-CSRTT. Of interest, we found that expression of 2 chemokines, CCL2 and CXCL10, correlated with the median stimulus length in the 5-CSRTT. Furthermore, we found that acute adult supplementation of methyl donors increased motivation in HFD-fed offspring and those who previously received supplementation with methyl donors. These data point to early life as a sensitive time during which dietary methyl donor supplementation can alter PFC-dependent cognitive behaviors.—McKee, S. E., Grissom, N. M., Herdt, C. T., Reyes, T. M. Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet–fed dams.
Keywords: folate, one carbon metabolism, vitamin B12, choline, operant
Over the past 3 decades, overweight and obesity rates have more than doubled (1, 2). Pregnancy is a particularly vulnerable time during which women are susceptible to excessive weight gain, as >75% of women who are pregnant will gain excessive weight during pregnancy (3). This maternal obesity can lead to an increased risk for neurologic disorders in offspring, such as attention deficit/hyperactivity disorder (ADHD), autism, and schizophrenia (4–7). Excessive gestational weight gain can be modeled in mice by feeding dams a 60% high-fat diet (HFD) from the onset of breeding through pregnancy and lactation (8, 9). Furthermore, we have previously shown that offspring from dams that were fed an HFD have cognitive and executive function deficits (e.g., impulsivity) in an operant 5-choice serial reaction timed task (5-CSRTT) (10).
DNA methylation—the addition of a methyl group to a cytosine base within the DNA sequence—is an epigenetic mark that, in part, regulates gene expression (11). DNA methylation is hypothesized to link the gestational environment with adult disease (12) via persistent changes in gene expression. In autism, schizophrenia, and ADHD, aberrant DNA methylation and methyltransferase function have been implicated in development of these diseases and cognitive dysfunction (13–15). In our mouse model, we have shown that offspring who were exposed to maternal HFD have whole-genome DNA hypomethylation in multiple brain regions, including the prefrontal cortex (PFC), along with promoter-specific hypomethylation and dysregulated gene expression (9). In addition, expression of 2 methyltransferase enzymes, DNA methyltransferase 1 and catechol-o-methyltransferase, in the PFC correlate with overall poor performance in operant behavioral tasks (10), which suggests that maternal HFD exposure disrupts methyltransferase function, possibly leading to altered gene expression and behavioral deficits.
Methylation reactions, including DNA methylation, require dietary consumption of a group of nutrients known as methyl donors—folate, vitamin B12, methionine, zinc, betaine, and choline (16, 17). Pregnant obese women have lower plasma concentrations of methyl donors and this is correlated with levels found in the fetus (18). The explanation for these lower levels is unclear, but may include differences in bioavailability or metabolism of methyl donors in the context of higher adiposity (19) or decreased intake of recommended multivitamins (20). Lowered concentrations could be one mechanism by which aberrant DNA methylation is induced, which results in increased disease risk for offspring. Conversely, methyl donor supplementation (MS) can alter DNA methylation, RNA gene expression, and behavior in mice (12, 21, 22), and supplementation of maternal HFD with methyl donors during pregnancy has been shown to ameliorate palatable food preference, increase locomotor activity and gene expression (23), alter promotor-specific methylation and gene expression (24), and prevent metabolic disease (25, 26). Beyond the early life window, MS in adults has been shown to affect cognitive end points. Adult MS can improve memory function (27) and has been used to target synaptic dysfunction in Alzheimer’s disease (28) as well as slow memory decline and improve processing speed in some populations (29).
Given that MS during pregnancy or in adulthood alters gene expression and cognitive and behavioral end points, we wanted to determine whether early life supplementation (e.g., in adolescence, when the PFC is still developing) could alter performance in a cognitive task. The human brain continues to develop extensively after birth (30), and the PFC is the last brain region to mature (31, 32), not reaching functional capacity until late adolescence (33). Behavioral tasks, such as the 5-CSRTT, can be used to assess PFC-mediated executive function. Deficits in PFC-mediated executive function are central to ADHD, autism, and schizophrenia (34–36); therefore, in the present studies, we used the 5-CSRTT behavioral task to determine whether early life MS could block or attenuate PFC-mediated behavioral deficits that are associated with maternal HFD exposure. In addition, targeted gene expression analysis of the PFC was used to identify potential molecular mechanisms that underlie behavioral changes, with a focus on genes related to neurotransmission, neuroinflammation, and epigenetics.
MATERIALS AND METHODS
Animals and experimental model
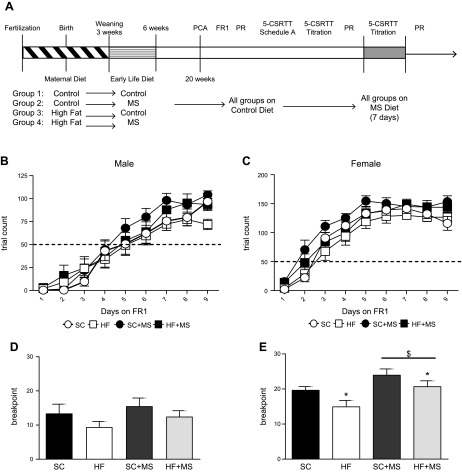
All animals were cared for according to the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. An experimental timeline is given in Fig. 1A. C57BL/6J virgin females were bred with DBA/2J males. B6D2F1/J hybrid mice were used in our work as the hybrid background was more similar to heterogeneity that was observed in humans, as opposed to a pure inbred strain. All animals were fed control diet before breeding (Table 1). From the onset of breeding through pregnancy and lactation, dams (n = 10/group) were fed 1 of 2 purified diets (Test Diet, Richmond, IN, USA), standard control diet (SC) or 60% HFD (9, 37). Specific diet formulations are shown in Table 1. Litters were culled to 8–9 pups, as necessary, to equalize access to nutrition throughout lactation. At weaning, 1–2 offspring of each sex from each litter were randomly assigned to 1 of 2 diets (to control for litter or maternal effects), control or control diet plus MS (Test Diet), and were group housed with 5 animals per cage. At 6 wk, all offspring were returned to control diet for the remainder of the experiment. (Specific litter information: litter numbers; SC: n = 8, HFD: n = 10; pups reared; SC: n = 67, HFD: n = 86; initial experimental group size; male: SC: n = 15, HFD: n = 15, SC + MS: n = 15, HFD + MS: n = 15; female: SC: n = 15, HFD: n = 13, SC + MS: n = 15, HFD + MS: n = 18.)
Figure 1.
Maternal and postnatal diet alter cognition. A) Mouse dams are fed either SC or HFD throughout gestation and lactation. Offspring were weaned either onto SC or MS diets, creating 4 experimental groups. Male and female offspring were then tested in a series of operant tasks. B, C) Neither maternal nor postnatal diet impacted animals’ ability to learn the FR1 task (male, left; female, right). D) Male offspring motivation is not altered by diet. E) Female HFD-fed offspring are less motivated to work for reinforcer on a PR task. Female offspring supplemented with methyl donors are more motivated to work for reinforcer. (n: male: SC = 15, HFD = 15, SC + MD = 14, HFD + MS = 15; female: SC = 14, HFD = 13, SC + MS = 15, HFD + MS = 18 in all figures.) PCA, pavlovian conditioned approach. *P < 0.05 main effect of maternal diet; $P < 0.05 main effect of MS.
TABLE 1.
Diet information
| Variable | SC (no. 5755) | HFD (58G9) | MS (no. 5AAX) |
|---|---|---|---|
| Fat (%) | 22.1 | 59.9 | 23.2 |
| Carbohydrate (%) | 59.6 | 21.4 | 51.7 |
| Protein (%) | 18.3 | 18.6 | 19.7 |
| Choline chloride (ppm) | 1400 | 1784 | 11,900 |
| Betaine (%) | ND | ND | 1.50 |
| Folic acid (ppm) | 4.2 | 5.1 | 15,004 |
| Vitamin B12 (μg/kg) | 24 | 25 | 72 |
| l-Methionine (%) | 0.15 | 0.19 | 0.90 |
| Zinc (ppm) | 27 | 35 | 178 |
ND, not detected.
Behavior
Operant behavior testing occurred as previously described (10). In brief, male and female animals were group housed (5 mice/cage) in a reverse light 12-h light/dark cycle (0900 lights off), so testing could be performed during the active period (lights off). Animals were food restricted to 90% of free feeding weight. Before training, animals were pre-exposed to the palatable reinforcer (Yoohoo; Mott’s, Plano, TX, USA) for 24 h to decrease neophobic responses and ensure that all animals consumed the reinforcer.
Operant behavior training
Male and female animals were trained simultaneously in a 9-hole operant chamber (Lafayette Instruments, Lafayette, IN, USA). The back of the chamber contained 9 holes with recessed lights visible to the animal. Chambers detected nosepokes to any of the 9 holes by infrared beam breaks. A magazine, where reinforcer was deposited, was located at the front of the chamber. Infrared beam breaks upon magazine entry were also recorded. Magazine training (pavlovian conditioned approach) was administered for the first 14 d. In magazine training, the back center light was turned on for 8 s, then terminated. Upon termination, a droplet of reinforcer was delivered to the magazine at the front of the chamber (30 trials/30 min training session).
After magazine training, fixed ratio 1 (FR1) training began. The back center hole (hole 5) remained lit until one nosepoke was made to this hole. The light was then terminated and the reinforcer was dispensed into the magazine. When the animal completed a magazine entry, the cycle repeated. Responses to the other 8 holes did not initiate reinforcer. Each animal completed one 30-min session each day and responses were recorded each day. Criterion for task acquisition was 50 nosepoke responses within the 30-min session. Animals that failed to acquire nosepoke response were eliminated from FR1 and progressive ratio (PR) analyses (number of animals that failed to learn: male: 1 HFD, 3 HFD + MS; female: 1 HFD, 1 SC + MS).
PR testing occurred after 2.5 wk of FR1 training. The number of nosepokes to dispense reinforcer now increased arithmetically every third trial (i.e., 1, 1, 1, 2, 2, 2, 4, 4, 4, 7, 7, 7...). Sessions continued for 60 min or until the animal made no nosepoke response for 5 min. Breakpoint was measured at the trial in which the animal no longer made a nosepoke for the reinforcer.
Animals that met the FR1 criterion continued onto 5-CSRTT schedule A training, which has been previously described (10, 38). In brief, animals initiated training by completion of a magazine entry. This triggered an intertrial interval (ITI) by which the animal waited for the illumination of one of the 5 odd-numbered holes at the back of the chamber. The animal was then required to nosepoke the illuminated hole for completion of a correct trial. A premature trial was recorded if an animal made a response during ITI. If an animal responded to an unilluminated nokepoke hole, then an incorrect trial was recorded. If no response was made, an omitted trial was recorded. Only a response at the correct hole during the 8-s stimulus or a 2-s limited hold after stimulus termination was reinforced. Performance measures are reported for the first day at which the criterion (>20 responses, with 50% correct) was met.
Once the criterion was met for schedule A training, animals were transitioned to 5-CSRTT titration schedule, a more challenging version of the task, as described in Martin et al. (39). In brief, in contrast to schedule A training in which the stimulus duration and ITI remained constant throughout the session, within a single session of the titration schedule, stimulus duration is titrated on the basis of an animals’ individual performance. Specifically, after a successful magazine entry to initiate training for the session, the first light stimulus was 10 s (stimulus duration). Upon each correct nosepoke, reinforcer was received and the stimulus duration for the subsequent trial was shortened one step down in the series (10, 8, 6, 4, 2, 1, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, and 0.1 s, respectively). Incorrect and omitted responses elicited a lengthened stimulus duration that was one step up in the series for the subsequent trial. Whereas premature responses maintained the same stimulus duration, ITI remained constant for this training. Median stimulus duration (median of stimulus lengths for all trials) and shortest cue duration (shortest stimulus duration reached within a single training session) were calculated for each animal. Performance measures are reported for d 1 of titration training.
Adult MS
Upon completion of 5-CSRTT titration training, PR was run to evaluate baseline motivation before acute MS. All animals were then fed MS diet for 7 d—each weekday running 5-CSRTT training to maintain operant responding—and then tested in PR after acute supplementation. Each animals’ breakpoint was compared before and after supplementation to assess differences in motivation as a result of acute MS. Only animals that successfully performed schedule A training were included in the analysis (animals not included: HFD, 2; SC + MS, 1; HFD + MS, 1; 1 additional SC + MS animal was not included because the chamber malfunctioned).
Gene expression in PFC
After completion of operant training, female animals were sacrificed. Brains were placed in RNAlater and stored at −20C. The medial PFC was dissected from a 2-mm coronal slice from bregma +2.3 to 0.3, and DNA and RNA were extracted by using AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA, USA). cDNA (220 μg/10 μl) was synthesized by using High Capacity Reverse Transcriptase kit (Applied Biosystems, Foster City, CA, USA). Concentrated cDNA was used in a specific target preamplification according to manufacturer recommendations (Fluidigm, South San Francisco, CA, USA). In brief, TaqMan assays for 32 genes were pooled to a final concentration of ×0.2. Of pooled assay mix, 2 μl was combined with 2 μl cDNA and 4 μl 2× TaqMan Preamp Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). Preamplification reaction was cycled according to manufacturer protocol in a 750 fast quantitative PCR machine. Samples were then diluted 1:50 using 1× Tris-EDTA. Samples were then run on a 96.96 Dynamic Array Integrated Fluidic Circuit on the Biomark HD machine (Fluidigm). We were interested in the expression of genes involved in DNA methylation, inflammation, and reward and learning behaviors, as these genes are known to be altered after HFD exposure (Table 2). Gene expression values were correlated with median stimulus duration in the 5-CSRTT titration schedule, an end point that was increased by maternal HFD and normalized by MS. Expression of targets was normalized to the mean of the housekeeping gene ACTB (expression was not changed across conditions), expressed as respiratory quotient values, and analyzed by 2-way ANOVA. Nonparametric Spearman correlations were calculated between each animal’s performance for all behaviors and the expression of each gene as 2−ΔCt.
TABLE 2.
Gene list
| Gene | Assay ID |
|---|---|
| ACTB | Mm00607939_s1 |
| ADORA2a | Mm00802075_m1 |
| C1Qa | Mm00432142_m1 |
| CCL2 | Mm00441242_m1 |
| CD86 | Mm00444543_m1 |
| COMT | Mm00514377_m1 |
| CXCL10 | Mm00445235_m1 |
| CXCL5 | Mm00436451_g1 |
| DDARP-32 | Mm00454892_m1 |
| DNMT1 | Mm00599763_m1 |
| DNMT3A | Mm00432881_m1 |
| DRD1A | Mm01353211_m1 |
| DRD2 | Mm00438545_m1 |
| GADD45B1 | Mm00435123_m1 |
| GAPDH | Mm99999915_g1 |
| GRM5 | Mm00690332_m1 |
| HDAC2 | Mm00515108_m1 |
| HDAC5 | Mm01246076_m1 |
| IL-10 | Mm01288386_m1 |
| IL-1β | Mm01336189_m1 |
| IL-6 | Mm00446190_m1 |
| MECP2 | Mm01193537_g1 |
| MTR | Mm01340053_m1 |
| NGP | Mm01250218_m1 |
| OPRD1 | Mm00443063_m1 |
| OPRK1 | Mm01230885_m1 |
| PNMT | Mm00476993_m1 |
| SET7 | Mm00499823_m1 |
| SLC6A3 | Mm00438388_m1 |
| TH | Mm00447557_m1 |
| TLR4 | Mm00445273_m1 |
| TNF-α | Mm00443258_m1 |
Data analysis
Two-way ANOVA (maternal diet × supplementation) was used to evaluate results using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). Repeated measures by time were conducted for FR1 training. Bonferroni post hoc planned comparisons were conducted when appropriate. Comparisons of interest were control vs. HFD-fed offspring (to determine the effect of maternal HFD), and unsupplemented vs. supplemented (to determine the effect of supplementation). Interactions and main effects are described in Results, and significant post hoc comparisons are indicated in the figures, with P ≤ 0.05 considered significant.
RESULTS
To determine whether early life MS can alter operant behavior in offspring from dams that were fed 60% HFD throughout pregnancy, we supplemented offspring with dietary methyl donors from age 3–6 wk and tested them in a series of operant behavior tasks (Fig. 1A). Male mice from all dietary conditions reached the criterion around d 5 of FR1 training (Fig. 1B), whereas all females met the criterion around d 3 (Fig. 1C) with no significant difference between diet groups in either sex. After all animals had learned the FR1 task, a PR task was used to assess motivation. In male offspring, motivation was unchanged by maternal (F1,51 = 2.396; P = 0.128) or postnatal (F1,51 = 1.319; P = 0.256) diet (Fig. 1D); however, female HFD-fed offspring showed decreased motivation (lower breakpoint) compared with offspring from SC dams (main effect of maternal diet, F1,54 = 5.91; P = 0.019; Fig. 1E). Of interest, we found that female offspring provided with MS during early life had increased motivation to work for reinforcer compared with unsupplemented females (main effect of MS, F1,54 = 9.32; P = 0.004; Fig. 1E).
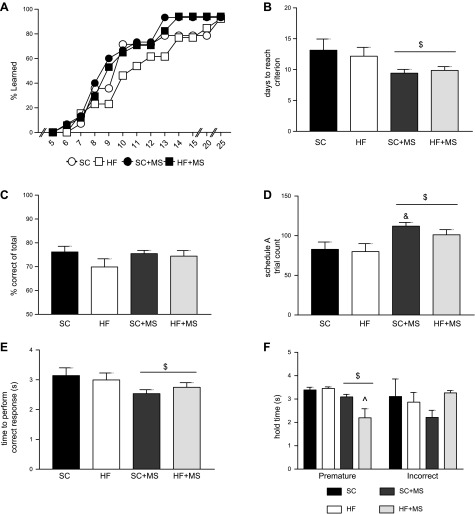
To evaluate executive function, animals were tested in the 5-CSRTT task. As a result of an insufficient number of control males that acquired 5-CSRTT schedule A tasks, we were unable to move forward with the male analysis; therefore, from this point forward, all studies were completed only in female offspring. In female offspring, we found that between d 9 and 13, fewer of the HFD-fed offspring had learned the task, but over the entire course of the learning period, this difference only resulted in a trend toward a differential learning rate between groups (Mantel-Cox log-rank test: χ2 (3) = 6.209; P = 0.1; Fig. 2A). Of note, MS female offspring reached the criterion in fewer training sessions than did unsupplemented females (main effect of MS, F1,51 = 7.16; P = 0.01; Fig. 2B). All female offspring performed ∼75% correct responses (Fig. 2C), and there were no differences in error type (data not shown). MS significantly increased the total number of trials performed during the session (main effect of MS, F1,51 = 10.64; P = 0.002; Bonferroni post hoc SC vs. SC + MS t51 = 2.77; P < 0.05; Fig. 2D) and decreased the time required to complete a correct response (main effect of MS, F1,51 = 4.52; P = 0.038; Fig. 2E). MS also decreased the amount of time to perform a premature trial error when this error type was performed (main effect of MS, F1,50 = 9.75; P = 0.003; Bonferroni post hoc HFD vs. HFD + MS; P = 0.006; Fig. 2F), whereas the time to perform an incorrect response was not affected.
Figure 2.
Postnatal MS enhances the ability to learn operant behavioral task. A) Rates at which the criterion was reached. Criterion was reached when animals performed 20 trials with 50% correctness in the 30-min session. B) Postnatal MS decreased the number of training sessions for animals to reach criterion. C) All offspring performed with around 70% correctness once the criterion was met. D) MS significantly increased the number of trials performed during the session. E) MS significantly decreased the amount of time required to perform a correct response. F) MS significantly decreased the amount of time to perform a premature error. (n: SC = 14, HF = 11, SC + MS = 14, HFD + MS = 16 in all figures.) $P < 0.05 main effect of MS; &P < 0.05 SC vs. SC + MS; ^P < 0.05 HFD vs. HFD + MS.
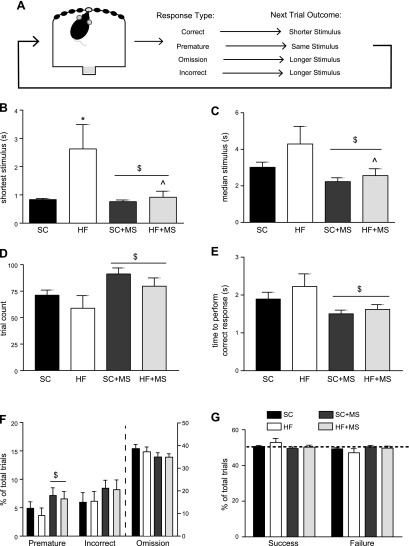
After 3 consecutive days of criterion performance on 5-CSRTT schedule A, females were moved onto the more difficult 5-CSRTT titration schedule. On this schedule, the response performed by the animal directly affected stimulus length for the subsequent trial (Fig. 3A), which is in contrast to schedule A, in which stimulus length remained constant throughout the 30-min training session. This titration schedule identified each animal’s individual performance limit (shortest stimulus duration) by titrating between a stimulus length at which a correct response was possible and a stimulus length below the performance limit (39). Of interest, we found that HFD-fed females were unable to reach the shortest stimulus length reached by the SC-fed females (Fig. 3B), and this was ameliorated by MS (interaction of maternal diet and MS, F1,46 = 4.793; P = 0.034; Bonferroni post hoc SC vs. HFD; t46 = 3.16; P = 0.014; HFD vs. HFD + MS; t46 = 3.26; P = 0.011). We also found that MS decreased the median stimulus needed to perform a correct response (main effect of MS, F1,46 = 6.58; P = 0.014; Bonferroni post hoc HFD vs. HFD + MS; t46 = 2.52; P = 0.031; Fig. 3C). In addition, similar to the observation in schedule A, MS females completed more total trials than unsupplemented females (main effect of MS, F1,46 = 6.61; P = 0.013; Fig. 3D), and the time to complete successful trials for MS females was decreased compared with unsupplemented females (main effect of MS, F1,46 = 6.62; P = 0.017; Fig. 3E). Unlike in schedule A, the titration schedule increased the percentage of premature errors performed by MS females (main effect of MS, F1,46 = 4.36; P = 0.042), but did not significantly change incorrect or omission errors (Fig. 3F). The titration task is designed to detect the performance limit of each animal (e.g., an equal number of success and failures); therefore, as expected, all animals performed approximately 50% of trials successfully within this titration schedule (Fig. 3G).
Figure 3.
HFD-fed offspring require a longer stimulus to perform correct responses. A) As shown in the schematic, on the 5-CSRTT titration training, response performed within each trial directly impacted the stimulus length of the subsequent trial. This allowed each animal to reach an individual set point for the task. B) HFD-fed offspring required the longest stimulus to perform a correct trial; however, this was ameliorated by MS. C) MS significantly decreased the median stimulus needed to perform a correct response. D) MS increased the number of trials performed during the 30-min session. E) MS decreased the time to complete a successful trial within this schedule. F) MS significantly increased the number of premature errors, but did not alter incorrect or omission errors. G) As designed, animals performed approximately 50% of trials successfully within this titration schedule. (n: SC = 11, HFD = 10, SC + MS = 13, HFD + MS = 16 in all figures.) $P < 0.05 main effect of MS; *P < 0.05 SC vs. HFD; ^P < 0.05 HFD vs. HFD + MS.
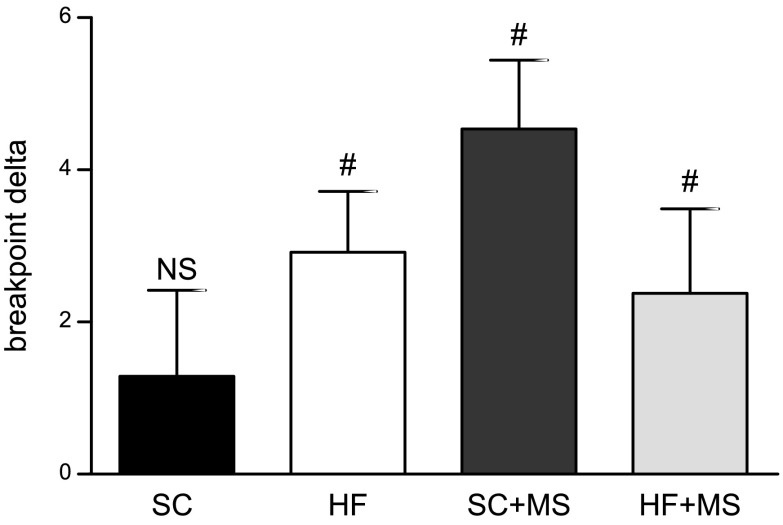
After completion of operant training, we wanted to determine if acute adult exposure to MS would alter motivation apart from cognitive performance (e.g., memory or learning). After 5-CSRTT was completed, females were tested in PR while being fed the control diet to establish a baseline. This baseline PR performance did not differ from the initial PR conducted before 5-CSRTT testing (data not shown). All animals were then fed MS diet for 7 d, during which time animals continued daily 5-CSRTT testing to maintain operant responding, before testing PR for a second time (Fig. 1A). We found that 7 d of acute MS increased the number of trials performed across all groups except for controls compared with their own baseline (SC, t13 = 1.137; P = 0.27; HF, t13 = 3.636; P = 0.009; SC + MS, t12 = 5.025; P < 0.001; HFD + MS, t15 = 2.132; P < 0.05; Fig. 4).
Figure 4.
Acute adult MS alters motivation. At the conclusion of cognitive testing, motivation was again tested with the PR task. After this testing, animals were given acute access to MS for 7 d, during which maintenance 5-CSRTT was performed, before motivation was again assessed with PR. Acute MS increased the motivation of HFD-fed offspring and offspring that had previous supplementation of methyl donors early in life. Motivation remained unchanged in control offspring. (n: SC = 14, HFD = 11, SC + MS = 13, HFD + MS = 16.) NS, not significant. #P < 0.05 compared with 0.
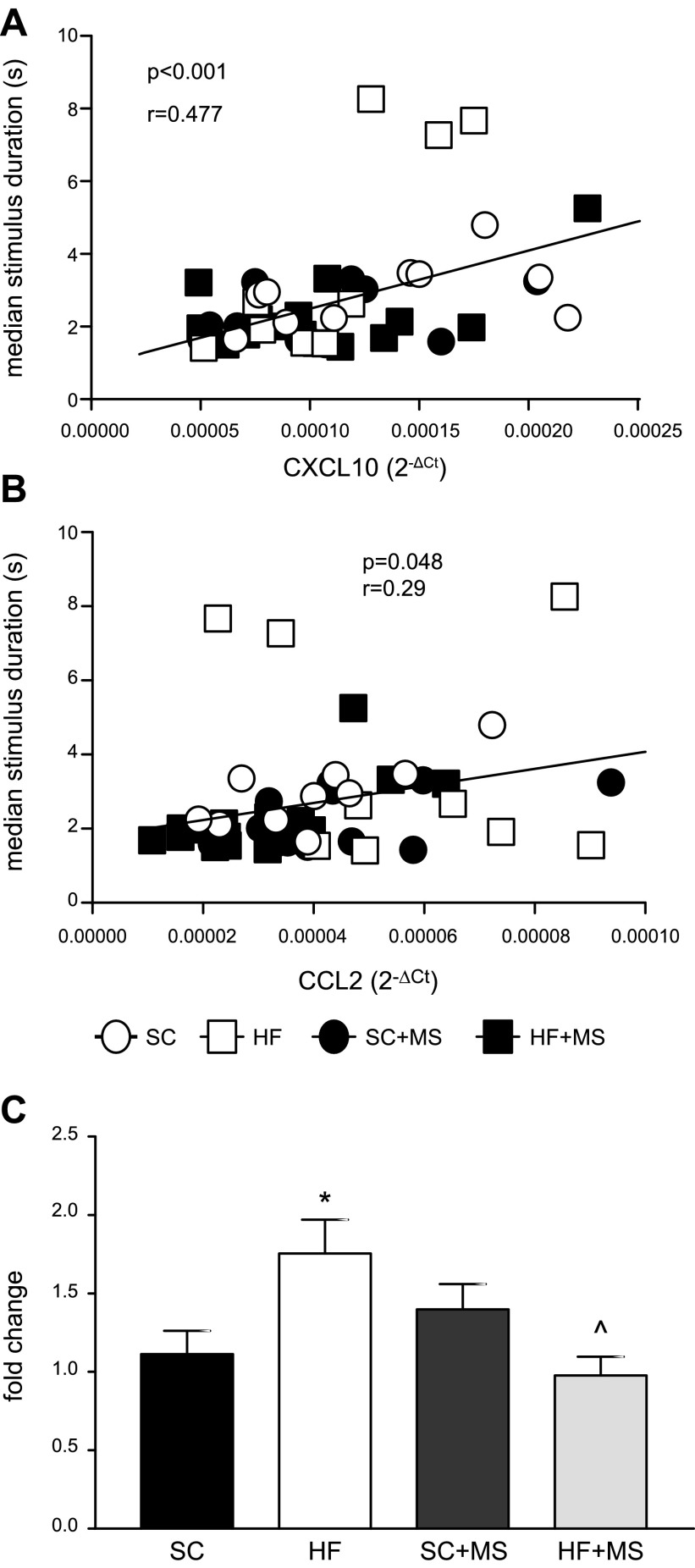
After completion of all operant training, we screened for genes (Table 2) with expression changes altered by either maternal or postnatal diet and correlated with median stimulus duration. We identified 2 chemokines, CXCL10 (Fig. 5A) and CCL2 (also known as MCP-1; Fig. 5B), whose expression levels correlated with median stimulus duration on the 5-CSRTT titration schedule. Expression of both chemokines was positively correlated with stimulus length (CXCL10: r = 0.477, P < 0.001; CCL2: r = 0.291, P = 0.047). We also found that overall expression levels of CCL2 was increased in HFD-fed offspring and this increase was attenuated by MS (interaction of maternal diet and MS, F1,48 = 11.15; P = 0.002; Bonferroni post hoc SC vs. HFD; t48 = 2.75; P < 0.05; HFD vs. HFD + MS; t48 = 3.44; P = 0.007; Fig. 5C). There were no group differences in CXCL10 expression (data not shown). No other genes correlated with stimulus duration, and only 1 other gene was affected by HFD or MS, with TNF-α levels increased by MS (F1,48 = 4.093; P = 0.049; data not shown).
Figure 5.
HFD-fed offspring have increased expression of CCL2 in the PFC which is attenuated by MS. At the conclusion of operant testing, RNA gene expression was measured in the PFC. A, B) Expression of 2 chemokines, CXCL10 (A) and CCL2 (B), correlated with median stimulus duration on the 5-CSRTT titration schedule. C) HFD-fed offspring have increased CCL2 gene expression; MS attenuated this increase. (n: SC = 13, HFD = 11, SC + MS = 13, HFD + MS = 15 in (A); animals that did not reach the titration schedule were left out of correlation analysis (B, C): n: SC = 11, HFD = 10, SC + MS = 13, HFD + MS = 15.) *P < 0.05 SC vs. HFD; ^P < 0.05 HFD vs. HFD + MS.
DISCUSSION
MS has been implicated in altering behavior, gene expression, and DNA methylation when administered either during pregnancy or in late adulthood. We hypothesized that because the PFC continues to develop after birth, exposure to methyl donors in the postnatal period would alter PFC-dependent cognitive performance. We found that in mice, MS during age 3–6 wk can both reverse certain HFD-induced deficits as well as improve other cognitive end points that were not affected by HFD. By using—for the first time, to our knowledge, in mice—a 5-CSRTT titration schedule, we were able to determine individual limits of performance for each animal. Incorporation of this more difficult version of the 5-CSRTT proved to be valuable in identifying parameters affected by both maternal HFD and MS.
Consumption of an HFD during pregnancy has been shown to affect offspring cognition—for example, it can impair spatial learning (40, 41) and worsen memory deficits (42). Here, by using a more difficult version of the 5-CSRTT, the titration protocol, we demonstrate that maternal HFD impairs cognitive performance in female offspring such that the stimulus length required for a correct response was significantly longer in HFD-fed offspring. Of importance, MS was able to reverse this deficit. In HFD-fed offspring, the shortest stimulus length obtained matched the time required to perform a correct response, which potentially indicated that an illuminated cue was necessary to complete a correct response. In all other groups, the shortest stimulus reached was shorter than the time required to perform a correct response, which signified that a correct response could be performed after the light cue was terminated. These data indicate that HFD-fed offspring could have either a deficit in working memory or a slower reaction time to the task. Neurons in the PFC have been implicated in working memory, specifically in the encoding and retention of temporal information (43). This type of working memory is critical to the 5-CSRTT titration schedule. As the stimulus duration shortens, animals must remember where the light cue was previously seen. Synaptic plasticity also contributes to working memory (44), and synapse number and signaling across synapses are 2 ways to measure synaptic function. Maternal HFD has been shown to adversely affect both synaptic stability (45) and dendritic complexity in new neurons (40). Conversely, it is known that increased plasma concentration of specific methyl donor nutrients, which are precursors for synapses, can promote synthesis of new brain synapses (46, 47) and stimulate neurite outgrowth (48) and neurogenesis (49). Because MS contains multiple precursors for brain synapse development, one possible mechanism by which MS improved the HFD phenotype is via an increase in synapse number and signaling; however, further studies will be required to support this hypothesis.
Of interest, we found that gene expression of 2 chemokines, CCL2 and CXCL10, was positively correlated with median stimulus length in the 5-CSRTT titration task, with higher chemokine levels associated with poorer performance. In addition, overall expression of CCL2 was increased in HFD-fed offspring, and this increase was normalized with early life exposure to MS. We hypothesize that increased expression of chemokines, particularly CCL2, negatively affect cognition, an idea with additional support in the literature. CCL2 overexpression in a mouse model of Alzheimer’s disease was found to accelerate deficits in spatial and working memory (50). In addition, in humans, increased expression of these chemokines has been associated with neurocognitive deficits in patients with Alzheimer’s disease (51) and cognitive status in patients with Parkinson’s disease (52). Beyond their classically defined function in the immune system, to attract and activate immune cells, there is an increasing appreciation for the importance of chemokine action within the CNS (53). CCL2, specifically, is known to modulate neuronal function (54, 55) and can alter membrane resistance in dopaminergic neurons (56). Behavioral effects of CCL2 have also been noted, as chronic infusion of CCL2 was shown to increase sweetened ethanol consumption in an operant self-administration task (57). Of importance, it has been shown that prenatal fat exposure disrupts proper functioning of the CCL2 chemokine system in embryonic neurons in the hypothalamus (58). Collectively, these findings support the conclusion that early life exposure to HFD or MS can affect the CCL2 chemokine system, which represents a potential mechanistic link between these dietary manipulations and effects on cognition in adulthood.
In addition to improving HFD-driven phenotypes, early life MS also increased motivation and performance (independent of HFD). MS increased PR breakpoint and increased the number of trials performed within a session on both 5-CSRTT training schedules, as well as decreasing the days required to learn the 5-CSRTT and 5-CSRTT reaction time. These data indicate increased overall motivation and cognitive performance in MS animals; however, these increases in performance were not without some cost. MS also increased the number of impulsive premature errors in the titration experiment and decreased the time to make an impulsive error in the standard 5-CSRTT. The mechanism whereby MS affects cognitive performance remains unknown. It is possible that MS increases acetylcholine concentration during development by providing the precursor choline (59) and, in turn, could enhance cholinergic signaling pathways that are known, in part, to modulate learning and memory (60, 61). Furthermore, MS has the potential to broadly affect gene expression via alteration of the methylation status of target genes or regulatory regions. We previously reported maternal HFD leads to global hypomethylation within the PFC, which is reversed by MS (23), and that high gene expression within the PFC, canonically associated with decreased promoter methylation, was related to poor executive function performance (10). Therefore, it is possible that MS leads to increased methylation of specific gene promoter regions within the PFC, which leads to reduced gene expression and improvements in motivation and performance. However, it is important to note that assessments of global hypomethylation include DNA methylation in many genomic regions outside of the promoter regions (i.e., intergenic and intronic regions in which methylation affects overall stability of the genome); therefore, at the level of individual gene promoters, both hypo- and hypermethylation will exist. Identification of target genes affected by MS in the PFC is an important next step.
It has been shown previously that adult MS, with either single or multiple nutrients, increased memory retention and decreased and/or slowed progression of dementia (27, 29). Our data add to this literature by demonstrating an acute positive effect on motivation. Whereas the effects of adolescent supplementation on both behavior and gene expression persisted well into adulthood, it is unclear how long the effects of acute supplementation on motivation may last. Of interest, motivation increased in all experimental groups but not controls, which suggested that prior exposure to either HFD or MS is required for the beneficial effects of adult supplementation. Whereas the precise mechanism of acute MS action on cognition remains unclear, the current data demonstrate that early life exposure to either HFD or MS increases responsiveness to later life MS.
Addition of folic acid to many grain products was mandated by the U.S. Food and Drug Administration in 1996 to decrease the risk of spina bifida and other neural tube closure defects. More recently, a critical role for folate is increasingly being appreciated with regard to adverse neurodevelopmental outcomes. Folate in the brain prevents accumulation of homocysteine by remethylation of homocysteine to methionine. Epidemiologic studies have linked folate deficiency with homocysteine accumulation in stroke and in patients with Alzheimer’s disease and Parkinson’s disease (62), whereas experimentally in rats, folate deficiency led to both increased homocysteine as well as learning and memory deficits (63). Folate insufficiency can be caused by decreased dietary folate consumption, genetic defects, or blockage of folate transport to the brain. Folate receptor α (FRα) auto-Abs block the transport of folate into the brain and have been associated with cerebral folate deficiency–related developmental disorders, autism, and schizophrenia (64–66). Exposure to FRα auto-Abs during gestation have been associated with increased anxiety-like behaviors in mice (67), and folinic acid supplementation during gestation can alter adult cognition, specifically ameliorating adult communication and sociability deficits in mice that were exposed to FRα auto-Abs (66). Use of various other methyl donors postnatally has generally been limited to preventing or slowing cognitive decline (29). Our findings indicate that early life postnatal consumption of a cocktail of methyl vitamins, possibly via vitamin or supplementation protocol, could affect learning and cognition into adulthood and may be particularly beneficial to children exposed to HFD and/or obesity during gestation. These data also provide support for the hypothesis that adult MS remains a potential mechanism by which cognition could be altered in the adult or aging population. Whereas folic acid is the most broadly used supplement clinically, the present studies provided a cocktail of methyl vitamins, as this is important for proper functioning of the entire 1-carbon metabolism cycle, helping to catalyze enzymes reliant on folate, vitamin B12, and choline (68). Whether the same beneficial effects would be found with the use of single nutrient supplements (e.g., only folic acid or choline) remains to be determined.
We have previously reported that offspring of a maternal HFD show deficits acquiring motivated behavior (10). Here, we saw no differences in time to acquire motivated behavior in either sex. This could be a result of differences in the study design, including age of animals during training, housing conditions, or dietary differences. In the previous study, restriction began at 12 wk, whereas in the current study, restriction began at 20 wk. Age of testing has been shown to be a factor in locomotor activity, social behavior, depression-related behavior, and spatial and cued fear memory (69), whereas the age of food restriction has been shown to alter overall goal and sign tracking in an operant task, with adolescence being a more vulnerable time period than adulthood (70). It is possible that cortical circuits that underlie learning and motivation are more vulnerable to food restriction that occurs at an earlier age. Housing conditions were another key difference between our previous and current study. In the previously reported study, male and female experiments were performed sequentially, whereas in the current study, male and female experiments were performed simultaneously in the same room. It is possible that housing both sexes in the same room instead of in separate rooms within an experiment could affect behavioral results (71). Presence of female odors could distract male mice enough to displace their motivation from performing the operant task to acquisition of a mate (72, 73). This could also explain the inability of control male offspring to successfully learn the 5-CSRTT task, which precluded their further behavioral testing. As such, it is important to note that these findings apply only to females, and it will be important to confirm these findings in males. Finally, differences in diets could account for differential behavioral findings. Previously, we used defined diets during pregnancy and lactation, and animals were then weaned onto the house chow. In the current study, defined diets were used throughout the entire study. Lab Diet 5001 (previous weaning diet) contains 13% fat, 57% carbohydrate, and 30% protein with more than double the amount of all methyl donor nutrients, whereas Test Diet 5755 (current weaning diet) contains 22% fat, 59% carbohydrate, and 18% protein. Nutrient composition differences between regular chow and defined diet are significant and are a confounding factor overlooked by approximately 75% of diet studies (74). Furthermore, it is also important to note that the HFD used here also has lower carbohydrate content than the control diet, so any observed effects of HFD may be related to increased fat content or decreased carbohydrate content. There are also various increases in micronutrient concentration per gram of diet in the HFD; however, mice consume less total volume of HFD to compensate for excess calories, so intake of the micronutrients is likely to be similar.
In sum, by using a novel variation of the 5-CSRTT schedule, we have shown that female HFD-fed offspring fail to reach the same performance standard (stimulus length duration) as control offspring, and early life MS ameliorated this deficit possibly via modulation of the chemokines CCL2 and CXCL10. Furthermore, MS improved a number of cognitive and motivational end points, which led MS female offspring to learn more rapidly, perform more trials, and display a reduced reaction time; therefore, MS in early life has the potential to both reverse negative outcomes associated with HFD during pregnancy, as well as promote an overall increase in cognitive performance. Future studies will be directed at identifying the molecular mechanisms by which MS alters these cognitive endpoints. Deciphering these mechanisms could point toward novel therapeutic targets to improve human cognition.
AUTHOR CONTRIBUTIONS
S. E. McKee, N. M. Grissom, and T. M. Reyes designed research; S. E. McKee and C. T. Herdt analyzed data; S. E. McKee performed research; S. E. McKee and T. M. Reyes wrote the paper; N. M. Grissom and C. T. Herdt provided analytic tools.
ACKNOWLEDGMENTS
The authors acknowledge the technical support of Landis Walsh, Matt Marini (both from the University of Pennsylvania), and Amanda Fritsch (University of Cincinnati). This work was supported by the U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences (Grant ES019851 to S.E.M.) and NIH National Institute of Mental Health (Grant MH087978 to T.M.R.). The authors declare no conflicts of interest.
Glossary
- 5-CSRTT
5-choice serial reaction timed task
- FRα
folate receptor α
- FR1
fixed ratio 1
- HFD
high fat diet
- ITI
intertrial interval
- MS
methyl donor supplementation
- PFC
prefrontal cortex
- PR
progressive ratio
- SC
standard control diet
REFERENCES
- 1.Ogden C. L., Carroll M. D., Kit B. K., Flegal K. M. (2012) Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 82, 1–8 [PubMed] [Google Scholar]
- 2.Ogden C. L., Carroll M. D., Kit B. K., Flegal K. M. (2014) Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schack-Nielsen L., Michaelsen K. F., Gamborg M., Mortensen E. L., Sørensen T. I. (2010) Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int. J. Obes. 34, 67–74 [DOI] [PubMed] [Google Scholar]
- 4.Camprubi Robles M., Campoy C., Garcia Fernandez L., Lopez-Pedrosa J. M., Rueda R., Martin M. J. (2015) Maternal diabetes and cognitive performance in the offspring: a systematic review and meta-analysis. PLoS One 10, e0142583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Lieshout R. J., Taylor V. H., Boyle M. H. (2011) Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes. Rev. 12, e548–e559 [DOI] [PubMed] [Google Scholar]
- 6.Krakowiak P., Walker C. K., Bremer A. A., Baker A. S., Ozonoff S., Hansen R. L., Hertz-Picciotto I. (2012) Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–e1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera H. M., Christiansen K. J., Sullivan E. L. (2015) The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 9, 194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grissom N., Bowman N., Reyes T. M. (2014) Epigenetic programming of reward function in offspring: a role for maternal diet. Mamm. Genome 25, 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vucetic Z., Kimmel J., Totoki K., Hollenbeck E., Reyes T. M. (2010) Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151, 4756–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grissom N. M., Herdt C. T., Desilets J., Lidsky-Everson J., Reyes T. M. (2015) Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology 40, 1353–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones P. A. (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492 [DOI] [PubMed] [Google Scholar]
- 12.Waterland R. A., Jirtle R. L. (2003) Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 23, 5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng Y. L., An R., Shin J., Song H., Ming G. L. (2013) DNA modifications and neurological disorders. Neurotherapeutics 10, 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loke Y. J., Hannan A. J., Craig J. M. (2015) The role of epigenetic change in autism spectrum disorders. Front. Neurol. 6, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuch V., Utsumi D. A., Costa T. V. M. M., Kulikowski L. D., Muszkat M. (2015) Attention deficit hyperactivity disorder in the light of the epigenetic paradigm. Front. Psychiatry 6, 126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox J. T., Stover P. J. (2008) Folate-mediated one-carbon metabolism. Vitam. Horm. 79, 1–44 [DOI] [PubMed] [Google Scholar]
- 17.Anderson O. S., Sant K. E., Dolinoy D. C. (2012) Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 23, 853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen S., Iyer C., Meydani S. N. (2014) Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J. Perinatol. 34, 105–111 [DOI] [PubMed] [Google Scholar]
- 19.Da Silva V. R., Hausman D. B., Kauwell G. P., Sokolow A., Tackett R. L., Rathbun S. L., Bailey L. B. (2013) Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int. J. Obes. 37, 1608–1610 [DOI] [PubMed] [Google Scholar]
- 20.Masho S. W., Bassyouni A., Cha S. (2016) Pre-pregnancy obesity and non-adherence to multivitamin use: findings from the National Pregnancy Risk Assessment Monitoring System (2009-2011). BMC Pregnancy Childbirth 16, 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooney C. A., Dave A. A., Wolff G. L. (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 132, 2393S–2400S [DOI] [PubMed] [Google Scholar]
- 22.O’Neill R. J., Vrana P. B., Rosenfeld C. S. (2014) Maternal methyl supplemented diets and effects on offspring health. Front. Genet. 5, 289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlin J., George R., Reyes T. M. (2013) Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One 8, e63549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordero P., Campion J., Milagro F. I., Martinez J. A. (2013) Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: effect of dietary methyl donor supplementation. Mol. Genet. Metab. 110, 388–395 [DOI] [PubMed] [Google Scholar]
- 25.Seferovic M. D., Goodspeed D. M., Chu D. M., Krannich L. A., Gonzalez-Rodriguez P. J., Cox J. E., Aagaard K. M. (2015) Heritable IUGR and adult metabolic syndrome are reversible and associated with alterations in the metabolome following dietary supplementation of 1-carbon intermediates. FASEB J. 29, 2640–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodspeed D., Seferovic M. D., Holland W., Mcknight R. A., Summers S. A., Branch D. W., Lane R. H., Aagaard K. M. (2015) Essential nutrient supplementation prevents heritable metabolic disease in multigenerational intrauterine growth-restricted rats. FASEB J. 29, 807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L., Chen Y., Wang W., Xiao Z., Hong Y. (2016) Multi-vitamin B supplementation reverses hypoxia-induced tau hyperphosphorylation and improves memory function in adult mice. J. Alzheimers Dis. 54, 297–306 [DOI] [PubMed] [Google Scholar]
- 28.Van Wijk N., Broersen L. M., de Wilde M. C., Hageman R. J. J., Groenendijk M., Sijben J. W. C., Kamphuis P. J. G. H. (2014) Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J. Alzheimers Dis. 38, 459–479 [DOI] [PubMed] [Google Scholar]
- 29.Morris M. S. (2012) The role of B vitamins in preventing and treating cognitive impairment and decline. Adv. Nutr. 3, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X., Nardelli J. (2016) Cellular and molecular introduction to brain development. Neurobiol. Dis. 92, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., Nugent T. F. III, Herman D. H., Clasen L. S., Toga A. W., Rapoport J. L., Thompson P. M. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 101, 8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sowell E. R., Delis D., Stiles J., Jernigan T. L. (2001) Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J. Int. Neuropsychol. Soc. 7, 312–322 [DOI] [PubMed] [Google Scholar]
- 33.Spear L. P. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 [DOI] [PubMed] [Google Scholar]
- 34.Duncan J. (2013) The structure of cognition: attentional episodes in mind and brain. Neuron 80, 35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floresco S. B., Jentsch J. D. (2011) Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology 36, 227–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bari A., Robbins T. W. (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79 [DOI] [PubMed] [Google Scholar]
- 37.Grissom N. M., Reyes T. M. (2013) Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int. J. Dev. Neurosci. 31, 406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young J. W., Light G. A., Marston H. M., Sharp R., Geyer M. A. (2009) The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One 4, e4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin T. J., Grigg A., Kim S. A., Ririe D. G., Eisenach J. C. (2015) Assessment of attention threshold in rats by titration of visual cue duration during the five choice serial reaction time task. J. Neurosci. Methods 241, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tozuka Y., Kumon M., Wada E., Onodera M., Mochizuki H., Wada K. (2010) Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 57, 235–247 [DOI] [PubMed] [Google Scholar]
- 41.Lu J., Wu D. M., Zheng Y. L., Hu B., Cheng W., Zhang Z. F., Shan Q. (2011) Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav. Immun. 25, 1658–1667 [DOI] [PubMed] [Google Scholar]
- 42.Martin S. A. L., Jameson C. H., Allan S. M., Lawrence C. B. (2014) Maternal high-fat diet worsens memory deficits in the triple-transgenic (3xTgAD) mouse model of Alzheimer’s disease. PLoS One 9, e99226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai Y., Takahashi S., Inoue M. (2004) Stimulus duration in working memory is represented by neuronal activity in the monkey prefrontal cortex. Eur. J. Neurosci. 20, 1069–1080 [DOI] [PubMed] [Google Scholar]
- 44.Mayford M., Siegelbaum S. A., Kandel E. R. (2012) Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 4, a005751–a005751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatanaka Y., Wada K., Kabuta T. (2016) Maternal high-fat diet leads to persistent synaptic instability in mouse offspring via oxidative stress during lactation. Neurochem. Int. 97, 99–108 [DOI] [PubMed] [Google Scholar]
- 46.Wurtman R. J., Cansev M., Sakamoto T., Ulus I. (2010) Nutritional modifiers of aging brain function: use of uridine and other phosphatide precursors to increase formation of brain synapses. Nutr. Rev. 68, S88–S101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cansev M., Wurtman R. J., Sakamoto T., Ulus I. H. (2008) Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement. 4, S153–S168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujii A., Matsumoto H., Yamamoto H. (1996) Effect of vitamin B complex on neurotransmission and neurite outgrowth. Gen. Pharmacol. 27, 995–1000 [DOI] [PubMed] [Google Scholar]
- 49.Craciunescu C. N., Albright C. D., Mar M. H., Song J., Zeisel S. H. (2003) Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 133, 3614–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiyota T., Yamamoto M., Xiong H., Lambert M. P., Klein W. L., Gendelman H. E., Ransohoff R. M., Ikezu T. (2009) CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One 4, e6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westin K., Buchhave P., Nielsen H., Minthon L., Janciauskiene S., Hansson O. (2012) CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS One 7, e30525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rocha N. P., Scalzo P. L., Barbosa I. G., Souza M. S., Morato I. B., Vieira É. L. M., Christo P. P., Teixeira A. L., Reis H. J. (2014) Cognitive status correlates with CXCL10/IP-10 levels in Parkinson’s disease. Parkinsons Dis. 2014, 903796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mélik-Parsadaniantz S., Rostène W. (2008) Chemokines and neuromodulation. J. Neuroimmunol. 198, 62–68 [DOI] [PubMed] [Google Scholar]
- 54.Gosselin R. D., Varela C., Banisadr G., Mechighel P., Rostene W., Kitabgi P., Melik-Parsadaniantz S. (2005) Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J. Neurochem. 95, 1023–1034 [DOI] [PubMed] [Google Scholar]
- 55.Guyon A., Conductier G., Rovere C., Enfissi A., Nahon J. L. (2009) Melanin-concentrating hormone producing neurons: activities and modulations. Peptides 30, 2031–2039 [DOI] [PubMed] [Google Scholar]
- 56.Guyon A., Skrzydelski D., De Giry I., Rovère C., Conductier G., Trocello J. M., Daugé V., Kitabgi P., Rostène W., Nahon J. L., Mélik Parsadaniantz S. (2009) Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience 162, 1072–1080 [DOI] [PubMed] [Google Scholar]
- 57.Valenta J. P., Gonzales R. A. (2016) Chronic intracerebroventricular infusion of monocyte chemoattractant protein-1 leads to a persistent increase in sweetened ethanol consumption during operant self-administration but does not influence sucrose consumption in Long-Evans rats. Alcohol. Clin. Exp. Res. 40, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poon K., Mandava S., Chen K., Barson J. R., Buschlen S., Leibowitz S. F. (2013) Prenatal exposure to dietary fat induces changes in the transcriptional factors, TEF and YAP, which may stimulate differentiation of peptide neurons in rat hypothalamus. PLoS One 8, e77668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen E. L., Wurtman R. J. (1976) Brain acetylcholine: control by dietary choline. Science 191, 561–562 [DOI] [PubMed] [Google Scholar]
- 60.Savelkoul P. J. M., Janickova H., Kuipers A. A. M., Hageman R. J. J., Kamphuis P. J., Dolezal V., Broersen L. M. (2012) A specific multi-nutrient formulation enhances M1 muscarinic acetylcholine receptor responses in vitro. J. Neurochem. 120, 631–640 [DOI] [PubMed] [Google Scholar]
- 61.Klinkenberg I., Sambeth A., Blokland A. (2011) Acetylcholine and attention. Behav. Brain Res. 221, 430–442 [DOI] [PubMed] [Google Scholar]
- 62.Mattson M. P., Shea T. B. (2003) Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 26, 137–146 [DOI] [PubMed] [Google Scholar]
- 63.Berrocal-Zaragoza M. I., Sequeira J. M., Murphy M. M., Fernandez-Ballart J. D., Abdel Baki S. G., Bergold P. J., Quadros E. V. (2014) Folate deficiency in rat pups during weaning causes learning and memory deficits. Br. J. Nutr. 112, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 64.Frye R. E., Delhey L., Slattery J., Tippett M., Wynne R., Rose S., Kahler S. G., Bennuri S. C., Melnyk S., Sequeira J. M., Quadros E. (2016) Blocking and binding folate receptor alpha autoantibodies identify novel autism spectrum disorder subgroups. Front. Neurosci. 10, 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramaekers V. T., Thöny B., Sequeira J. M., Ansseau M., Philippe P., Boemer F., Bours V., Quadros E. V. (2014) Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. Mol. Genet. Metab. 113, 307–314 [DOI] [PubMed] [Google Scholar]
- 66.Desai A., Sequeira J. M., Quadros E. V. (2016) Prevention of behavioral deficits in rats exposed to folate receptor antibodies: implication in autism. [E-pub ahead of print] Mol. Psychiatry doi: 10.1038/mp.2016.153 [DOI] [PubMed] [Google Scholar]
- 67.Sequeira J. M., Desai A., Berrocal-Zaragoza M. I., Murphy M. M., Fernandez-Ballart J. D., Quadros E. V. (2016) Exposure to folate receptor alpha antibodies during gestation and weaning leads to severe behavioral deficits in rats: a pilot study. PLoS One 11, e0152249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selhub J., Paul L. (2011) Folic acid fortification: why not vitamin B12 also? Biofactors 37, 269–271 [DOI] [PubMed] [Google Scholar]
- 69.Shoji H., Takao K., Hattori S., Miyakawa T. (2016) Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain 9, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson R. I., Bush P. C., Spear L. P. (2013) Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behav. Brain Res. 257, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beatty W. W. (1979) Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm. Behav. 12, 112–163 [DOI] [PubMed] [Google Scholar]
- 72.Kavaliers M., Choleris E. (2013) Neurobiological aspects of the effects of anticipation of interaction with a female on male cognitive performance. Arch. Sex. Behav. 42, 331–333 [DOI] [PubMed] [Google Scholar]
- 73.Kavaliers M., Choleris E., Colwell D. D. (2001) Brief exposure to female odors “emboldens” male mice by reducing predator-induced behavioral and hormonal responses. Horm. Behav. 40, 497–509 [DOI] [PubMed] [Google Scholar]
- 74.Warden C. H., Fisler J. S. (2008) Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 7, 277 [DOI] [PMC free article] [PubMed] [Google Scholar]