Abstract
Many studies demonstrate that activation of aldehyde dehydrogenase 2 (ALDH2) protects against oxidative stress via detoxification of cytotoxic aldehydes, and could attenuate cardiac, cerebral, lung and renal ischaemia–reperfusion (I/R) injuries. However, the effect of ALDH2 in intestinal I/R is unknown. The present study was set up to determine whether an ALDH2 agonist, Alda-1, could alleviate intestinal injury after gut I/R. In a mouse model of intestinal I/R injury, histological grading, proinflammatory cytokines, oxidative stress, cellular apoptosis, chemokine contents, ALDH2 activity, 4-hydroxy-trans-2-nonenal (4-HNE) and malondialdehyde (MDA) were evaluated. The results indicated that I/R treatment conferred elevation in pathological scores, proinflammatory cytokines, oxidative stress, cellular apoptosis and chemokine levels, accompanied by accumulated 4-HNE and MDA. No significant changes in ALDH2 activity were observed after I/R. However, Alda-1 pretreatment significantly decreased these injurious indicators, concomitant with up-regulated ALDH2 activity, and lessened 4-HNE and MDA accumulation. Taken together, our results implicate activation of ALDH2 by Alda-1 in the significant abatement intestinal I/R injury.
Keywords: 4-hydroxy-trans-2-nonenal, Alda-1, ALDH2, intestine, ischaemia–reperfusion injury, multiple organ dysfunction syndrome
Clinical perspectives
-
•
Many studies demonstrate that activation of aldehyde dehydrogenase 2 (ALDH2) protects against oxidative stress via detoxification of cytotoxic aldehydes and could attenuate cardiac, cerebral, lung and renal ischaemia-reperfusion (I/R) injuries; However, its effect in intestinal I/R is unknown.
-
•
This research for the first time demonstrates Alda-1 exerts protective effects against gut I/R injury.
-
•
Alda-1 may serve as a novel treatment for patients with intestinal I/R in the future.
Introduction
Intestinal ischaemia–reperfusion (I/R) injury is a life-threatening condition resulting from mesenteric artery blocking, intestinal transplantation and abdominal trauma [1]. The injury is caused paradoxically not only by the interruption of the blood supply in the ischaemic state, but also by the restoration of blood flow in the reperfusion phase [2]. The local intestinal damage can contribute to multiple organ dysfunction syndrome (MODS) and systemic inflammatory response syndrome (SIRS), leading to remote organ injury with a high mortality rate of approximately 40% [3].
Oxidative stress is one of the most crucial factors in the pathogenesis of intestinal I/R injury. Previous studies have shown that the imbalance between oxygen delivery and oxygen demand induces the formation of reactive oxygen species (ROS) [4] and the accumulation of reactive aldehydes by lipid peroxidation in mitochondria [5]. These toxic reactive aldehydes, including malondialdehyde (MDA) [6] and 4-hydroxy-trans-2-nonenal (4-HNE) [7], can form adducts with proteins and DNA, and exert a detrimental effect on the intestine, further exacerbating the injury. Therefore, agents aiming at oxidative stress, especially the reactive aldehydes mediated by lipid peroxidation, have become a novel target for prevention and treatment of intestinal I/R injury.
Aldehyde dehydrogenase 2 (ALDH2) is the chief enzyme in mitochondria to catalyse conversion of acetaldehyde (high toxicity) to carboxylic acid (low toxicity), and it also detoxifies the ROS-generated aldehyde adducts [8]. The identification of ALDH2 in I/R injuries originates from high-throughput proteomics screening, published in the journal Science, of cardiac tissue proteins after reperfusion, in which a small-molecule activator of ALDH2 (Alda-1) can reduce the infarct size by 60% [9], demonstrating a central role of ALDH2 in cardiac I/R injury for the first time. Since then, more subsequent studies have repeatedly validated the effectiveness of ALDH2 activation in cardioprotection [10–14]. Apart from its application in cardiac research, the ALDH2 agonist has also been reported to confer protection against ischaemic injury in the brain [15,16], lungs [17] and kidneys [18], through clearance of reactive aldehydes, such as 4-HNE and MDA.
Although ALDH2 activation is demonstrated to protect against I/R injuries in organs outside the gut, no publication addresses its effect on I/R injury in the intestine to the best of our knowledge. Given the role of ALDH2 in lipid peroxidation and its successful application in other organs with alleviating effects, it is rational to hypothesize that ALDH2 may have a therapeutic effect in intestinal I/R. Therefore, the present study was established to investigate whether activating ALDH2 can attenuate the intestinal injuries in a murine model of gut I/R.
Materials and methods
Animals and experimental grouping
The experiment in the present study was approved by the animal ethical committee of Peking Union Medical College Hospital, Beijing, China, and the experimental procedures were performed in strict compliance with the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, China, 1998). Efforts were made to minimize the animals’ pain.
Male C57BL/6 mice (aged 8–12 weeks) were purchased from Charles River. They were housed under standard conditions of room temperature (25±2°C), humidity (60%) and a 12-h dark–light cycle with free access to water and chow for acclimatization of at least 2 weeks. The experimental design comprised three groups: (i) the I/R+Alda-1 group (n=6) underwent I/R by clamping the superior mesenteric artery for 60 min, followed by 120 min of reperfusion, and were pretreated with Alda-1 (10 mg/kg) intraperitoneally (i.p.) 1 h before ischaemia; (ii) the I/R+vehicle group (n=5) underwent I/R for the same duration but were treated with vehicle (dimethyl sulphoxide, DMSO); and (iii) the sham group (n=5) underwent laparotomy but without occlusion of the artery. For vehicle control, equal volumes of DMSO (solution of Alda-1) were injected into the I/R + vehicle group i.p.. The schematic panel is shown in Figure 1A. Alda-1 was purchased from Calbiochem (Merck KGaA).
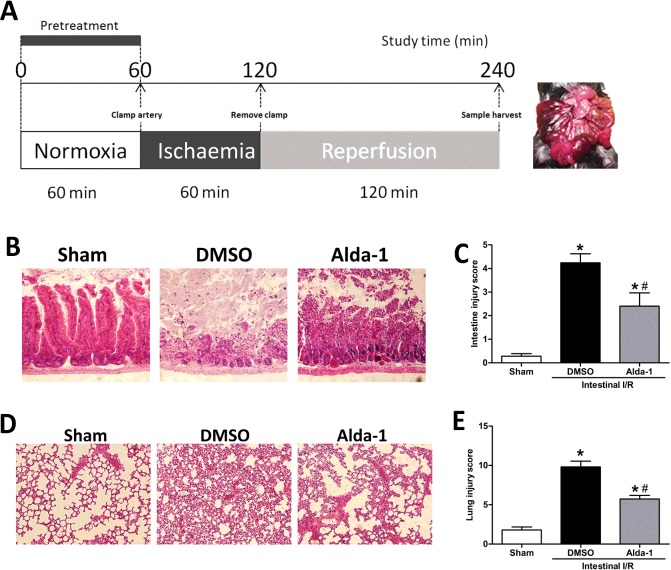
Figure 1. Alteration of gut and lung morphology by Alda-1 after intestinal I/R injury.
(A) Schematic panel, (B) representative H&E section of intestinal tissue, (C) histological grading of intestine injury, (D) morphological lung H&E section and (E) histological grading of lung. Original magnification ×200. Data were given as means±S.D.s (n=5–6/group). Pathological scores were compared using the Kruskal–Wallis test and the Mann–Whitney U test. #P<0.05 vs DMSO and *P<0.05 vs sham.
Surgical protocol
The mice were fasted, but allowed free access to water, 12 h before the experiment. The anaesthesia was achieved with a sodium pentobarbital injection (50 mg/kg, i.p.). Body temperature was maintained using a heating pad at 36°C, and was monitored with a rectal probe. After induction of anaesthesia (sodium pentobarbital 50 mg/kg), a midline laparotomy was performed to expose the intestine. Then, the superior mesenteric artery was isolated and blocked with a microvascular clamp for 60 min to induce ischaemia. Evidence of ischaemia was based on the immediate blanching of the whole intestine and disappearance of a pulse in or peristalsis of the intestinal tract. At the end of ischaemia, reperfusion was initiated by removal of the clamp. The return of a pink colour and the enhanced intestinal peristalsis were assumed to signal reperfusion of the intestine. The abdomen was temporarily covered with a sterile plastic wrap to minimize evaporative loss. The reperfusion was induced for 120 min. At the end of the experiment, the mice were euthanized with an overdose of anaesthetic drugs. The I/R time was set as previously described [19]. Blood samples were obtained by cardiac puncture and were centrifuged at 3000g for 10 min to separate the serum. Serum and tissue samples (brain, heart, lung, liver, intestine and kidney) were frozen immediately using liquid nitrogen, and stored at −80°C until analysis. In addition, sections of lung and intestine samples were immersed in formalin for histopathology.
Histopathological examination
Intestinal and lung tissues fixed in formalin were embedded in paraffin. Tissues blocks were sectioned at a 5-μm thickness, transferred to glass slides and stained with haematoxylin and eosin (H&E). Two blinded, experienced pathology investigators evaluated the sections under a light microscope. The degree of intestinal injury was based on a grading scale described previously by Chiu et al. [20]: (i) grade 0–normal mucosal villi; (ii) grade 1–development of oedema in the subepithelial space, usually at the apex of the villus, often with capillary congestion; (iii) grade 2–extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria; (iv) grade 3–massive epithelial lifting down the sides of villi with a few tips possibly being denuded; (v) grade 4–denuded villi with lamina propria and dilated capillaries exposed and increased cellularity of the lamina propria possibly noted; and (vi) grade 5–digestion and disintegration of the lamina propria and the presence of haemorrhage and ulceration. A minimum of five randomly chosen fields from each mouse were evaluated and averaged to represent the grade for each animal. The lung injury was determined semiquantitatively to the level of absent, mild, moderate or severe damage (score 0–3) by assessing the alveolar congestion, haemorrhage, infiltration of neutrophils and thickness of the alveolar wall/hyaline membrane [21]. Five high-magnification fields were randomly selected and graded for the average lung injury score in each animal.
Measurement of cytokines
Frozen tissues were cut into 1- to 2-mm pieces and homogenized. Then the samples were submitted to one cycle of freeze–thawing to break the cell membrane. The final homogenate was centrifuged at 5000g for 5 min to obtain the supernatants for cytokine measurements. The cytokines in serum or tissue were determined using enzyme-linked immunosorbent assay (ELISA) kits specific for mouse tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, macrophage inflammatory protein (MIP)-2, monocyte chemoattractant protein (MCP)-1, chemokine (C-X-C motif) ligand 1 (CXCL1) and myeloperoxidase (MPO) (R&D Systems Inc.) according to the manufacturer's instructions. The concentrations of nitric oxide (NO), inducible NO synthase (iNOS), H2O2, alanine transaminase (ALT), aspartate transaminase (AST) and creatinine were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Co.). Serum lipopolysaccharide (LPS) levels were determined using limulus amoebocyte lysate assay kits (BioEndo Technology). The tissue cytokines were normalized using total proteins.
Determination of ALDH2 activity
ALDH2 activity was determined in the samples, using an ALDH2 activity assay kit (Genmed Scientifics Inc.), according to the manufacturer's protocol. In brief, the activity was estimated by monitoring the conversion of oxidized nicotinamide adenine dinucleotide (NAD+) to reduced nicotinamide adenine dinucleotide (NADH) at an absorbance of 340 nm for 5 min. The final output of ALDH2 activity was expressed as micromoles of NAD/minute per mg of protein.
Assays for 4-HNE and MDA contents
For determination of the 4-HNE protein adducts content, a commercially available ELISA kit (Cell Biolabs, Inc.) was used according to the manufacturer's instruction. In principle, the measurement of 4-HNE protein adducts in samples was achieved by comparing their absorbance with that of a known standard curve. The level of MDA was evaluated using an MDA detecting kit (Abcam) according to manufacturer's protocols. Concentrations were normalized using total proteins.
Western blotting
Total proteins were extracted from tissues and were boiled with a five times loading buffer for 5 min. Then, 50 μg of each protein was equally loaded, separated by sodium dodecyl sulfate (SDS)/6–10% polyacrylamide gel electrophoresis (PAGE) (depending on the molecular mass), and transferred on to nitrocellulose membranes (Millipore). The membranes were blocked with 5% skimmed milk and incubated overnight with one of the following primary antibodies: rabbit monoclonal anti-ALDH2 antibody (1:1000 dilution, v/v, Abcam), rabbit polyclonal anti-nuclear factor (NF)-κ B p65 antibody (1:1000, v/v, Abcam), rabbit monoclonal anti-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB) α antibody (1:1000, v/v, Abcam), rabbit polyclonal anti-caspase-3 antibody (1:500, v/v, Abcam), mouse monoclonal anti-B-cell lymphoma (Bcl)-2 antibody (1:500, v/v, Abcam), mouse polyclonal anti-Bcl-2 associated X protein (Bax) antibody (1:300, v/v, Abcam), rabbit polyclonal anti-TLR-4 antibody (1:500, v/v, Abcam) and rabbit polyclonal anti-β-actin antibody (1:2000, v/v, Abcam). After washing to remove the former antibody binding, the membranes were incubated with the goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated secondary antibodies (depending on the primary antibody) for 2 h. Bands were detected using a chemiluminescent peroxidase substrate (ECL, Amersham) and normalized to relative changes with the internal loading control.
Statistical analysis
Data were expressed as means±S.D.s. Statistical comparisons were all performed with the software package SPSS 19.0 (IBM Corp.). Differences across groups were compared using one-way analysis of variance (ANOVA) followed by a post-hoc least significant difference (LSD) test to analyse the differences between individual groups. Pathological scores across groups were tested by a Kruskal–Wallis test, followed by a Mann–Whitney U test. Statistical significance was set at a two-tailed P<0.05.
Results
Alda-1 protects against morphological injuries in the intestine and lung
To investigate the effects of Alda-1 on local intestine after I/R, we conducted histological analysis of the H&E staining of the gut. In the I/R group, severe intestinal crypt injury, accompanied by widespread mucosal destruction and disintegrated intestinal villi, was observed 2 h after reperfusion. However, in the Alda-1 treatment group, the local intestinal damage was remarkably ameliorated, evidenced by the integrity of the villi and the preserved intestinal structures. In the sham group, there was no sign of denudation of villi or extensive oedema, suggesting a normal intestinal appearance (Figure 1B). Similarly, the pathological score of the intestine showed that Alda-1 administration before ischaemia significantly lowered the intestinal injury compared with the vehicle-treated group (Figure 1C), demonstrating a protective role of Alda-1 in the local intestine after injury.
As the lungs are the distant organs most severely affected by intestinal I/R injury [22], we evaluated the lung injury through H&E staining. In the sham group, there were no exudates or haemorrhage, and the structures of the alveolar wall were normal. However, in the I/R group, the lung tissues were damaged with alveolar congestion, exudates, haemorrhage and infiltration of neutrophils (Figure 1D). Pretreatment with Alda-1 significantly decreased these injurious signs with well-aerated alveoli and little intra-alveolar haemorrhage or few exudates. The pathological grading of the lung in the I/R group was significantly elevated over that of the sham group. Alda-1 treatment significantly decreased the lung injury score by 41% compared with the I/R group (Figure 1E).
Alda-1 reduces systemic inflammatory response markers
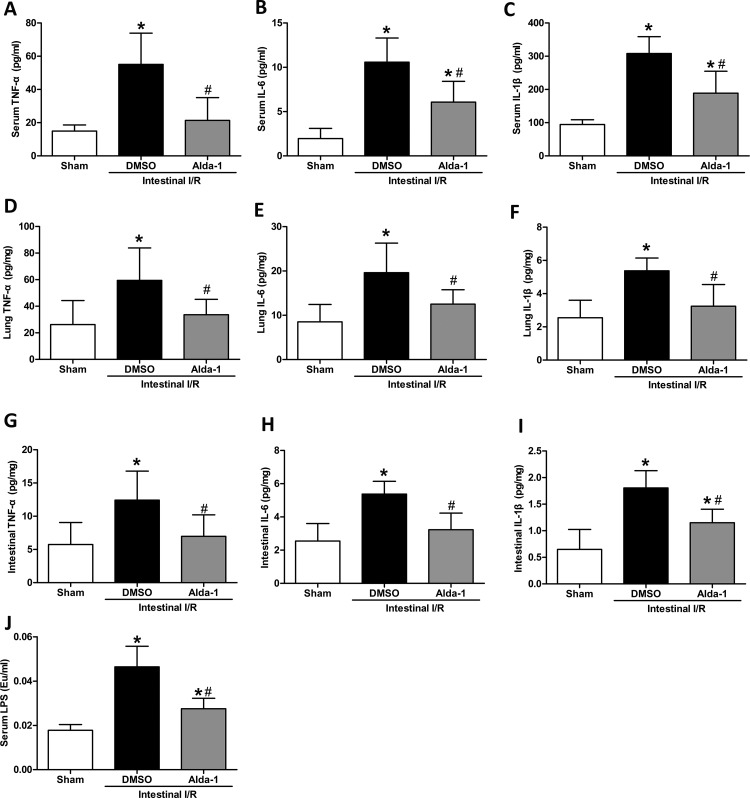
The proinflammatory cytokines are pivotal in the induction of MODS from the local intestine to distant organs [23]. We measured the level of TNF-α, IL-6 and IL-1β in the serum to observe the effect of Alda-1. Although serum levels of TNF-α, IL-6 and IL-1β were increased significantly after intestinal I/R (TNF-α by 3.7-fold, IL-6 by 5.4-fold and IL-1β by 3.3-fold), Alda-1 treatment significantly reduced the proinflammatory response by reducing TNF-α, IL-6 and IL-1β by 61, 43 and 39%, respectively (Figure 2A–C). We then investigated whether the production of these cytokines in tissues was affected by Alda-1. In agreement with the trend in the serum, the tissue cytokines of the lung and intestine also exhibited significantly decreased expression in the Alda-1 group than in the vehicle group (Figure 2D–I). Other organs, including the brain, heart and kidney, showed a suppressive effect, although some of the indicators did not reach significance (Table 1). In addition, the serum LPS levels were significantly blunted by Alda-1 after intestinal I/R (Figure 2J).
Figure 2. Changes of proinflammatory cytokines in serum, lung and intestine by Alda-1 treatment after intestinal I/R.
The superior mesenteric artery was clamped for 60 min, followed by 120 min of reperfusion. Serum and tissue cytokines were measured using ELISA. Data are presented as means±S.D.s (n=5–6/group) and compared using one-way ANOVA and LSD tests. #P<0.05 vs DMSO and *P<0.05 vs sham. Abbreviation: Eu, endotoxin unit.
Table 1. Proinflammatory cytokine suppression in distant organs after intestinal I/R.
The systemic inflammatory response variables TNF-α, IL-6 and IL-1β in the sham, DMSO (vehicle) and Alda-1 groups were assessed using ELISA in mice that underwent 60 min of ischaemia followed by 120 min of reperfusion. Data are denoted as means±S.D.s (n=5–6/group).
| Ischaemia–reperfusion | ||||
|---|---|---|---|---|
| Organ | Cytokine (pg/mg) | Sham | DMSO (vehicle) | Alda-1 |
| Brain | TNF-α | 21.03±7.37 | 64.29±16.72* | 49.43±6.18*# |
| IL-6 | 5.00±2.85 | 11.64±2.63* | 6.79±2.82# | |
| IL-1β | 2.33±0.63 | 7.19±0.68* | 5.83±1.04*# | |
| Heart | TNF-α | 36.69±22.52 | 94.52±40.19* | 42.57±13.82# |
| IL-6 | 14.13±7.60 | 26.03±14.29 | 21.19±9.38 | |
| IL-1β | 3.69±1.83 | 6.21±1.19* | 4.15±1.82 | |
| Kidney | TNF-α | 108.66±7.38 | 153.18±6.83* | 114.79±6.74# |
| IL-6 | 47.06±6.00 | 65.21±12.19* | 33.29±7.64*# | |
| IL-1β | 20.54±9.17 | 64.15±9.86* | 58.48±10.32* | |
#P<0.05 vs DMSO and *P<0.05 vs sham using one-way ANOVA and LSD tests.
Administration of Alda-1 modifies apoptotic variables
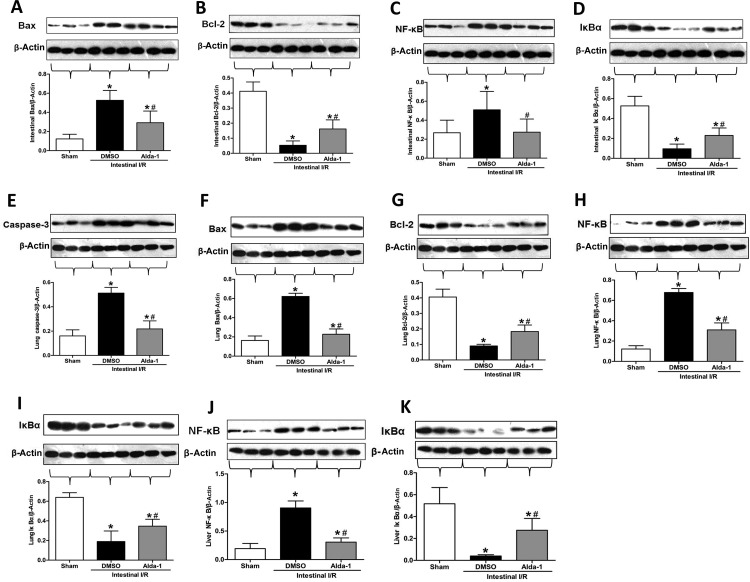
Apoptosis has been implicated in the regulation of tissue architecture and homoeostasis in intestinal I/R [24]. To evaluate the effect of Alda-1 on apoptosis, we measured the protein expression of caspase-3, Bcl-2, Bax, NF-κB and IκBα by Western blotting. In intestinal tissue, the expression of Bax and NF-κB, was significantly increased after intestinal I/R, whereas treatment with Alda-1 dramatically mitigated the expression levels similar to sham animals (Figure 3A and C). Meanwhile, intestinal expression of Bcl-2 and IκBα was significantly decreased after I/R, and Alda-1 markedly elevated the expression compared with the vehicle groups (Figure 3B and D). In the lung tissues, similar expression trends could be observed for caspase-3, Bcl-2, Bax, NF-κB and IκBα (Figure 3E–I). In addition, in liver tissues, Alda-1 pretreatment could significantly reduce the level of NF-κB by 66% and increase the level of IκBα by 6.9-fold (Figure 3J and K).
Figure 3. Effect of Alda-1 treatment on apoptotic marker expression.
Tissue protein expression was measured using Western blotting: (A) intestinal Bax, (B) intestinal Bcl-2, (C) intestinal NF-κB, (D) intestinal IκBα, (E) lung caspase-3, (F) lung Bax, (G) lung Bcl-2, (H) lung NF-κB, (I) lung IκBα, (J) liver NF-κB and (K) liver IκBα. Representative blots are presented and β-actin is used as a loading control. Each bar represents means±S.D.s (n=5–6/group). Data were compared using one-way ANOVA followed by the LSD tests. #P<0.05 vs DMSO and *P<0.05 vs sham.
Effects of Alda-1 on ALDH2 activity and aldehyde accumulation
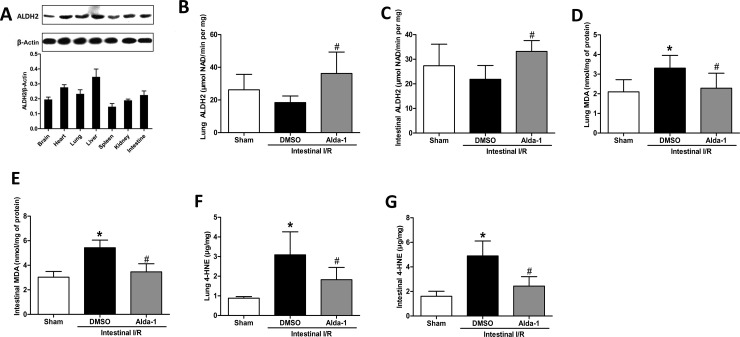
To monitor the effect of Alda-1 on ALDH2 activity, we first examined the tissue-specific expression of ALDH2 in normal mice using Western blotting (Figure 4A). In the examined tissues, the liver, heart, lung and intestine showed relatively high expressions of ALDH2, whereas the kidney, brain and spleen showed relatively low expressions of ALDH2. Then, to investigate whether the activity of ALDH2 was changed by Alda-1 treatment, we evaluated its activity in the lung and intestine 2 h after I/R. Compared with the vehicle-treated group, the administration of Alda-1 significantly increased the activity of ALDH2 in the lung and intestine (Figure 4B and C).
Figure 4. Alteration of ALDH2 activity and toxic aldehyde by Alda-1 pretreatment after intestinal I/R.
(A) Expression of ALDH2 in various tissues was measured by Western blotting. Representative blots against ALDH2 and β-actin are shown. ALDH2 activity was observed in (B) lung and (C) intestine at 2 h after intestinal I/R or sham operation. Aldehyde accumulation was evaluated by determining the MDA and 4-HNE levels in the lung (D and F) and intestine (E and G). Data were expressed as means±S.D.s (n=5–6/group) and compared using one-way ANOVA and LSD tests. #P<0.05 vs DMSO and *P<0.05 vs sham.
The accumulation of MDA and 4-HNE aldehydes is responsible for the toxic effects caused by I/R. As shown in Figure 4(D–G), there was a significant increase of both MDA and 4-HNE in the lung and intestinal tissue after I/R compared with the sham-operated group. This increase was dampened by Alda-1 treatment, demonstrating an effective clearance of toxic aldehydes.
Alda-1 alleviates chemokine levels
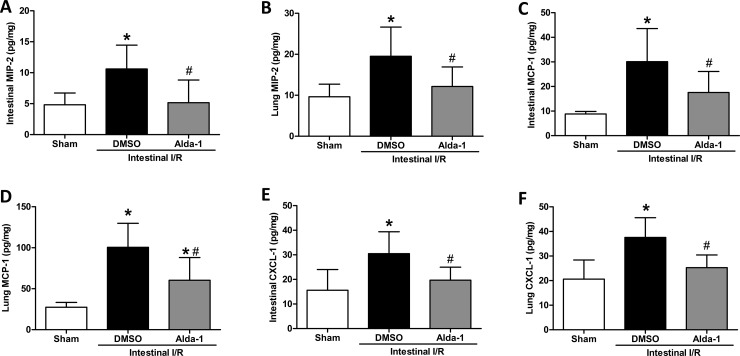
To characterize the effect of Alda-1 on chemokine expression in intestinal I/R, several chemokines, such as MIP-2, MCP-1 and CXCL-1, were evaluated after 2 h of I/R. MIP-2, an index of a neutrophil chemotactic factor, was remarkably elevated in intestinal and lung tissues after I/R, which was prevented by Alda-1 treatment, evidenced by a 51% and 38% reduction in the intestine and lung, respectively (Figure 5A and B). Similarly, there was also a significant inhibition of MCP-1 expression in the intestine and lung (Figure 5C and D). Moreover, Alda-1 administration elicited a notable decrease of CXCL-1 in the gut and lung after I/R (Figure 5E and F). These results indicate that production of chemotactic factors can be alleviated by ALDH2 activation through Alda-1.
Figure 5. Effect of Alda-1 on chemokine production in gut and lung after I/R.
The superior mesenteric artery was occluded for 60 min, followed by 120 min of reperfusion, to achieve intestinal I/R. (A) Intestinal MIP-2, (B) lung MIP-2, (C) intestinal MCP-1, (D) lung MCP-1, (E) intestinal CXCL-1 and (F) lung CXCL-1 were measured using ELISA in the sham, DMSO (vehicle) and Alda-1-treated groups. Bar graphs show the means±S.D.s. #P<0.05 vs DMSO and *P<0.05 vs sham by one-way ANOVA and LSD tests.
Treatment with Alda-1 suppresses oxidative stress
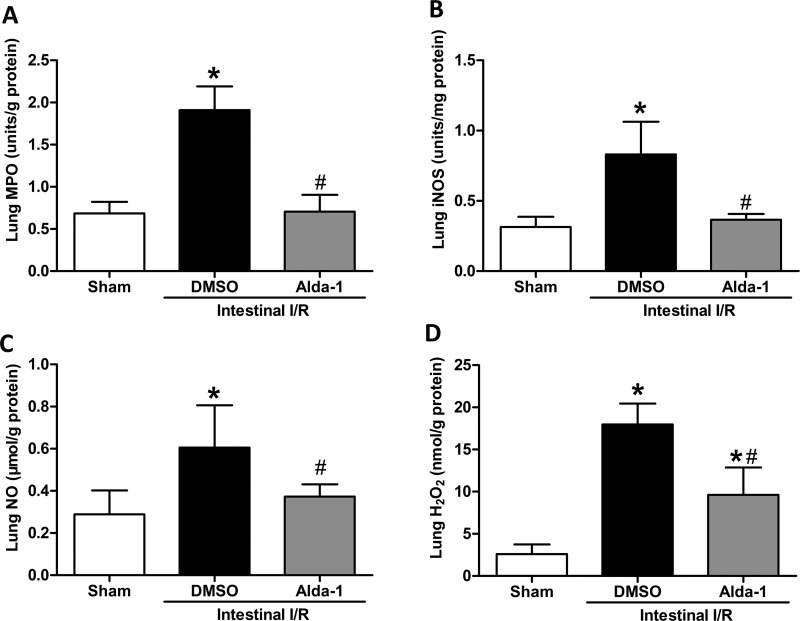
Oxidative stress, an important facet of I/R, can contribute to acute lung injury with a high mortality rate. We therefore assayed oxidative stress markers in the lung tissue. Compared with the sham-operated mice, the mice subjected to intestinal I/R showed substantially elevated expression of MPO, iNOS, NO and H2O2 in the lung tissues, whereas Alda-1 pretreatment significantly reversed the increase of these markers (MPO by 63%, iNOS by 56%, NO by 38% and H2O2 by 47%) compared with the vehicle-treated mice (Figure 6), revealing a notable effect of Alda-1 on oxidative stress.
Figure 6. Suppressive effects of Alda-1 on oxidative stress after intestinal I/R.
Mice were subjected to 60 min of ischaemia by clamping the superior mesenteric artery and then 120 min of reperfusion. Sham surgery involved the same surgical procedure except for clamping the artery. (A) MPO, (B) iNOS, (C) NO and (D) H2O2 levels were assessed in the lung tissues. Data are given as means±S.D.s (n=5–6 per group). #P<0.05 vs DMSO and *P<0.05 vs sham by one-way ANOVA and LSD tests.
Alda-1 improves kidney and liver organ injury variables
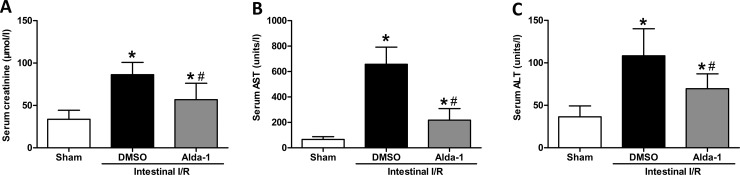
To assess the effect of Alda-1 administration on intestinal I/R-induced organ injury in the kidney and liver, circulating levels of creatinine, AST and ALT were determined. Mice subjected to I/R, 2 h after the intestinal I/R, developed markedly renal and hepatic dysfunction, evidenced by a dramatic rise in serum creatinine, AST and ALT above the sham levels. However, Alda-1 treatment protected against both hepatic and renal dysfunction, as indicated by a significant reduction in serum creatinine, AST and ALT (Figure 7).
Figure 7. Alda-1 reduces kidney and liver injury variables after intestinal I/R.
Serum (A) creatinine, (B) AST and (C) ALT levels were measured in mice exposed to Alda-1, vehicle or sham treatment (n=5–6/group). Data are presented as means±S.D.s and compared using one-way ANOVA and LSD tests. #P<0.05 vs DMSO and *P<0.05 vs sham.
Effects of Alda-1 on TLR-4 expression
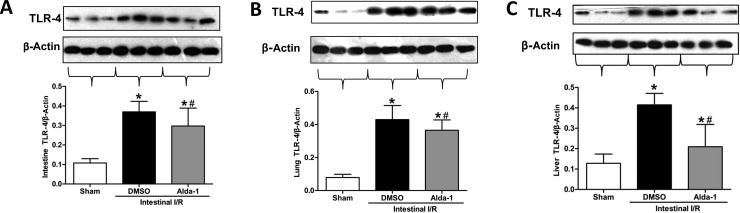
As toll-like receptor (TLR) 4 is a crucial pathway in acute intestinal and lung injury upon gut I/R [25,26], we conducted a pilot investigation of whether TLR-4 expression is affected by Alda-1 administration in the gut, lung and liver tissues after I/R (Figure 8). Consistent with previous reports [27], the vehicle-treated group exhibited a significantly up-regulated expression of TLR-4 in the gut, lung and liver tissues compared with the sham surgery group. However, the induction was significantly mitigated by Alda-1 pretreatment (Figure 8), suggesting an involvement of TLR-4 in the effect of Alda-1 after IR.
Figure 8. Effects of Alda-1 on TLR-4 expression in the intestine, lung and liver after gut I/R.
TLR-4 expression in (A) the intestine, (B) the lung and (C) liver tissues was determined using Western blotting. Representative blots were presented and β-actin was used as a loading control. Data are given as means±S.D.s (n=5–6/group) and compared using one-way ANOVA and LSD tests. #P<0.05 vs DMSO and *P<0.05 vs sham.
Discussion
In the present study, we have demonstrated that treatment with Alda-1, an ALDH2 activator, alleviated not only the structural disruptions in the lung and intestine after gut I/R but also the inflammatory responses and oxidative stress indicators. Moreover, Alda-1 exerted its effect through clearance of toxic aldehyde accumulation and inhibition of apoptosis. More importantly, TLR-4 pathways were probably involved. To the best of our knowledge, this is the first study to demonstrate that ALDH2 activation by Alda-1 can mitigate intestinal I/R injury as well as acute lung injury in mice.
Intestinal I/R is a devastating clinical condition with a high mortality rate. The pathological mechanism of intestinal I/R-caused multiple organ failure remains complicated and even now is not thoroughly understood. One of the widely accepted views is the gut-origin hypothesis [28]. The translocation of gut bacteria, resulting from damage to the intestinal mucosal barrier, triggers a systemic inflammatory response and oxidative stress, thus leading to distant organ injuries, especially in the lung. Reactive aldehydes derived from lipid peroxidation, such as MDA and 4-HNE, have been identified in almost all tissues that are submitted to ischaemia, indicating its central role in I/R. Thus, pharmacological agents aimed at the aldehydes may be a helpful strategy to reduce the I/R injury.
ALDH2 is the crucial enzyme for metabolizing aldehydes. It converts acetaldehyde to acetic acid, and thereby lowers the toxicity. In 2008, Chen et al. [9] used an unbiased proteomic approach to detect differentially expressed proteins in cardiac I/R, and found that the phosphorylation state of ALDH2 was consistently correlated with cardioprotection after ischaemic injury. They then proved the sufficiency of ALDH2 in cardioprotection by applying a selective ALDH2 activator, Alda-1, before ischaemia, which expectedly decreased the size of the myocardial infarct by about 60%. Their findings are the first revelation about ALDH2 in I/R injury. Since then, many studies have consistently demonstrated the protective effects of Alda-1 in cardiac injury and other I/R injury models, including the brain [15], lung [17] and kidney [18]. However, its effect on gut I/R remains unknown. The present study was designed to explore the role of Alda-1 on alleviating intestinal I/R, including injury to the lung, in mice, and the possible signalling pathway involved.
While gut ischaemia by itself can cause local damage, the subsequent activated SIRS can contribute to distant organ injuries with lung as the most vulnerable organ. Thus, we focused mainly on the intestine and the lung. We found that the morphological structures of the two organs were well preserved by Alda-1 compared with the vehicle. As the inflammatory cytokines in SIRS, such as TNF-α, IL-1β and IL-6, are key mediators linking the gut and the lung [28], we measured their levels in the circulation after I/R. In agreement with our expectation, the serum levels of TNF-α, IL-1β and IL-6 were significantly suppressed by Alda-1 treatment, paralleling the remarkably decreased morphological damage in the lung and intestine. Our findings were consistent with the reported anti-inflammatory property of Alda-1 [17,29]. In a mouse model of lung I/R, the TNF-α and IL-1β were significantly reversed from the I/R-induced elevation to levels similar to that of control groups [17]. Similarly, in a rat model of myocardial infarction, the number of TNF-α-positive cells was significantly decreased by Alda-1 treatment [29]. In addition to the modulated inflammatory response, involvement of oxidative stress is another important aspect in the pathogenesis of I/R. ROS, including iNOS and hydrogen peroxide, have been demonstrated to worsen the injury after intestinal I/R [30,31]. We detected that Alda-1 pretreatment could significantly reduce the levels of NO, iNOS and H2O2 in lung tissues, which suggests its antioxidant effects in I/R. In support of our results are the publications showing that activation of ALDH2 by another agonist, lipoic acid, reversed the production of ROS [32,33], implying that there may be an inverse correlation between ALDH2 activity and ROS.
Apoptosis is considered to be the main contributor to cellular death after I/R injury [34]. In the present study, we used caspase-3, Bax and Bcl-2 as indicators to evaluate apoptosis in the lung and intestinal tissue. As expected, in this research the apoptosis was significantly reduced by Alda-1 treatment. Our results are consistent with those of previous studies. In renal I/R, it was observed that increased expression of ALDH2 reduced renal cell apoptosis, evidenced by the significantly changed caspase-3, Bax and Bcl-2 levels [18]. In cerebral I/R, treatment with Alda-1 significantly down-regulated the activity of caspase-3 [16]. Furthermore, in models of parkinsonism, activation of ALDH2 could suppress rotenone-induced augmentation of Bax and caspase-3 [35]. Although we do not have direct data, such as immunohistological evaluation, showing that the apoptotic cell is significantly reduced by Alda-1, the reports of others using terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labelling (TUNEL) staining [15,17] have clearly revealed that Alda-1 administration was significantly associated with a decreased number of apoptotic cells in organic I/R injury. Taken together, we can infer that blunted apoptosis is a possible explanation for the alleviating effects of Alda-1 in intestinal I/R injuries. Actually, the activation of apoptosis can be triggered by 4-HNE, the mechanism of which was related to activation of NF-κB and its adduction with the c-Jun N-terminal kinase [36]. Theoretically, accelerating clearance of 4-HNE is likely to lighten the severity of apoptosis. We found that 4-HNE was significantly decreased with Alda-1 pretreatment in gut I/R, a result in good accordance with previous publications [15]. Consequently, the decreased apoptotic variables can also be understood from the perspective of 4-HNE clearance.
NF-κB is a classic transcriptional factor regulating proinflammation, cell proliferation and differentiation. Previous studies reported that 4-HNE was capable of up-regulating formation of NF-κB [37], which makes it quite reasonable to speculate that clearance of 4-HNE through Alda-1 supplementation might decrease the expression of NF-κB. We found that Alda-1 significantly lessened the expression of NF-κB along with the corresponding down-regulated 4-HNE. Similar supportive results can be seen in research on atherosclerotic plaques in which a negative association between ALDH2 activation and NF-κB was observed [38]. Translocation of NF-κB can be caused by activation of TLR-4 [39], a crucial pathway exerting a significant function on the lesion pathogenesis through the cascade inflammatory response [40]. TLR-4 binds to bacterial LPS, and activates the c-Jun N-terminal kinase, mitogen-activated protein kinase and NF-κB pathways, all of which are pivotal pathways for inflammatory immune responses in I/R injury [41]. As such, we examined TLR-4 expression and found that TLR-4 was significantly reduced in the lung, intestine and liver tissues after I/R. The decreased expression, in line with observed anti-inflammation results, coincides with the existing publication showing that a TLR-4 mutant conferred protection against haemorrhagic intestinal injury [42]. The underlying mechanisms of the effect of Alda-1 on TLR-4 might be explained by the preserved intestinal structures, resulting from clearance of 4-HNE and relief of oxidative stress damage by Alda-1, largely inhibiting the bacterial translocation into blood, and thereby lessening LPS conjugation with TLR-4. Published reports concluded that ALDH2 gene expression enhancement was concurrent with a decrease in TLR-4 gene expression in non-alcoholic steatohepatitis [43] and Alda-1 could suppress TLR-induced TNF production in haematopoietic stem cells [44], unveiling a potential triad among ALDH2 (Alda-1), TLR-4 and inflammation.
A noteworthy point to mention is that ALDH2 activity was not significantly affected by gut ischaemia treatment alone–not using the ALDH2 agonist. Albeit the mean value of ALDH2 activity in the intestinal I/R group was lower than in the sham group, this trend did not reach statistical significance. A possible explanation for this phenomenon is that ALDH2 is an intrinsic enzyme in the living organism, which will not be affected by I/R alone. Even if the enzyme's substrates (such as 4-HNE) have an exaggerated accumulation in I/R state, the enzyme that catalyses these toxic aldehydes still remains at a relatively constant level with unremarkably changed activities. Under such circumstance, the clearing capability of ALDH2 for aldehydes falls far behind the generation of aldehydes under I/R. This could account for the seemingly paradoxical coexistence of the insubstantial fluctuation of ALDH2 activity and the dramatic enhancement of 4-HNE and MDA after I/R. In this condition, promoting ALDH2 activities through in vitro agonists is a beneficial strategy to facilitate the clearance of aldehyde and thus reduce the injury. To date, similar findings have been reported in cardiac [9], cerebral [16] and lung [17] organs that I/R treatment alone did not incur a significant ALDH2 activity modification, but ALDH2 activation by Alda-1 could alleviate I/R injury.
There could be several limitations to the present study. First, we solely investigated the effect of Alda-1 at the animal level; however, the clinical investigations, especially large prospective studies or multiple phase clinical trials, are more important for their translational application. Second, the exact molecular pathways underlying the effect of Alda-1 on I/R with different cells or in various organs need more elucidation in the future. However, we believe that our findings could provide helpful hints for future research. Third, for translation into clinical practice, pre-loading with Alda-1 is suitable for planned ischaemia (bowel transplantation, abdominal surgery requiring temporary clamping of intestinal blood supply). However, it may not be applicable to unplanned ischaemia (traumatic or embolic upset of intestinal blood supply). To solve this problem, subsequent translational studies need to comprehensively enrol emergency ischaemia, if possible. Last, but not least, although the ALDH2 activity was evaluated, we did not measure the protein expression of ALDH2 using Western blotting. Several studies so far have confirmed that ALDH2 protein expression has not displayed significant differences across sham, I/R or Alda-1 groups [9,10,12,18]. In contrast, it is the ALDH2 activity that may be responsible for the subsequent function. Therefore, the present study did not concentrate on the protein expression of ALDH2 and detected only ALDH2 activity.
In summary, the most striking finding of the present study is that we, for the first time, demonstrated that Alda-1, as an ALDH2 activator, can alleviate intestinal I/R injury in a murine model via clearance of 4-HNE and MDA. Oxidative stress, inflammatory response, apoptosis, NF-κB and TLR-4 pathways also participated in the protective effects. The current data suggest that Alda-1 may serve as a novel treatment for patients with intestinal I/R in the future.
Abbreviations
- 4-HNE
4-hydroxy-trans-2-nonenal
- ALDH2
aldehyde dehydrogenase 2
- ALT
alanine transaminase
- ANOVA
analysis of variance
- AST
aspartate transaminase
- Bax
Bcl-2 associated X protein
- Bcl
B-cell lymphoma
- CXCL1
chemokine (C-X-C motif) ligand 1
- DMSO
dimethyl sulphoxide
- ELISA
enzyme-linked immunosorbent assay
- H&E
haematoxylin and eosin
- IκB
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor
- IL
interleukin
- iNOS
inducible NO synthase
- I/R
ischaemia–reperfusion
- i.p.
intraperitoneally
- LPS
lipopolysaccharide
- LSD
least significant difference
- MCP
monocyte chemoattractant protein
- MDA
malondialdehyde
- MIP
macrophage inflammatory protein
- MODS
multiple organ dysfunction syndrome
- MPO
myeloperoxidase
- NAD+
oxidized nicotinamide adenine dinucleotide
- NF
nuclear factor
- NO
nitric oxide
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
- TLR
toll-like receptor
- TNF
tumour necrosis factor
Author contribution
Q. Zhu conceived of the idea, conducted the experiments and analysed the data. G. He assisted in experimental conception, provided critical advice and reviewed the final draft of the manuscript. J. Wang collected the data and participated in data interpretation. Y. Wang assisted in performing the experiments and collected the samples. W. Chen provided technical support. All authors approved the final manuscript as submitted and agree to be responsible for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [grant no. 30940069].
Competing interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Wu M.C., Brennan F.H., Lynch J.P., Mantovani S., Phipps S., Wetsel R.A.. et al. (2013) The receptor for complement component C3a mediates protection from intestinal ischemia–reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl. Acad. Sci. U.S.A. 110, 9439–9444 10.1073/pnas.1218815110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L.W., Egan L., Li Z.W., Greten F.R., Kagnoff M.F. and Karin M. (2003) The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat. Med. 9, 575–581 10.1038/nm849 [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M.. et al. (2005) Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693 10.1056/NEJMoa050333 [DOI] [PubMed] [Google Scholar]
- 4.Wang G., Yao J., Li Z., Zu G., Feng D., Shan W.. et al. (2016) miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid. Redox. Signal. 24, 961–973 10.1089/ars.2015.6492 [DOI] [PubMed] [Google Scholar]
- 5.Cerqueira N.F., Hussni C.A. and Yoshida W.B. (2005) Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir. Bras. 20, 336–343 [DOI] [PubMed] [Google Scholar]
- 6.Horton J.W. and Walker P.B. (1993) Oxygen radicals, lipid peroxidation, and permeability changes after intestinal ischemia and reperfusion. J. Appl. Physiol. 74, 1515–1520 [DOI] [PubMed] [Google Scholar]
- 7.Lee H., Ko E.H., Lai M., Wei N., Balroop J., Kashem Z.. et al. (2014) Delineating the relationships among the formation of reactive oxygen species, cell membrane instability and innate autoimmunity in intestinal reperfusion injury. Mol. Immunol. 58, 151–159 10.1016/j.molimm.2013.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C.H., Cruz L.A. and Mochly-Rosen D. (2015) Pharmacological recruitment of aldehyde dehydrogenase 3A1 (ALDH3A1) to assist ALDH2 in acetaldehyde and ethanol metabolism in vivo. Proc. Natl. Acad. Sci. U.S.A. 112, 3074–3079 10.1073/pnas.1414657112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.H., Budas G.R., Churchill E.N., Disatnik M.H., Hurley T.D. and Mochly-Rosen D. (2008) Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 321, 1493–1495 10.1126/science.1158554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes K.M., Campos J.C., Bechara L.R., Queliconi B., Lima V.M., Disatnik M.H.. et al. (2014) Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc. Res. 103, 498–508 10.1093/cvr/cvu125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes K.M., Bechara L.R., Lima V.M., Ribeiro M.A., Campos J.C., Dourado P.M.. et al. (2015) Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post-myocardial infarction cardiomyopathy: benefits of Alda-1. Int. J. Cardiol. 179, 129–138 10.1016/j.ijcard.2014.10.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji W., Wei S., Hao P., Xing J., Yuan Q., Wang J.. et al. (2016) Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front. Pharmacol. 7, 101. 10.3389/fphar.2016.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita K., Miyazaki H., Saruwatari J., Oniki K., Kumagae N., Tanaka T.. et al. (2015) Combined effects of current-smoking and the aldehyde dehydrogenase 2*2 allele on the risk of myocardial infarction in Japanese patients. Toxicol. Lett. 232, 221–225 10.1016/j.toxlet.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 14.Hu Y., Yan J.B., Zheng M.Z., Song X.H., Wang L.L., Shen Y.L.. et al. (2015) Mitochondrial aldehyde dehydrogenase activity protects against lipopolysaccharideinduced cardiac dysfunction in rats. Mol. Med. Rep. 11, 1509–1515 [DOI] [PubMed] [Google Scholar]
- 15.Guo J.M., Liu A.J., Zang P., Dong W.Z., Ying L., Wang W.. et al. (2013) ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 23, 915–930 10.1038/cr.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S.H., Zhang H.F., Yang Z.B., Li T.B., Liu B., Lou Z.. et al. (2014) Alda-1 reduces cerebral ischemia/reperfusion injury in rat through clearance of reactive aldehydes. Naunyn Schmiedebergs Arch. Pharmacol. 387, 87–94 10.1007/s00210-013-0922-8 [DOI] [PubMed] [Google Scholar]
- 17.Ding J., Zhang Q., Luo Q., Ying Y., Liu Y., Li Y.. et al. (2016) Alda-1 attenuates lung ischemia–reperfusion injury by reducing 4-hydroxy-2-nonenal in alveolar epithelial cells. Crit. Care Med. 44, e544–e552 10.1097/CCM.0000000000001563 [DOI] [PubMed] [Google Scholar]
- 18.Zhong Z., Hu Q., Fu Z., Wang R., Xiong Y., Zhang Y.. et al. (2016) Increased expression of aldehyde dehydrogenase 2 reduces renal cell apoptosis during ischemia/reperfusion injury after hypothermic machine perfusion. Artif. Organs 40, 596–603 10.1111/aor.12607 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X.Y., Liu Z.M., Zhang H.F., Li Y.S., Wen S.H., Shen J.T.. et al. (2015) Decreased PD-1/PD-L1 expression is associated with the reduction in mucosal immunoglobulin A in mice with intestinal ischemia reperfusion. Dig. Dis. Sci. 60, 2662–2669 10.1007/s10620-015-3684-y [DOI] [PubMed] [Google Scholar]
- 20.Chiu C.J., McArdle A.H., Brown R., Scott H.J. and Gurd F.N. (1970) Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 101, 478–483 10.1001/archsurg.1970.01340280030009 [DOI] [PubMed] [Google Scholar]
- 21.He X., Han B., Mura M., Xia S., Wang S., Ma T.. et al. (2007) Angiotensin-converting enzyme inhibitor captopril prevents oleic acid-induced severe acute lung injury in rats. Shock 28, 106–111 10.1097/SHK.0b013e3180310f3a [DOI] [PubMed] [Google Scholar]
- 22.Ware L.B. and Matthay M.A. (2000) The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 10.1056/NEJM200005043421806 [DOI] [PubMed] [Google Scholar]
- 23.Wu S., Zhu X., Jin Z., Tong X., Zhu L., Hong X.. et al. (2015) The protective role of montelukast against intestinal ischemia-reperfusion injury in rats. Sci. Rep. 5, 15787. 10.1038/srep15787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda H., Suzuki Y., Suzuki M., Koike M., Tamura J., Tong J.. et al. (1998) Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 42, 530–537 10.1136/gut.42.4.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arumugam T.V., Okun E., Tang S.C., Thundyil J., Taylor S.M. and Woodruff T.M. (2009) Toll-like receptors in ischemia-reperfusion injury. Shock 32, 4–16 10.1097/SHK.0b013e318193e333 [DOI] [PubMed] [Google Scholar]
- 26.Watson M.J., Ke B., Shen X.D., Gao F., Busuttil R.W., Kupiec-Weglinski J.W.. et al. (2008) Intestinal ischemia/reperfusion injury triggers activation of innate toll-like receptor 4 and adaptive chemokine programs. Transplant. Proc. 40, 3339–3341 10.1016/j.transproceed.2008.07.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatum P.J., Harmon C.M., Lorenz R.G. and Dimmitt R.A. (2010) Toll-like receptor 4 is protective against neonatal murine ischemia-reperfusion intestinal injury. J. Pediatr. Surg. 45, 1246–1255 10.1016/j.jpedsurg.2010.02.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deitch E.A. (1992) Multiple organ failure. Pathophysiology and potential future therapy. Ann. Surg. 216, 117–134 10.1097/00000658-199208000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X., Hua Y., Chen H., Yang H., Zhang T., Huang G.. et al. (2015) Aldehyde dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt/beta-catenin signaling pathway. Ther. Clin. Risk Manag. 11, 1371–1381 10.2147/TCRM.S88297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinrinmade J.F., Akinrinde S.A., Odejobi A. and Oyagbemi A.A. (2015) Evidence of attenuation of intestinal ischemia-reperfusion injury following pre-treatment with methanolic extracts from Chromolena odorata in rats. J. Compl. Integr. Med. 12, 23–32 [DOI] [PubMed] [Google Scholar]
- 31.Impellizzeri D., Cordaro M., Campolo M., Gugliandolo E., Esposito E., Benedetto F.. et al. (2016) Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (BJe) in a mouse model of intestinal ischemia/reperfusion injury. Front. Pharmacol. 7, 203. 10.3389/fphar.2016.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L., Liu B., Dai Z., Zhang H.F., Zhang Y.S., Luo X.J.. et al. (2012) Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur. J. Pharmacol. 678, 32–38 10.1016/j.ejphar.2011.12.042 [DOI] [PubMed] [Google Scholar]
- 33.Li R.J., Ji W.Q., Pang J.J., Wang J.L., Chen Y.G. and Zhang Y. (2013) Alpha-lipoic acid ameliorates oxidative stress by increasing aldehyde dehydrogenase-2 activity in patients with acute coronary syndrome. Tohoku J. Exp. Med. 229, 45–51 10.1620/tjem.229.45 [DOI] [PubMed] [Google Scholar]
- 34.Grootjans J., Hodin C.M., de Haan J.J., Derikx J.P., Rouschop K.M., Verheyen F.K.. et al. (2011) Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology 140, 529–539 10.1053/j.gastro.2010.10.040 [DOI] [PubMed] [Google Scholar]
- 35.Chiu C.C., Yeh T.H., Lai S.C., Wu-Chou Y.H., Chen C.H., Mochly-Rosen D.. et al. (2015) Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp. Neurol. 263, 244–253 10.1016/j.expneurol.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoeb M., Ansari N.H., Srivastava S.K. and Ramana K.V. (2014) 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 21, 230–237 10.2174/09298673113209990181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breitzig M., Bhimineni C., Lockey R. and Kolliputi N. (2016) 4-Hydroxy-2-nonenal: a critical target in oxidative stress?. Am. J. Physiol. Cell Physiol. 311, C537–C543 10.1152/ajpcell.00101.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan C., Xing J.H., Zhang C., Zhang Y.M., Zhang L.T., Wei S.J.. et al. (2016) Aldehyde dehydrogenase 2 inhibits inflammatory response and regulates atherosclerotic plaque. Oncotarget 7, 35562–35576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Covert M.W., Leung T.H., Gaston J.E. and Baltimore D. (2005) Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science 309, 1854–1857 10.1126/science.1112304 [DOI] [PubMed] [Google Scholar]
- 40.Pope M.R., Hoffman S.M., Tomlinson S. and Fleming S.D. (2010) Complement regulates TLR4-mediated inflammatory responses during intestinal ischemia reperfusion. Mol. Immunol. 48, 356–364 10.1016/j.molimm.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben D.F., Yu X.Y., Ji G.Y., Zheng D.Y., Lv K.Y., Ma B.. et al. (2012) TLR4 mediates lung injury and inflammation in intestinal ischemia-reperfusion. J. Surg. Res. 174, 326–333 10.1016/j.jss.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 42.Reino D.C., Palange D., Feketeova E., Bonitz R.P., Xu D.Z., Lu Q.. et al. (2012) Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock 38, 107–114 10.1097/SHK.0b013e318257123a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker S.S., Baker R.D., Liu W., Nowak N.J. and Zhu L. (2010) Role of alcohol metabolism in non-alcoholic steatohepatitis. PLoS One 5, A82–A92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garbati M.R., Rathbun R.K., Yates J.E., Yang W. and Bagby G.C. (2014) ALDH1A1 dysfunction in FA-deficient macrophages contributes to aberrant activation of the innate immune response. Blood 124, 4107 [Google Scholar]