Abstract
Objective
To determine whether baseline nausea or prior triptan treatment for migraine impact the effectiveness of diclofenac potassium for oral solution in treating acute migraine.
Background
A great deal of variability exists in patients' response to migraine medications. Migraine‐associated nausea is common and debilitating and can reduce the effectiveness of oral medications. It may cause patients to delay taking oral medications, which is known to diminish therapeutic outcomes, or to avoid taking them altogether. Gastroparesis, which may be associated with nausea, also inhibits drug absorption, resulting in lower bioavailability. Studies have shown that having nausea at the time of drug administration predicts a poorer response to triptan treatment. It is of interest to understand how effective other migraine medications are in patients with a poor response to triptans.
Methods
Data from two randomized, double‐blind, placebo controlled trials were pooled and post hoc subgroup analyses were performed in patients with and without nausea at baseline, and in patients with and without prior triptan treatment. Efficacy assessments included the percentage of patients who, at 2 hours postdosing, were headache pain‐free (2hPF, primary endpoint), without photophobia, without phonophobia, without nausea, or without a severe degree of disability. A Cochran–Mantel–Haenszel test, stratified by analysis center was used to evaluate treatment effect. Effects of nausea or prior triptan use were determined using logistic regression with factors of treatment group, analysis center, nausea or prior triptan use at time of dosing, and interaction of treatment group by nausea or prior triptan use at time of dosing.
Results
The modified intent to treat population consisted of 1272 patients, 644 on active drug and 628 on placebo. The majority of patients (85%) were female. At the time of dosing, 783 (62%) patients reported nausea with the treated attack. Prior triptan use was recorded in 570 (45%). For headache pain, nausea, photophobia, and phonophobia, patients in the active treatment group had a statistically significantly better response than those receiving placebo, regardless of whether they had nausea at baseline. In logistic regression analysis only treatment group predicted a response for these parameters with no detectable group interaction. Baseline nausea, as well as treatment group, predicted whether patients recorded severe disability at 2 hours. While patients in the active treatment group were significantly more likely to be headache pain‐free at 2 hours after dosing, whether or not they had previously been treated with triptan, more triptan‐naïve patients (30%) than triptan‐experienced patients (20%) were headache pain‐free. Interestingly, in the placebo groups, triptan‐naïve patients were also more likely to be PF (14% vs 7%). In the logistic regression analysis, treatment group predicted a headache pain response, triptan use predicted a lack of response, and there was no interaction between the two. Prior triptan use did not predict any of the other outcome measures.
Conclusions
Nausea at the time of dosing does not diminish the effectiveness of diclofenac potassium for oral solution. The rapid absorption profile may enhance the effectiveness in patients with nausea. Prior triptan use predicted poorer headache response at 2 hours postdose, suggesting the possibility of a subset of patients who are more likely to be refractory to both triptans and diclofenac. Diclofenac potassium for oral solution is effective in triptan‐naïve patients but no reliable inference can be made from this study as to about how to order treatment.
Keywords: migraine, nausea, vomiting, triptan, diclofenac potassium, oral solution
Abbreviations
- 2hPF
headache pain‐free at 2 hours postdosing
- AE
adverse event
- CI
confidence interval
- DPOS
diclofenac potassium for oral solution
- LOCF
last observation carried forward
- mITT
modified intent to treat
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- WOCF
worst observation carried forward
INTRODUCTION
Migraine is a prevalent and debilitating condition. In a 2015 literature review and survey of publically available U.S. health surveillance databases, the overall age‐adjusted 3‐month prevalence of migraine in females was 19.1% and in males 9.0%.1 Headache or pain in the head accounted for 3% of all emergency department visits in 2009‐2010 in the United States.1
The mainstays of acute migraine treatment are nonsteroidal anti‐inflammatory drugs (NSAIDS) and triptans.2, 3 It is difficult to predict which medication will work for any given patient and many patients will attempt multiple pharmacological approaches.4, 5
Gastrointestinal symptoms of migraine, specifically nausea with or without vomiting, are common and debilitating.6 The causes of nausea may be multifactorial. Delayed gastric emptying (gastroparesis) has been implicated as one important cause.7, 8, 9, 10 However, the fact that migraine sufferers may also experience gastroparesis outside of a migraine attack with no relationship to nausea suggests that nausea may also (or alternatively) be caused by central brainstem processes.11
Nausea can reduce the effectiveness of oral medications to treat migraine. It may cause patients to delay taking oral medications,12 which is known to diminish therapeutic outcomes,13 or to avoid taking them altogether.12 Gastroparesis, which may be associated with nausea, also inhibits drug absorption, resulting in lower bioavailability of oral medications intended to treat migraine.8, 9, 10, 14, 15, 16
In a retrospective analysis of headache response in patients treated with sumatriptan and included in the Sumatriptan Naratriptan Aggregate Patient database, absence of baseline nausea was one of seven significant (P < .01) predictors of headache relief (n = 2389) and one of nine significant (P < .01) predictors of pain‐free response at 2 hours (n = 2657).17 In another analysis of pooled data from 10 double‐blind, placebo‐controlled, parallel‐group, multicenter studies (n = 8473), baseline nausea in eletriptan‐ or sumatriptan‐treated patients was one of the three strongest predictors of failure to achieve a 2‐hour pain‐free response (odds ratio, 1.24; 95% confidence interval [CI] 1.1‐1.4; P < .0004).18
CAMBIA® (diclofenac potassium for oral solution, DPOS) is an NSAID indicated for the acute treatment of migraine attacks with or without aura in adults 18 years of age or older.19 It is more rapidly absorbed than diclofenac potassium tablets and thus may have a more rapid onset of analgesia.20, 21 In two phase 3 multicenter, double‐blind, placebo‐controlled, clinical trials, the percentage of patients who were pain‐free 2 hours after dosing was significantly higher in patients taking DPOS than in those who received placebo.22, 23 In one of these trials, DPOS also provided better relief from nausea, photophobia, and phonophobia compared with placebo.23 In the other trial, DPOS was also statistically superior to diclofenac potassium tablets with respect to frequency of patients who were pain‐free at 2 hours, those with a sustained pain‐free response, and with a sustained headache response.22
Given the evidence that baseline nausea predicts a poorer response to migraine treatment with triptans, we sought to understand whether a similar phenomenon applies to treatment with DPOS. Here, we present a post hoc subanalysis of data from the two phase 3 trials evaluating the association between nausea at the time of DPOS treatment, and headache response, presence of phono‐ and photophobia, nausea, and overall disability at 2 hours after DPOS treatment. We also evaluate whether an association exists between prior triptan treatment for migraine and these same parameters.
METHODS
Data from two randomized, or parallel group,23 placebo‐controlled, phase 3 clinical studies were pooled for this post hoc analysis (Fig. 1). One study was a double‐blind, cross‐over (patients were switched between tablet and powder) study conducted in 21 centers in five European countries,22 and the other study was a parallel group study conducted in 23 U.S. centers.23 The U.S. study was a single migraine attack study,23 whereas the European study was a treatment of three separate migraine attacks each preceded by a symptom‐free and acute headache medication‐free period of 48 hours.22
Figure 1.
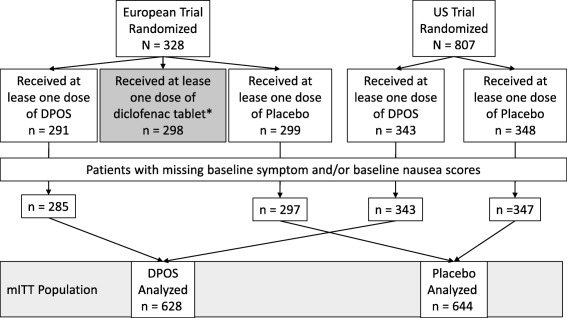
Simplified patient disposition. *This analysis does not include diclofenac tablet. For additional details please refer to the original publications of these studies.22, 23 DPOS, diclofenac potassium for oral solution.
Patients were aged 18 to 65 years, with at least a one year history of migraine attacks, with or without aura. In the European study, patients had to have two to six migraine attacks per month over the previous 3 months. In the U.S. study, patients had experienced one to six attacks per month for the prior 12 months, with 10 or fewer headache days per month. Prophylactic treatment for migraine was permitted provided patients had been on a consistent dosing regimen. In the U.S. study, patients with a history of vomiting 20% of the time during migraine attacks or who were usually so incapacitated as to require bed rest during the attack were excluded. Further inclusion/exclusion criteria have been previously published.22, 23
Patients received 50 mg DPOS or placebo that they were directed to take, in an outpatient setting, at the first sign of migraine. In the European study, an additional arm was included in which patients were treated with diclofenac potassium tablets. That treatment arm is not included in this post hoc analysis. Patients were requested not to take rescue medications within 2 hours after taking study medication.
All information was recorded using a standard headache diary. Patients recorded self‐evaluations of headache pain intensity, nausea, vomiting, photophobia, phonophobia, and ability to function at baseline (prior to taking study medication) and at 1, 2, and 8 hours postdose. Additional details have previously been published.22, 23
All clinical investigations were conducted in compliance with Good Clinical Practice Guidelines in accordance with the regulations of the Declaration of Helsinki, and pre‐approved by local ethics committees at individual study centers (institutional review boards). Study subjects provided informed consent prior to enrollment.
Subgroup analyses were performed in patients with or without nausea at baseline and in patients with or without prior triptan treatment. Patients may have received triptan treatment at any time prior to the study up to 7 days preceding the migraine attack to be evaluated for frovatriptan, or 24 hours prior the attack for sumatriptan, zolmitriptan, naratriptan, and rizatriptan. Prespecified efficacy assessments included the percentage of patients who, at 2 hours were (a) headache pain‐free (primary endpoint), (b) without photophobia (secondary endpoint), (c) without phonophobia (secondary endpoint), (d) without nausea (exploratory endpoint), and (e) without a severe degree of disability (exploratory endpoint) all at 2 hours postdosing.
Statistical Analyses
The efficacy analysis included a modified intent to treat (mITT) population that excluded patients with missing baseline symptom and/or baseline nausea scores. Analyses were conducted on the available data, and no formal a priori power calculation was used to guide sample size. All statistical analyses were performed using SAS version 9. A Cochran–Mantel–Haenszel test, stratified by analysis center, was used to evaluate treatment effect. Effects of nausea or prior triptan use were determined using one‐tailed logistic regression with factors of treatment group, analysis center, nausea or prior triptan use at time of dosing, and interaction of treatment group by nausea or prior triptan use at time of dosing. An α ≤ .05 was considered significant. Last observation carried forward (LOCF) was used to impute missing observations. Worst observation carried forward (WOCF) was used to impute/replace values after rescue medication use.
Adverse events were collected in the safety population, which included all patients who received at least one dose of study drug or placebo.
RESULTS
Patient Demographics and Characteristics
The pooled mITT population included 1272 patients; 628 received DPOS and 644 received placebo. Patient demographics and baseline characteristics are shown in Table 1. The majority of patients (85%) were female. At the time of dosing, 783 (62%) patients recorded experiencing nausea. Prior triptan use was recorded in 570 (45%).
Table 1.
Demographic and Baseline Characteristics
| DPOS | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
|
Triptan‐Experienced n = 286 |
Triptan Naïve n = 342 |
Nausea
†
n = 391 |
No Nausea n = 237 |
Triptan‐Experienced n = 284 |
Triptan Naïve n = 360 |
Nausea
†
n = 392 |
No Nausea n = 252 |
|
| Age, mean (SD) | 42.4 (11.4) | 37.7 (11.1) | 39.8 (11.2) | 40.1 (12.0) | 42.7 (11.0) | 37.3 (11.0) | 39.4 (11.0) | 40 (12.3) |
| Male, n (%) | 36 (12.6) | 54 (15.8) | 45 (11.5) | 45 (19.0) | 31 (12.0) | 62 (17.2) | 40 (10.2) | 56 (22.2) |
| Race, n (%) | ||||||||
| White | 276 (96.5) | 284 (83.0) | 351 (89.8) | 209 (88.2) | 266 (93.7) | 306 (85.0) | 350 (89.3) | 222 88.1) |
| Black/African American | 5 (1.7) | 48 (14.0) | 31 (7.9) | 22 (9.3) | 13 (4.6) | 47 (13.1) | 34 (8.7) | 26 (10.3) |
| Asian | 2 (0.7) | 1 (0.3) | 1 (0.3) | 2 (0.8) | 0 | 3 (0.8) | 3 (0.8) | 0 (.0) |
| Other | 3 (1) | 9 (2.6) | 8 (2.0) | 4 (1.7) | 5 (1.8) | 4 (1.1) | 5 (1.3) | 4 (1.6) |
| Presence of nausea, n (%) | 174 (60.8) | 217 (63.5) | 391 (100.0) | 0 (.0) | 167 (58.8) | 225 (62.5) | 392 (100.0) | 0 (.0) |
| Presence of photophobia, n (%) | 239 (83.6) | 278 (81.3) | 335 (85.7) | 182 (76.8) | 233 (82.0) | 280 (77.8) | 323 (82.4) | 190 (75.4) |
| Presence of phonophobia, n (%) | 219 (76.6) | 253 (74.0) | 298 (76.2) | 174 (73.4) | 217 (76.4) | 271 (75.3) | 307 (78.3) | 181 (71.8) |
| Vomiting, n (%) | 10 (3.5) | 20 (5.8) | 26 (6.6) | 4 (1.7) | 13 (4.6) | 24 (6.7) | 30 (7.7) | 7 (2.8) |
†At time of dosing. DPOS = diclofenac potassium for oral solution.
Impact of Baseline Nausea on Treatment Response
In patients with baseline nausea, 50% of DPOS‐treated patients and 35% of those who received placebo were nausea‐free 2 hours post‐treatment (P < .001). Treatment‐emergent nausea was observed in 12% of those treated with DPOS and 18% of those who received placebo (P = .048). More DPOS‐treated patients were headache pain‐free at 2 hours postdose compared with placebo‐treated patients, regardless of whether they had nausea at baseline (25% vs 9% among those with nausea and 26% vs 13% of those without nausea, P < .001 for both). When accounting for the factors of treatment group, analysis center, nausea at time of dosing, and treatment group by nausea, only treatment group (P < .001) predicted a 2hPF response (Fig. 2A).
Figure 2.
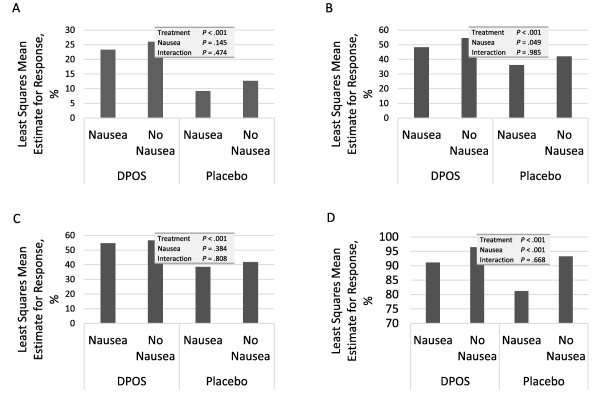
Impact of nausea at baseline on headache pain response, photophobia, phonophobia, and overall degree of disability (mITT population). (A) Percentage of patients who were headache‐free at 2 hours. (B) Percentage of patients without photophobia at 2 hours. (C) Percentage of patients without phonophobia at 2 hours. (D) Percentage of patients without severe disability at 2 hours. DPOS, diclofenac potassium for oral solution.
Among patients with baseline nausea, 45% of DPOS‐treated patients had no photophobia at 2 hours compared with 33% for placebo (P = .002). Corresponding values for those without nausea were 54% and 43% (P = .009). After adjusting for covariates, both treatment group (P < .001) and baseline nausea (P = .049) predicted a photophobia‐free response (Fig. 2B).
Absence of phonophobia at 2 hours was recorded for 51% of DPOS‐treated patients with baseline nausea and 36% of those receiving placebo (P < .001). In those without baseline nausea, these values were 57% and 44% (P = .002). Only treatment group (P < .001) predicted phonophobia at 2 hours (Fig. 2C).
Both baseline nausea (P < .001) and treatment group (P < .001) predicted degree of disability at 2 hours (Fig. 2D). Among those with baseline nausea, 10% of those treated with DPOS and 19% of those receiving placebo reported a severe degree of disability. In those with no baseline nausea, the corresponding values were 4% and 8%.
Treatment Response in Patients with Prior Triptan Treatment for Migraine
In patients with prior triptan use, 20% of patients in the DPOS group and 7% of those in the placebo group were headache pain‐free at 2 hours following dosing (P < .001). In triptan‐naïve patients, 30% and 14% in the DPOS and placebo groups, respectively, were headache pain‐free (P < .001). While treatment group predicted a response, triptan use predicted a lack of response (Fig. 3A). No interaction among variables was detected in the regression analysis.
Figure 3.
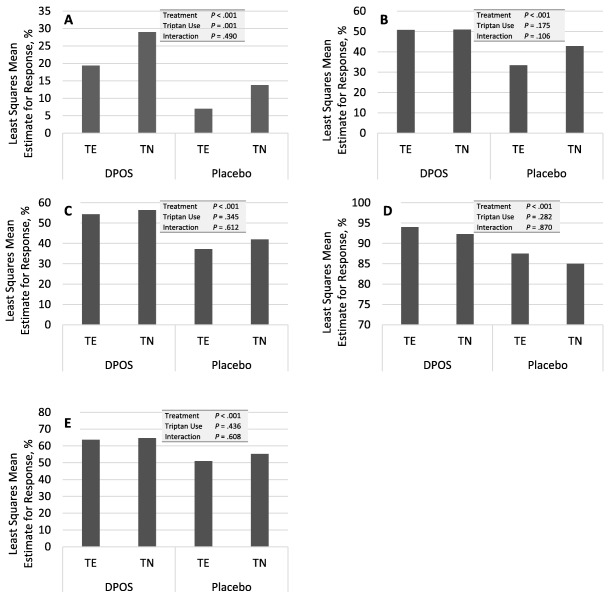
Impact of prior triptan use on headache pain response, photophobia, phonophobia, and overall degree of disability (mITT population). (A) Percentage of patients who were headache‐free at 2 hours. (B) Percentage of patients without photophobia at 2 hours. (C) Percentage of patients without phonophobia at 2 hours. (D) Percentage of patients without severe disability at 2 hours. (E) Percentage of patients nausea‐free at 2 hours. DPOS, diclofenac potassium for oral solution; TE, triptan‐experienced; TN, triptan‐naïve.
Prior triptan use did not predict relief from nausea, photophobia, phonophobia, nor degree of disability at 2 hours postdose (Fig. 3B,C,D,E). In triptan‐experienced patients, 63% of DPOS‐treated, and 51% of placebo patients were nausea‐free at 2 hours (P = .002). Among those who were triptan‐naïve, 66% and 56% were nausea‐free at 2 hours (P = .013). In the triptan experienced group, 47% and 31% were photophobia‐free in the DPOS and placebo group, respectively (P < .001). Among those with no prior triptan use, these values were 49% and 42% (P = .037). Corresponding values for those who were phonophobia‐free at 2 hours were 50% and 35% in the triptan‐experienced group (P < .001) and 56% and 43% in the triptan‐naïve group (P < .001).
Adverse Events and Treatment‐Related Adverse Events
Table 2 shows pooled adverse events (AE) reported in ≥1% of patients in the two phase 3 trials. The most commonly occurring AEs were gastrointestinal disorders. Nausea was reported as an AE in 4% of patients receiving DPOS and 3% of those receiving placebo. Nausea was reported as possibly or probably treatment‐related in 3% and 2%, respectively.
Table 2.
Adverse Events and Treatment‐Related Adverse Events with Incidences ≥1% (Safety Population)
| n (%) | DPOS (n = 634) | Placebo (n = 646) |
|---|---|---|
| Adverse Events | ||
| Gastrointestinal disorders | 51 (8) | 48 (7) |
| Diarrhea | 3 (<1) | 8 (1) |
| Dyspepsia | 7 (1) | 6 (<1) |
| Nausea | 25 (4) | 18 (3) |
| Vomiting | 8 (1) | 5 (<1) |
| Nervous system disorders | 19 (3) | 19 (3) |
| Dizziness | 7 (1) | 7 (1) |
| Somnolence | 4 (<1) | 8 (1) |
| Psychiatric disorders | 9 (1) | 1 (<1) |
| Treatment‐Related Adverse Events | ||
| Patients with 1 or more events | 56 (9) | 45 (7) |
| Gastrointestinal disorders | 39 (6) | 31 (5) |
| Nausea | 17 (3) | 14 (2) |
| Nervous system disorders | 12 (2) | 10 (2) |
| Dizziness | 7 (1) | 3 (<1) |
DISCUSSION AND CONCLUSIONS
Variability in the effectiveness of migraine medications in individual patients confounds treatment. A key contributing factor to variability, at least for orally administered triptans, may be inefficient absorption, particularly in patents with gastroparesis.8, 9, 10, 14, 15, 16 Baseline nausea is often associated with gastroparesis and is one factor that predicts the effectiveness of triptans.17, 18 In this study using pooled data from two phase 3 randomized controlled trials, we investigated whether nausea similarly predicted a response to DPOS, a rapidly absorbed oral formulation of diclofenac potassium.20, 21
Baseline nausea was present in more than half (62%) of the 1272 patients included in this analysis. For headache pain, nausea, photophobia, and phonophobia, all at 2 hours postdose, patients treated with DPOS had a statistically significantly better response than those receiving placebo, regardless of whether they had nausea at baseline. In a logistic regression analysis, accounting for factors of treatment group, analysis center, nausea at time of dosing, and treatment group by nausea, only treatment group predicted a response for these parameters with no detectable interaction among variables. In contrast, baseline nausea, as well as treatment group, did predict whether patients recorded severe disability at 2 hours (with no interaction between the two). This result may not be surprising as nausea has previously been demonstrated to independently contribute to disability associated with migraine and its impact.24 Despite this fact, in this analysis more than 90% of patients with nausea in the DPOS group were without severe disability 2 hours postdose. These results demonstrate that nausea at the time of dosing does not diminish the effectiveness of DPOS relative to placebo. As nausea may be induced by headache‐associated gastroparesis, the rapid absorption profile of DPOS20, 21 may be beneficial in this regard.
Treatment‐emergent nausea was less frequent in patients treated with DPOS (12%) than in those who received placebo (18%). In another published post hoc analysis of data from five randomized, double‐blind clinical trials using triptans to treat patients with migraine, rates of treatment‐emergent nausea were 5‐13% in those treated with rizatriptan 10 mg, 10‐20% in those treated with sumatriptan 25 mg, 50 mg, or 100 mg, naratriptan 2.5 mg, or zolmitriptan 2.5 mg, and 11‐18% of those receiving placebo.25 In contrast to the current study, in which the incidence of treatment‐emergent nausea was significantly lower for DPOS vs placebo, the difference in incidence for triptan treatments vs placebo, with the exception of sumatriptan 25 mg, did not reach statistical significance.25
In this study nausea was reported as an AE at similar low frequencies for DPOS and placebo (4% and 3%, respectively). It is notable that the incidence of treatment‐emergent nausea recorded as an investigator‐elicited outcome in these studies was higher than the incidence of nausea recorded as an AE. The reason for the differences are unclear, but may reflect the severity of the nausea and/or may reflect elicited vs spontaneous mentions of nausea. The incidence of nausea as an AE in the post hoc analysis of triptan trials was 5‐7% for rizatriptan, 9% for sumatriptan 100 mg, 6% for sumatriptan 50 mg, 5% for sumatriptan 25 mg, 3% for naratiptan, 4% for zolmitriptan, and 1‐5% for placebo.25
A systematic review of reports of measures of persistence and/or switching patterns in migraineurs who were prescribed triptans, indicated that the proportion of who remained persistent for up to six refills of an index triptan ranged from 3.2% to 12.6%. The one‐year probability of discontinuation was estimated to be between 30% and 60%.26 In a recently published multicenter, cross‐sectional survey (N = 276) performed at U.S. tertiary care headache clinics, multivariate modeling showed that the strongest correlate of triptan discontinuation was lack of efficacy (odds ratio = 17; 95% CI, 8.8‐33.0).27 It was therefore of interest to investigate how patients enrolled in the phase 3 trials of DPOS and who had previously been treated with a triptan responded to DPOS. While patients treated with DPOS were significantly more likely to be headache pain‐free at 2 hours after dosing, whether or not they had previously been treated with triptan, triptan‐naïve patients (30%) were more likely to be PF than triptan‐experienced patients (20%). Interestingly, in the placebo groups, triptan‐naïve patients were also more likely to be PF (14% vs 7%). In the logistic regression analysis, treatment group predicted a response, triptan use predicted a lack of response, and there was no interaction between the two. These results suggest there may be a subset of patients who are more likely to be refractory to both triptans and diclofenac. However, this notion would require more rigorous investigation in an appropriately designed trial. Prior triptan use did not predict any of the other outcome measures (photophobia, phonophobia, severe disability, or nausea at 2 hours). No reliable inference can be made from this study as to about how to order treatment.
This study has several limitations. Nausea was scored as an all or none variable, and variations in nausea severity are not accounted for. In addition, in the U.S. trial, patients were excluded if they experiencing vomiting during ≥20% of migraine attacks, or were taking medications that could cause nausea and vomiting. These exclusion criteria do not limit the statistical validity of this study, but may limit generalization in the real world.
STATEMENT OF AUTHORSHIP
Category 1
-
(a) Conception and Design
Richard Lipton, Pete Schmidt, Hans‐Christoph Diener
-
(b) Acquisition of Data
Richard Lipton, Hans‐Christoph Diener
-
(c) Analysis and Interpretation of Data
Richard Lipton, Pete Schmidt, Hans‐Christoph Diener
Category 2
-
(a) Drafting the Manuuscript
Pete Scmidt
-
(b) Revising It for Intellectual Content
Pete Schmidt, Richard Lipton, Hans‐Christoph Diener
Category 3
-
(a) Final Approval of the Completed Manuscript
Pete Schmidt, Richard Lipton, Hans‐Christoph Diener
Acknowledgments
Funding for this analysis provided by Depomed, Inc. Statistical analyses were performed by QST Consultations, LTD. Medical writing provided by Pamela Foreman, PhD, of Depomed, Inc.
Conflict of Interest: Yes.
Disclosures: RBL receives research support from the NIH: PO1 AG003949 (Program Director), RO1AG025119 (Investigator), RO1AG022374‐06A2 (Investigator), RO1AG034119 (Investigator), RO1AG12101 (Investigator), and the National Headache Foundation; serves on the editorial boards of Neurology and as senior advisor to Headache. He has reviewed for the NIA and NINDS, holds stock options in eNeura Therapeutics; serves as consultant, advisory board member, or has received honoraria from: Alder, Allergan, American Headache Society, Autonomic Technologies, Avanir, Boston Scientific, Colucid, Dr. Reddy's, Electrocore, Eli Lilly, eNeura Therapeutics, Informa, Merck, Novartis, Pfizer, Teva, Vedanta. He receives royalties from Wolff's Headache, 8th Edition, Oxford Press University, 2009. PS is an employee of Depomed, Inc. HCD received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Böhringer Ingelheim, Bristol‐Myers Squibb, Chordate, Coherex, CoLucid, Electrocore, GlaxoSmithKline, Grünenthal, Janssen‐Cilag, Labrys Biologics, Lilly, La Roche, 3M Medica, Medtronic, Menerini, Minster, MSD, Neuroscore, Novartis, Johnson & Johnson, Pierre Fabre, Pfizer, Schaper and Brümmer, Sanofi, St. Jude, Teva, and Weber & Weber. Financial support for research projects was provided by Allergan, Almirall, AstraZeneca, Bayer, Electrocore, GSK, Janssen‐Cilag, MSD, and Pfizer. Headache research at the Department of Neurology in Essen is supported by the German Research Council (DFG), the German Ministry of Education and Research (BMBF), and the European Union. HCD has no ownership interest and does not own stocks of any pharmaceutical company.
Funding: Funding for this analysis provided by Depomed, Inc.
Clinical Trial Registration: NCT00330850
REFERENCES
- 1. Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache. 2015;55:21‐34. [DOI] [PubMed] [Google Scholar]
- 2. Becker WJ. Acute migraine treatment in adults. Headache. 2015;55:778‐793. [DOI] [PubMed] [Google Scholar]
- 3. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3‐20. [DOI] [PubMed] [Google Scholar]
- 4. Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: Experience from 3 centers in US and Sweden. Headache. 2007;47:475‐479. [DOI] [PubMed] [Google Scholar]
- 5. Katic BJ, Rajagopalan S, Ho TW, Chen YT, Hu XH. Triptan persistency among newly initiated users in a pharmacy claims database. Cephalalgia. 2011;31:488‐500. [DOI] [PubMed] [Google Scholar]
- 6. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 7. Boyle R, Behan PO, Sutton JA. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br J Clin Pharmacol. 1990;30:405‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tokola RA. The effect of metoclopramide and prochlorperazine on the absorption of effervescent paracetamol in migraine. Cephalalgia. 1988;8:139‐147. [DOI] [PubMed] [Google Scholar]
- 9. Tokola RA, Neuvonen PJ. Effects of migraine attack and metoclopramide on the absorption of tolfenamic acid. Br J Clin Pharmacol. 1984;17:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volans GN. Migraine and drug absorption. Clin Pharmacokinet. 1978;3:313‐318. [DOI] [PubMed] [Google Scholar]
- 11. Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: More than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57‐63. [DOI] [PubMed] [Google Scholar]
- 12. Gallagher RM, Kunkel R. Migraine medication attributes important for patient compliance: Concerns about side effects may delay treatment. Headache. 2003;43:36‐43. [DOI] [PubMed] [Google Scholar]
- 13. Valade D. Early treatment of acute migraine: New evidence of benefits. Cephalalgia. 2009;29(Suppl. 3):15‐21. [DOI] [PubMed] [Google Scholar]
- 14. Aurora S, Kori S, Barrodale P, Nelsen A, McDonald S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007;47:1443‐1446. [DOI] [PubMed] [Google Scholar]
- 15. Aurora SK, Papapetropoulos S, Kori SH, Kedar A, Abell TL. Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia. 2013;33:408‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomsen LL, Dixon R, Lassen LH, et al. 311C90 (Zolmitriptan), a novel centrally and peripheral acting oral 5‐hydroxytryptamine‐1D agonist: A comparison of its absorption during a migraine attack and in a migraine‐free period. Cephalalgia. 1996;16:270‐275. [DOI] [PubMed] [Google Scholar]
- 17. Christoph‐Diener H, Ferrari M, Mansbach H. Predicting the response to sumatriptan: The Sumatriptan Naratriptan Aggregate Patient Database. Neurology. 2004;63:520‐524. [DOI] [PubMed] [Google Scholar]
- 18. Diener HC, Dodick DW, Goadsby PJ, Lipton RB, Almas M, Parsons B. Identification of negative predictors of pain‐free response to triptans: Analysis of the eletriptan database. Cephalalgia. 2008;28:35‐40. [DOI] [PubMed] [Google Scholar]
- 19. CAMBIA® . Diclofenac Potassium for Oral Solution [package insert]. Newark, CA: Depomed, Inc; 2014. [Google Scholar]
- 20. Reiner V, Reiner A, Reiner G, Conti M. Increased absorption rate of diclofenac from fast acting formulations containing its potassium salt. Arzneimittelforschung. 2001;51:885‐890. [DOI] [PubMed] [Google Scholar]
- 21. Marzo A, Dal Bo L, Verga F, et al. Pharmacokinetics of diclofenac after oral administration of its potassium salt in sachet and tablet formulations. Arzneimittelforschung. 2000;50:43‐47. [DOI] [PubMed] [Google Scholar]
- 22. Diener HC, Montagna P, Gacs G, et al. Efficacy and tolerability of diclofenac potassium sachets in migraine: A randomized, double‐blind, cross‐over study in comparison with diclofenac potassium tablets and placebo. Cephalalgia. 2006;26:537‐547. [DOI] [PubMed] [Google Scholar]
- 23. Lipton RB, Grosberg B, Singer RP, et al. Efficacy and tolerability of a new powdered formulation of diclofenac potassium for oral solution for the acute treatment of migraine: Results from the International Migraine Pain Assessment Clinical Trial (IMPACT). Cephalalgia. 2010;30:1336‐1345. [DOI] [PubMed] [Google Scholar]
- 24. Lipton RB, Buse DC, Saiers J, Fanning KM, Serrano D, Reed ML. Frequency and burden of headache‐related nausea: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:93‐103. [DOI] [PubMed] [Google Scholar]
- 25. Lipton RB, Pascual J, Goadsby PJ, et al. Effect of rizatriptan and other triptans on the nausea symptom of migraine: A post hoc analysis. Headache. 2001;41:754‐763. [DOI] [PubMed] [Google Scholar]
- 26. Messali AJ, Yang M, Gillard P, et al. Treatment persistence and switching in triptan users: A systematic literature review. Headache. 2014;54:1120‐1130. [DOI] [PubMed] [Google Scholar]
- 27. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache. 2014;54:278‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]


