H2A.Z is removed from nucleosomes localized in genes upon transcriptional activation in response to drought stress conditions in Arabidopsis thaliana.
Abstract
The influence of the histone variant H2A.Z on transcription remains a long-standing conundrum. Here, by analyzing the actin-related protein6 mutant, which is impaired in H2A.Z deposition, and by H2A.Z profiling in stress conditions, we investigated the impact of this histone variant on gene expression in Arabidopsis thaliana. We demonstrate that the arp6 mutant exhibits anomalies in response to osmotic stress. Indeed, stress-responsive genes are overrepresented among those hyperactive in arp6. In wild-type plants, these genes exhibit high levels of H2A.Z in the gene body. Furthermore, we observed that in drought-responsive genes, levels of H2A.Z in the gene body correlate with transcript levels. H2A.Z occupancy, but not distribution, changes in parallel with transcriptional changes. In particular, we observed H2A.Z loss upon transcriptional activation and H2A.Z gain upon repression. These data suggest that H2A.Z has a repressive role in transcription and counteracts unwanted expression in noninductive conditions. However, reduced activity of some genes in arp6 is associated with distinct behavior of H2A.Z at their +1 nucleosome, which exemplifies the requirement of this histone for transcription. Our data support a model where H2A.Z in gene bodies has a strong repressive effect on transcription, whereas in +1 nucleosomes, it is important for maintaining the activity of some genes.
INTRODUCTION
The organization of chromatin has profound implications for the regulation of gene expression in diverse biological processes, including genome stability, recombination, developmental reprogramming, and response to external stimuli (Feng et al., 2010; Soria et al., 2012; J. Zhu et al., 2013). The last of these processes is especially important for terrestrial plants, which as sessile organisms are inevitably exposed to daily and seasonal environmental changes (Kim et al., 2015). Plants respond to environmental stimuli by activation of signaling pathways that rapidly modify transcription rate of responsive genes and trigger physiological reactions. Thousands of genes are involved in the response; therefore, global genome regulation at the chromatin level is required to achieve the appropriate level of responsiveness (Y. Zhu et al., 2013; Probst and Mittelsten Scheid, 2015). This may be achieved by nucleosome remodeling and repositioning via covalent modifications of histone proteins, especially trimethylation of histone H3 lysine 4 (van Dijk et al., 2010; Kim et al., 2008) and redistribution of histone variants. Recent studies also suggest an important role for the H2A.Z histone variant in this process (Coleman-Derr and Zilberman, 2012).
H2A.Z is a conserved variant of histone H2A that has been implicated in different processes, such as transcriptional regulation, telomeric silencing, genome stability, cell cycle progression, DNA repair, and recombination (Zlatanova and Thakar, 2008). The distribution of this histone variant is conserved among eukaryotes. H2A.Z is highly enriched at the transcription start site (TSS) of a large set of genes across cell types, consistent with a role in the regulation of transcription. Genome-wide studies in yeast have shown that H2A.Z enrichment at promoter-distal nucleosomes is required for initiation of transcription, while being inversely correlated with transcript levels (Millar et al., 2006; Zhang et al., 2005; Guillemette et al., 2005; Rhee et al., 2014). In Drosophila melanogaster, the +1 nucleosome blocks RNA polymerase II transit, causing its increased stalling and backtracking at the promoters (Weber et al., 2014). Importantly, H2A.Z deposition at the +1 nucleosomes decreases RNA polymerase II (RNAPII) stalling, suggesting that its presence reduces the energy required for polymerase progression. H2A.Z levels negatively correlate with H3-H4 nucleosome turnover indicating that the nucleosomal H2A.Z facilitates RNAPII elongation without depletion of (H3-H4)2 tetramers.
In Arabidopsis thaliana, there are three functional genes encoding H2A.Z, HISTONE H2A 8 (HTA8), HTA9, and HTA11 (March-Díaz and Reyes, 2009). Both double and triple mutants are viable, although the last one shows severe developmental changes (Coleman-Derr and Zilberman, 2012). Deposition of H2A.Z into nucleosomes is performed by the specific chromatin remodeling complex SWI2/SNF2-Related 1 (SWR1). It has been shown that eliminating genes encoding key subunits of this complex, ACTIN-RELATED PROTEIN6 (ARP6; Choi et al., 2005; Deal et al., 2005) or PHOTOPERIOD-INDEPENDENT EARLY FLOWERING1 (PIE1; Noh and Amasino, 2003), results in inability to efficiently incorporate H2A.Z into nucleosomes and phenocopies the double mutant hta9 hta11 (March-Díaz et al., 2008), but not the triple mutant hta8 hta9 hta11 (Coleman-Derr and Zilberman, 2012). This may suggest that in the absence of a functional SWR1 complex, other mechanisms can occasionally incorporate H2A.Z into chromatin (Coleman-Derr and Zilberman, 2012; Hardy et al., 2009). However, as no other biological function of SWR1 has been identified, both arp6 and pie1 mutants have been widely used to study the effects of H2A.Z depletion from chromatin (Choi et al., 2013; Kumar and Wigge, 2010; Smith et al., 2010; Bieluszewski et al., 2015; Zilberman et al., 2008; Rosa et al., 2013).
Although the involvement of H2A.Z in the regulation of gene transcription has been extensively investigated, how H2A.Z influences gene expression in a physiological context-dependent manner has remained elusive. In this work, we present data suggesting that H2A.Z may have different effects on gene expression depending on its nucleosomal location within the gene body. At the +1 nucleosomes, H2A.Z may be required for transcriptional activity, likely by reducing the energy required by RNAPII to overcome the first nucleosomal barrier. This is consistent with previous results in Drosophila (Weber et al., 2014; Rhee and Pugh, 2012). On the other hand, H2A.Z has negative influence on transcription in nucleosomes located across gene bodies. Upon drought stress, H2A.Z is removed from induced genes independently of its location in the gene.
RESULTS
Plants Deficient in Nucleosomal H2A.Z Show a Distorted Germination Rate in Osmotic Stress Conditions
The H2A.Z variant is significantly enriched across gene bodies of responsive genes, suggesting a role in their transcriptional regulation (Coleman-Derr and Zilberman, 2012). To test this hypothesis, we sought to investigate whether H2A.Z-deficient mutants present an abnormal response to osmotic stresses. Delayed germination was observed for hta9 hta11, arp6, and pie1 mutants under control conditions suggesting that nucleosomal H2A.Z deficiency may affect seed germination (Figures 1A and 1D; Supplemental Table 1). Interestingly, we observed that the effect was stronger in the pie1-5 mutant than in hta9 hta11 and arp6 (Figures 1A and 1D). When NaCl-supplemented medium was used for germination (150 mM NaCl), the difference between arp6, pie1-5, and wild-type plants was enhanced significantly (Figures 1B and 1E; see Supplemental Table 1 for statistical analysis). In the arp6 and wild type comparison, we observed the highest difference at 96 h after stratification. For pie1-5, the highest difference was observed 144 h (radicle tip emergence) or 168 h (green cotyledon emergence) after stratification (Figures 1B and 1E). In these conditions, the difference between pie1-5 and arp6 was also increased. Surprisingly, the differences were not significant for radicle tip emergence in the hta9 hta11 double mutant, which developed green cotyledons faster than the wild type during the first 96 h after stratification (Figures 1B and 1E; Supplemental Table 1). Next, we tested the effect of sorbitol-supplemented media on plant germination. We observed a similar delay effect of this treatment on seed germination rate, which was highest between 72 and 96 h (radicle tips emergence) or 96 and 120 h (green cotyledon emergence) after stratification for all three mutants (Figures 1C and 1F; Supplemental Table 1). The double mutant hta9 hta11 exhibits a relatively mild phenotype (Figure 1). It should be noted that other phenotypic differences observed between mutants and the wild type were also reported to be smaller in hta9 hta11 than in arp6 and pie1 (March-Díaz et al., 2007; Deal et al., 2005; Choi et al., 2005; March-Díaz and Reyes, 2009). This indicates that hta9 hta11 is less representative of H2A.Z deposition deficiency than the other two, which is not surprising as the third H2A.Z-encoding gene, HTA8, remains fully functional in this line (March-Díaz et al., 2008). Interestingly, in the case of the pie1-5 mutant, the plants did not reach the wild-type germination rate defined as the radicle tip emergence percentage after 10 d (Supplemental Table 1). The difference observed between arp6 and pie1-5 phenotypes may be due to potential other functions of the PIE1 protein, which has been previously suggested (Jarillo and Piñeiro, 2015; Coleman-Derr and Zilberman, 2012; Lu et al., 2009; Berriri et al., 2016). For this reason, the arp6 mutant was used for further analyses. Collectively, our data indicate that H2A.Z deposition-deficient mutants show higher sensitivity to osmotic stress.
Figure 1.
Nucleosome H2A.Z-Depleted Plants Show Delayed Germination upon Osmotic Stress Conditions.
(A) to (C) Radicle tip emergence rate in the wild type, hta9 hta11, arp6, and pie1-5 on control media (A) and media containing 150 mM NaCl (B) and 300 mM sorbitol (C).
(D) to (F) Green cotyledon emergence on control media (D) and media containing 150 mM NaCl (E), and 300 mM sorbitol (F).
Transcriptional Changes in Response to Drought Stress in the Wild Type and the arp6 Mutant
To further investigate the impact of H2A.Z deficiency on the plant response to osmotic stress, we pursued drought stress, as severe drought changes the transcriptional activity of a large number of genes. As we believed that H2A.Z has a direct effect on gene transcriptional regulation, this would provide us with an opportunity to test, on a large scale, whether H2A.Z changes its distribution along the responsive genes during their activation or repression. The arp6 and wild-type plants did not show any obvious differences in sensitivity to drought; however, significant changes were observed at the level of gene expression.
First, we compared the transcriptional changes in response to drought stress between arp6 and wild-type plants. For clarity, genes that exhibit changes in transcript level in response to drought will be referred to as down- and upregulated, whereas genes that exhibit changes in transcript level in arp6 relative to the wild type will be referred to as hypo- and hyperactive. To minimize any potential secondary effects due to the early flowering phenotype of the arp6 mutant, we analyzed plants grown in short days for 30 d before the onset of drought conditions (arp6 plants flower in short-day photoperiod at 50 d from germination; Deal et al., 2005). Relative water content (RWC) was used as an indicator of stress progression, and after 7 d without watering, RWC of plants exposed to drought was ∼55% for arp6 and wild-type mutants and ∼85% for both genotypes grown in control conditions (Supplemental Figure 1A). In control conditions, we identified 1235 differentially expressed genes between arp6 and the wild type using an estimated false discovery rate of 5% and threshold log2 (arp6/wild type) > 0.5. Of these, 542 genes were hypoactive and 693 genes were hyperactive in arp6 (Supplemental Data Set 1A and Supplemental Figures 1B and 1C). A similar number of 1246 genes misregulated in arp6 were detected in stress conditions: 610 genes were hypoactive and 636 were hyperactive in comparison to wild-type plants (Supplemental Data Set 1B and Supplemental Figures 1B and 1C).
Analysis of the Gene Ontology (GO) term for biological function revealed that both in control and stress conditions the genes hypoactive in arp6 were significantly enriched for DNA metabolic process (P < 2.3E-04 and 4.4E-05 for control and stress, respectively). Moreover, Nitrogen compound metabolic process genes were enriched in stress conditions (P < 7.8E-04) and Response to DNA damage stimulus were enriched in control conditions (P < 2.79E-05) (Supplemental Data Sets 2A and 2B). The enrichment for these classes is indicative of the role of H2A.Z in the double-strand break repair process, which has been previously reported (Van et al., 2015; Xu et al., 2012).
Analysis of genes hyperactive in arp6 when compared with the wild type under drought conditions (636 genes) did not reveal any specific GO classes. This is in accordance with previous reports of the global effect of H2A.Z on gene expression. The germination assay and the transcriptome analysis were performed in different experimental setups; therefore, it is difficult to speculate whether the differences observed in germination upon osmotic stress result from distorted expression of single responsive genes. As we observed differences in control conditions between wild-type and arp6 mutant plants in both germination assays and the transcriptomic experiment, it is also possible that stress conditions additionally enhance distortion in seed germination already present in control conditions. By contrast, a similar comparison for control conditions (693 genes) showed overrepresentation of genes involved in Response to stimulus (P < 2.12E-09), particularly Response to chemical stimulus (P < 1.02E-06) (Supplemental Data Set 2C). This result suggests that ARP6 acts as a repressor of stress-responsive genes in control conditions.
In addition, we analyzed the expression of 27 genes selected from those identified by RNA-seq as hyperactive, hypoactive, or unaffected in our arp6 mutant (arp6suf3; Choi et al., 2005) in another arp6 allele (arp6-1; Deal et al., 2005) and in the pie1-5 mutant (Noh and Amasino, 2003). This analysis showed that all three mutant lines behave similarly when compared with wild-type plants (Supplemental Figure 2). This confirms that the arp6 allele used in this work is representative of other mutants affecting SWR1C-dependent deposition of H2A.Z.
Construction of H2A.Z-Tagged Lines and Analysis of Gene Expression in Plants Exposed to Water Deficit
To investigate H2A.Z distribution and occupancy in drought stress, we adapted the in vivo biotinylation system (Mito et al., 2005). We generated transgenic lines with Arabidopsis H2A.Z histone tagged with a peptide specifically recognized by BirA ligase (biotin ligase recognition peptide [BLRP]) and expressing BirA. A line expressing BirA, transformed with an empty vector with BLRP sequence, was used as a control in further analyses. We puri_fied biotinylated chromatin from rosette leaves following digestion with micrococcal nuclease (MNase) to mostly mononucleosomes. Comparison of H2A.Z deposition in those lines with published data on H2A.Z distribution in FLOWERING LOCUS C (Deal et al., 2007) confirmed that our tagged H2A.Z histone is deposited at the same sites as the native protein (Supplemental Figure 3A). We also performed a complementation test by crossing one of our lines with an hta9-1 hta11-1 mutant plant and observed restoration of wild-type flowering phenotype (Supplemental Figures 3B and 3C). This shows that the tagged H2A.Z is functional and may at least partially complement the native protein.
The 4-week-old H2A.Z-tagged plants were subjected to water deficit stress (see Methods). In this experiment, water content reached 45 to 55% in treated plants and ∼85% in control plants (Supplemental Figure 4). Rosette leaves were used to isolate chromatin for investigating nucleosome positioning (MNase-seq) and for affinity purification (ChAP) of H2A.Z-bound DNA followed by high-throughput sequencing (ChAP-seq; see below). To evaluate transcriptome changes in response to drought stress, we used the same plant material for RNA isolation. Genome-wide transcriptional changes were analyzed by RNA-sequencing. This resulted in identification of 3344 genes, for which we observed at least 2-fold change in transcription level when compared with control conditions. Of these, 2068 genes were downregulated, and 1276 genes were upregulated (Supplemental Data Set 3). As expected, the most overrepresented GO class for drought-induced genes was Response to stimulus (P < 2.9E-24), which includes Response to chemical stimulus (P < 6.77E-23), abiotic stimulus (P < 1.09E-22), stress (P < 1.07E-21), and endogenous stimulus (P < 2.16E-10) (Supplemental Data Set 4A). Among downregulated genes, the most overrepresented GO classes for biological processes included Photosynthesis (P < 1.14E-16) and a number of other photosynthesis-related GO classes, Metabolic process (P < 3.2E-10) connected primarily to pigment synthesis, and starch metabolism, Cell surface receptor-linked signaling pathway (P < 2.75E-09), and Response to abiotic stimulus (P < 5.14E-06) (Supplemental Data Set 4B).
The Level of H2A.Z Enrichment in Gene Bodies Correlates with the Fold of Transcriptional Response to Drought Stress
Previous work suggested that H2A.Z enrichment across gene bodies, rather than at the TSS, is correlated with higher measures of gene responsiveness (Coleman-Derr and Zilberman, 2012). However, the authors examined H2A.Z enrichment in control conditions, while applying measures of gene responsiveness from various published data gathered from different experimental conditions (e.g., different tissues). Therefore, it was not possible to test this relationship in detail and propose the actual role of H2A.Z in control of gene responsiveness. Our H2A.Z-ChAP and RNA-seq data obtained from both control and stress conditions allow us to analyze this relationship more specifically. For the purpose of this work, we defined gene drought-responsiveness as transcription fold change between control and drought stress conditions.
First, genes up- and downregulated in drought conditions were divided into subclasses according to the change in their expression (log2 fold change; from 1.5 to 3, n = 644, from 3 to 5, n = 358, and >5, n = 291 for upregulated, from −3 to −1.5, n = 896, from −5 to −3, n = 722, and <−5 n = 463 for downregulated). We observed that levels of H2A.Z positively correlate with gene responsiveness for both up- and downregulated gene groups along gene bodies, but not for TSS (Figures 2A to 2C; Supplemental Figure 5). Furthermore, we applied a method of Zilberman et al. (2007): For each expressed gene in which H2A.Z was detected (n = 16,332), we calculated H2A.Z enrichment scores by summing all H2A.Z enrichment values. This was done independently for TSS (positioned 50 to 180 bp downstream from TSS), transcription termination site (TTS; positioned 360 to 230 bp upstream from TTS), and exon start site (ESS; positioned 40 to 170 bp downstream from ESS; mean H2A.Z enrichment was used when a gene consisted of more than one exon). We grouped all H2A.Z-containing genes into percentiles based on their responsiveness calculated as a change in expression level (absolute values of log2 fold change in mRNA abundance between control and drought). Next, we calculated a total H2A.Z enrichment score by summing scores for all of the genes in each percentile. This was further used to calculate Spearman’s rank correlation coefficient. We observed that levels of H2A.Z correlate with the gene responsiveness in drought stress across gene bodies (ρ = 0.852 and 0.673; P = 2.29E-29 and 1.68E-14 for TTS and ESS, respectively) (Figure 2D). On the other hand, we observed a weak negative correlation for TSS (ρ = −0.539, P = 7.01E-9) (Figure 2D). This supports our hypothesis that H2A.Z enrichment significantly influences gene responsiveness via regulation of transcription elongation and termination, but not initiation. H2A.Z enrichment at TSS appears to play a different role in gene activation, likely by involvement in poising genes for transcription initiation (Zhang et al., 2005).
Figure 2.
H2A.Z Levels in Gene Bodies Correlate with Gene Responsiveness.
(A) and (B) H2A.Z profiles for three gene classes divided according to their change of transcript level (log2 fold change) are shown for genes induced (A) and repressed (B) in stress.
(C) Heat map visualization of H2A.Z enrichment (input [IP]) for the gene bodies represented in (A) and (B). Genes were sorted (top to bottom) from highest to lowest H2A.Z enrichment.
(D) Spearman rank correlation for H2A.Z level and expression change (absolute values from log2 fold change) for TSS, ESS, and TTS.
(E) Spearman rank correlation for H2A.Z level and expression level in control conditions for TSS, ESS, and TTS.
Importantly, when an H2A.Z enrichment score versus expression level in control conditions was calculated, weak negative correlations were observed (Spearman ρ = −0.401, −0.446, and −0.492, P = 3.61E-5, 3.29E-6, and 2.01E-7 for TSS, ESS, and TTS, respectively) (Figure 2E). Together, these data confirm that H2A.Z levels are relatively stable, permanent features of genes and have a repressive influence on gene transcription.
Genes Induced and Repressed in Stress Differ in Their Chromatin Structure
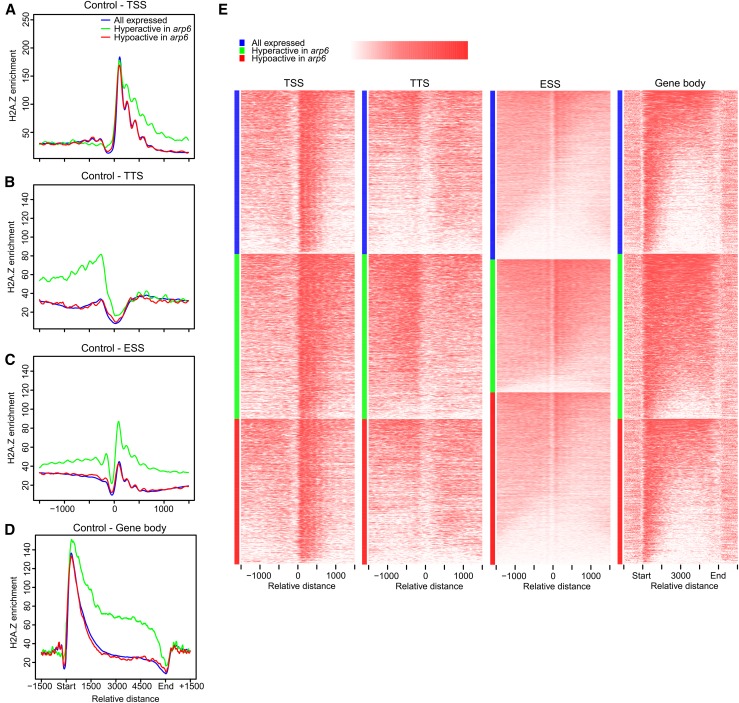
To investigate potential differences between genes upregulated and downregulated in stress, we grouped all of the Arabidopsis genes based on our RNA-seq results from the water deficit experiment into three categories: “upregulated in drought” (n = 1276), which have increased expression levels (log2 fold change > 2); “downregulated in drought” (n = 2068), which have reduced expression levels (log2 fold change < −2); and “all expressed” (n = 15,558), which provide a control set. Having defined these three groups of genes, we examined their H2A.Z pattern in the data obtained in control conditions (normal watering). As expected, we observed more H2A.Z across the gene bodies of up- and downregulated genes in comparison to “all expressed” genes (Figure 3A). Surprisingly, we detected differences in H2A.Z level in the +1 nucleosome between the three groups of genes. The genes “upregulated in drought” have significantly lower H2A.Z occupancy in the +1 nucleosome than “downregulated in drought” (P = 7.98E-13, Mann-Whitney test) and “all expressed” (P = 8.36E-12, Mann-Whitney Test) (Figure 3A). This is likely due to the fact that the +1 nucleosome is more “fuzzy” in this gene group than in the other two groups (Figures 3C and 3D). Moreover, we observed broader nucleosome-depleted regions (NDRs; a valley on plots on Figures 3C and 3D directly before a +1 nucleosome peak) in genes “upregulated in stress” (green lines) when compared with genes “downregulated in drought” and “all expressed” (red and blue lines, respectively, on Figures 3C and 3D). This might suggest that NDRs of genes induced in stress conditions have different chromatin structure than corresponding regions in constitutively active and stress-repressed genes.
Figure 3.
Distinct Pattern of H2A.Z in Constitutive, Stress-Induced, and Stress-Repressed Genes and Its Changes upon Drought Conditions.
(A) H2A.Z enrichment in control conditions (normalized H2A.Z-ChAP reads minus normalized MNase-seq [input]) relative to TSS, TTS, and across gene body is shown for genes upregulated (green) and downregulated (red) in drought stress in comparison with “all expressed genes” (blue).
(B) As for (A) but in stress conditions.
(C) and (D) Nucleosome distribution across TSS as determined by MNase-seq in control (C) and stress (D) conditions.
(E) Heat map visualization of H2A.Z enrichment (input [IP]) for the TSS/TTS/gene bodies represented in (A) and (B) and MNase-seq (from [C] and [D]). Genes were sorted (top to bottom) from highest to lowest H2A.Z enrichment (for TSS, TTS, and gene body) or nucleosome density (for MNase TSS). For the class “all expressed”, 2000 genes were randomly selected to compare similar number of elements as for other classes.
(F) The change in H2A.Z occupancy correlates with the change in expression level between control and drought conditions (Spearman rank correlation) for TSS, ESS, and TTS.
(G) Comparison of changes in H2A.Z enrichment between control (blue) and stress (green) conditions shown for all expressed, stress-induced, and stress-repressed genes. Plots show the same data as for (A), (B), and (E).
Transcriptional Response of Drought-Induced Genes Is Associated with Reduction of H2A.Z Occupancy across Their Bodies
Our data collected from both control and stress conditions provided an opportunity to investigate how H2A.Z levels change in relation to modification of gene transcription activity. We answer this question using several approaches. First, we performed differential binding analysis to identify loci exhibiting gain or loss of H2A.Z during drought stress. We identified 701 chromosomal regions that show a reduction in H2A.Z occupancy upon stress and 359 regions showing an increase in H2A.Z occupancy (Supplemental Data Sets 5A and 5B, respectively). Those regions corresponded to 512 and 329 genes, respectively, for which we performed GO classification. The GO overrepresented classes for genes showing reduction of H2A.Z levels in drought corresponded well to classification of genes induced in drought stress (Supplemental Data Set 6). GO analysis of genes corresponding to regions that increase H2A.Z levels upon drought did not produce a significant category, which is likely due to the smaller size of this group. This result suggests that H2A.Z is removed from nucleosomes during transcriptional activation.
Next, we analyzed changes in H2A.Z profiles in response to stress for genes upregulated and downregulated in drought and compared them to “all expressed”. When the profile of H2A.Z enrichment in the “all expressed” group was analyzed, no significant difference between control and stress conditions was observed (Supplemental Figures 6A and 7). Genes upregulated in stress showed a striking reduction in H2A.Z occupancy in stress conditions at the TSS, especially at the +1 position (Figures 3A, 3B, 3E, and 3G; Supplemental Figures 6B, 6D, and 7). Genes downregulated presented a less evident change in H2A.Z occupancy at the +1 nucleosome (Figures 3A, 3B, 3E, and 3G; Supplemental Figures 6C and 7). Across the gene body, genes “upregulated in stress” lost H2A.Z in drought conditions, while an opposite but less evident trend was observed for downregulated genes (Figures 3A, 3B, and 3E; Supplemental Figures 6B, 6D, and 7). This was further confirmed via ChAP followed by quantitative PCR (ChAP-qPCR) on selected genes in an independent drought stress experiment (Supplemental Figure 8). These experiments provided evidence for a statistically significant reduction in H2A.Z occupancy in upregulated genes; however, we failed to confirm that a significant gain of this histone variant occurs in repressed genes.
Finally, to statistically validate the relationship detected, we applied an approach based on H2A.Z enrichment scores. In this case, we sorted all the genes according to their difference in expression, and for each percentile calculated the difference between the H2A.Z enrichment score in control and stress conditions. We observed a strong negative correlation for TSS, ESS, and TTS (Spearman ρ = −0.944, −0.937, and −0.907, P = 6.96E-49, 1.55E-46, and 1.16E-38 for TSS, ESS, and TTS, respectively) (Figure 3F). This indicates that changes in H2A.Z levels are similar for both TSS and gene bodies. When this analysis was repeated separately for genes upregulated and downregulated in stress, the correlation was significantly stronger for the first group (e.g., for ESS upregulated ρ = −0.889, P = 1.30E-18, downregulated ρ = −0.631, P = 5.74E-6). Again this confirms that H2A.Z gain in repressed genes is less evident when compared with the opposite effect in induced genes, yet still significant.
Genes Hyperactive in arp6 Are Enriched for H2A.Z across Their Bodies in Wild-Type Plants
The expression data for arp6, the mutant impaired in H2A.Z deposition, can be compared with the genome-wide profiling of H2A.Z in wild-type plants to examine how nucleosomal H2A.Z deficiency affects transcription. If the transcriptional changes observed in arp6 result directly from the absence of H2A.Z in nucleosomes occupying the misregulated genes, then in the presence of intact ARP6, the pattern of H2A.Z distribution or occupancy in these genes should be distinct from that of genes which are transcriptionally unaffected in arp6 mutant.
To test this hypothesis, we performed a global analysis of H2A.Z enrichment across gene bodies in the BLRP-H2A.Z plants. Based on our RNA-seq data for arp6 mutants, we grouped all the genes into “hyperactive in arp6” (n = 693) and “hypoactive in arp6” (n = 542) and compared their wild-type nucleosome profile with the profile of “all expressed” genes (n = 15,558). We did not observe differences in the H2A.Z nucleosomal occupancy between “hypoactive in arp6” and “all expressed” gene groups (Figure 4). This suggests that most of the genes hypoactive in arp6 are indirectly affected by H2A.Z levels, as many of them are likely linked to a negative regulation of DNA repair pathways (Supplemental Data Sets 2A and 2B). On the other hand, genes “hyperactive in arp6” showed dramatically higher levels of H2A.Z across gene bodies in comparison to “all expressed” genes (Figure 4; Supplemental Figure 9A). We concluded that in the majority of genes “hyperactive in arp6”, H2A.Z has a direct effect on their expression by changing the property of underlying nucleosomes in the gene body, resulting in reduced transcriptional activity.
Figure 4.
Genes Hyperactive in arp6 Have Significantly Higher Levels of H2A.Z across Their Gene Bodies.
H2A.Z enrichment (normalized H2A.Z-ChAP reads minus normalized MNase-seq [input]) relative to TSS (A), TTS (B), ESS (C), and across gene body (D) is shown for genes upregulated (green) and downregulated (red) in arp6 in comparison with “all expressed” genes (blue).
(E) Heat map visualization of H2A.Z enrichment (input [IP]) for the TSS/TTS/ESS/gene bodies represented in (A) to (D). Genes were sorted (top to bottom) from highest to lowest H2A.Z enrichment. For the class “all expressed”, 1200 genes were randomly selected to compare similar number of elements as for “hyperactive in arp6” and “hypoactive in arp6” classes.
It is interesting to note that we did not observe significant differences in H2A.Z levels at the +1 nucleosome between the three gene groups (Figure 4A), which indicates that the presence of this histone variant around TSS is not decisive for expression level regulation. We repeated this analysis for the set of data that was obtained for drought stress conditions and observed virtually identical profiles of H2A.Z enrichment (Supplemental Figure 9B), providing further support for this interpretation.
Drought-Responsive Genes with Altered Expression in arp6 Show Different Patterns of H2A.Z
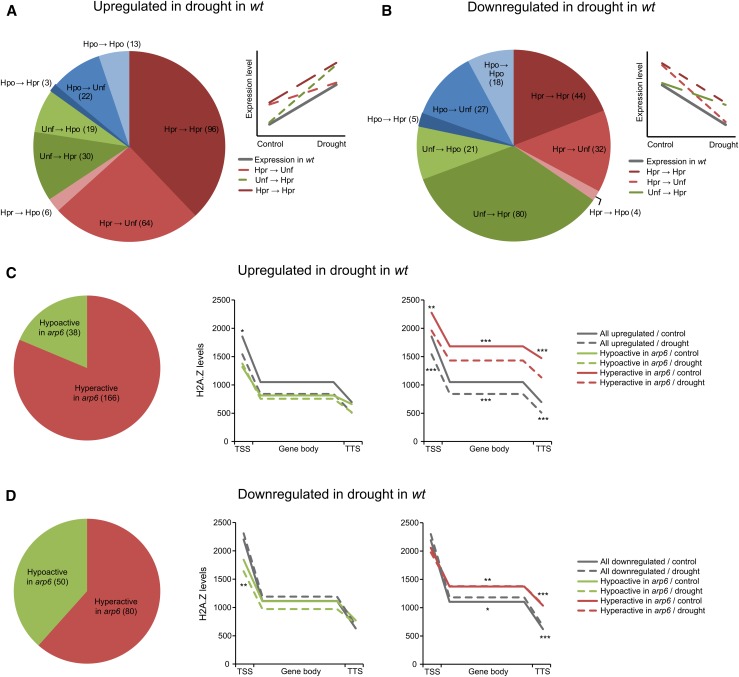
In order to take full advantage of our data set, we reanalyzed the data focusing on the drought responsive genes. We investigated the transcriptional behavior of genes responsive to drought stress that change their expression profile in the arp6 mutant. We identified 253 genes, which were both upregulated in drought in wild-type plants and showed altered expression in the arp6 mutant (either in control conditions or in stress) when compared with the wild type. When the genes were categorized by their expression profile in both control and stress conditions in arp6, the largest group consisted of genes hyperactive in both control and stress conditions (n = 96 genes, 37.9%) followed by genes hyperactive in control/unaffected in stress (n = 64 genes, 25.3%) and unaffected in control/hyperactive in stress (n = 30 genes, 11.9%) (Figure 5A). For genes downregulated in the wild type in stress conditions that displayed altered expression in the arp6 mutant, the largest group was those unaffected in control/hyperactive in stress (n = 80 genes, 34.6%) followed by genes hyperactive in both control and stress conditions (n = 44 genes, 19.0%) and hyperactive in control/unaffected in stress (n = 32 genes, 13.9%) (Figure 5B). This shows that lack of ARP6 protein results primarily in gene hyperactivity irrespective of whether they are upregulated or downregulated in stress.
Figure 5.
Analysis of H2A.Z Enrichment in Drought-Responsive Genes Misregulated in the arp6 Mutant.
(A) and (B) Classification of drought-upregulated (A) and drought-downregulated (B) genes according to their relative expression in arp6 mutant in control and stress conditions (control → stress). “Hpr”, “Unf”, and “Hpo” are used as abbreviations for genes hyperactive, unaffected, or hypoactive in arp6 relative to the wild type, respectively. Transcriptional profiles characteristic of three groups represented by the largest number of genes in each circle graph are shown schematically in the line graph on the right hand side of each circle graph.
(C) and (D) H2A.Z enrichment in genes upregulated (C) and downregulated (D) in drought, divided according to the directionality of their misregulation in arp6 in control conditions (hyper- or hypoactive in arp6). The sizes of the groups are compared in the circle graphs on the left in (C) and (D), and the number of genes in each group is given in parentheses. Note that each group covers three groups presented in (A) or (B), as this classification is based on control conditions only. The line plots show H2A.Z enrichment (mean values) at TSS, gene body (ESS), and TTS in control (red or blue solid lines) and drought conditions (red or blue dashed lines) compared with the same for all genes up- (C) or downregulated (D) in drought (gray lines). The significance of the difference in H2A.Z levels is indicated by asterisks for control (above the plots) and drought conditions (below the plots) (Mann-Whitney Test; *P < 0.05; **P < 0.01; ***P < 0.001).
To examine whether the observed alterations in gene transcriptional activity are a direct result of changes in H2A.Z levels, we compared H2A.Z profiles of genes upregulated in drought and misregulated in arp6 (n = 204) with all the genes upregulated in drought (n = 1844, from hereafter referred to as the “control group”). We divided them into two groups: “hyperactive in arp6 in control conditions” (n = 166, 81.4%) and “hypoactive in arp6 in control conditions” (n = 38, 18.6%). For these two gene groups, H2A.Z levels at TSS, gene body, and TTS were calculated based on our ChAP-seq data (Figure 5C). We observed that genes hypoactive in arp6 in control conditions had significantly lower H2A.Z levels in TSS in the wild type (Figure 5C). Importantly, those genes do not lose H2A.Z at TSS during their transcriptional activation, as do other genes upregulated in drought (compared with solid and dashed lines in Figure 5C). We suggest that those genes require some H2A.Z at the +1 nucleosome to stay active, and an inability to efficiently incorporate H2A.Z at this location in the arp6 mutant results in their transcriptional hypoactivity. By contrast, genes hyperactive in arp6 in control conditions show significantly higher H2A.Z levels across their whole length, indicating repressive effect on their transcription (Figure 5C).
Very similar results were obtained for genes downregulated in drought in wild-type plants (n = 2226; “control group”). Of them, 130 genes showed altered expression in the arp6 mutant in control conditions: 80 genes (61.5%) were hyperactive and 50 genes (38.5%) were hypoactive (Figure 5D). The hypoactive group has significantly less H2A.Z in TSS under stress conditions (Figure 5D). Moreover, this group of genes loses H2A.Z upon transcriptional repression, while other genes downregulated in drought behave the opposite (compared with solid and dashed lines in Figure 5D). This indicates that some genes “hypoactive in arp6” require H2A.Z for their activation. Genes hyperactive in arp6 in control conditions show significantly higher H2A.Z levels in gene body and TTS (Figure 5D), which is consistent with the repressive effect of H2A.Z on their expression.
These results are in line with the repressive role of gene body H2A.Z in the regulation of gene expression and suggest a direct effect of H2A.Z levels on expression of many drought-responsive genes. At the same time, presence of H2A.Z in +1 nucleosomes in some genes may be important for maintenance of transcriptional activity, as was previously demonstrated (Deal et al., 2007; Kumar and Wigge, 2010).
The arp6 Mutant Exhibits a Dramatic Reduction in H2A.Z Occupancy within Transcriptionally Active Genes
The previously presented interpretation of our results assumes that H2A.Z is largely absent from chromatin in the arp6 mutant. This assumption is based on the fact that the SWR1 complex is considered as the only chromatin remodeler capable of H2A.Z deposition (Billon and Côté, 2013). However, this was not examined in plants, with the exception of three genes linked with flowering regulation (FLC, MAF4, and MAF5; Deal et al., 2007; Zhang et al., 2015).
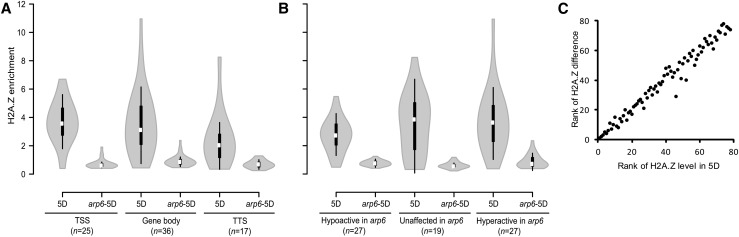
To investigate the extent to which lack of ARP6 protein affects H2A.Z deposition, we crossed the BLRP-H2A.Z line (5D) with the arp6 mutant. The selected homozygous mutant plants carrying a tagged H2A.Z gene (hereafter called arp6-5D) were used to isolate chromatin (MNase-based protocol) and to perform ChAP. We selected 30 genes from groups “hyperactive in arp6”, “hypoactive in arp6”, and “unaffected in arp6”, for which we performed ChAP-qPCR experiments. Altogether, 78 genomic regions (amplicons) were analyzed, which corresponded to TSS nucleosomes, gene body-located nucleosomes, or TTS nucleosomes. For 69 of them, the H2A.Z enrichment was significantly lower in the arp6-5D mutant than in the 5D line (Student’s t test), which is wild-type for ARP6 (Figure 6A; Supplemental Figure 10 and Supplemental Table 2). The difference in H2A.Z enrichment between 5D and arp6-5D lines was significant between all three genic region types (i.e., TSS, P = 2.0E-8; gene body, P = 2.45E-9; TTS, P = 1.6E-4, Mann-Whitney Test), indicating that lack of arp6 affects H2A.Z levels at different intergenic locations (Figure 6A). We observed a dramatic 76.5% reduction in H2A.Z enrichment in the arp6-5D when compared with the control 5D line, on average (Figure 6A). RT-qPCR analysis revealed that arp6-5D plants exhibit 3.3 times higher expression of tagged HTA11 than the 5D line; therefore, the difference in H2A.Z occupancy between the two lines is likely due to lack of ARP6 protein and not lower levels of tagged H2A.Z. Altogether, these results indicate that the arp6-dependent pathway is a major determinant of H2A.Z deposition in Arabidopsis.
Figure 6.
The ARP6-Dependent Pathway Is a Major Determinant of H2A.Z Enrichment within Arabidopsis Genes.
(A) and (B) H2A.Z is depleted from the chromatin in an arp6 mutant background irrespective of the genic location (A) and in genes hyperactive, hypoactive, and unaffected in arp6 (B).
(C) Spearman rank correlation showing the relationship between H2A.Z enrichment in the wild type (5D) and its depletion in arp6 background (arp6-5D).
In addition, for several genomic regions with the highest and lowest H2A.Z enrichment in the arp6-5D line, we performed negative controls using the arp6 line (without tagged H2A.Z). The differences between four regions with the highest, and three with the lowest, H2A.Z levels were significant in arp6-5D (P = 0.017; Mann-Whitney Test), but not between the same regions tested in the arp6 line (P = 0.362; Mann-Whitney Test). Therefore, it is possible that some limited H2A.Z deposition may occur independently of the ARP6 protein.
Characterization of H2A.Z depletion in regards to the gene transcriptional profile in the arp6 mutant indicates that all three gene groups, hyperactive, hypoactive, and unaffected in arp6, exhibit significant reduction in H2A.Z levels in an arp6 background (75.98%, P = 3.38E-08, 71.78%, P = 1.27E-08 and 82.53%, P = 2.18E-05, respectively; Mann-Whitney test) (Figure 6B; Supplemental Figure 10). When H2A.Z enrichment in the 5D line was compared between regions in genes hyperactive and hypoactive in the arp6 mutant, the first group show significantly higher enrichment (P = 0.035; Mann-Whitney test). However, when the same two groups of regions were compared in arp6-5D, no significant difference was observed (P = 0.438; Mann-Whitney test). Moreover, Spearman rank correlation indicated that the level of H2A.Z depletion in the arp6-5D line is strongly correlated with H2A.Z level in the 5D line (ρ = 0.985, P = 1.20E-59; Figure 6C). These results support our assumption that H2A.Z is largely absent from chromatin in the arp6 mutant, and the residual levels of H2A.Z remain evenly distributed and statistically indifferent.
DISCUSSION
In this work, we studied the role of the histone variant H2A.Z in transcriptional regulation using two approaches: by analyzing its distribution and occupancy in response to drought stress and by investigating its distribution in genes deregulated in the arp6 mutant. Although the repressive role of H2A.Z in transcription in Arabidopsis was previously suggested (March-Díaz et al., 2008; Kumar and Wigge, 2010; Smith et al., 2010), our work is the first to test changes in occupancy of this histone variant in genes during the response to stress on a genome-wide scale. Here, we present solid evidence for H2A.Z depletion from the gene body upon transcriptional activation (Figure 4; Supplemental Figures 6 to 8). H2A.Z deposition in genes repressed during stress was less evident, yet still notable and statistically significant (Figure 4; Supplemental Figures 6 to 8). Assuming that H2A.Z gain and loss accompanying transcriptional changes are active processes, this may suggest that they have different kinetics. This seems likely when one takes into account that H2A.Z deposition and eviction from nucleosomes depends on two different chromatin remodeling complexes, SWR1 and INO80 (Mizuguchi et al., 2004; Altaf et al., 2010; Luk et al., 2010; Yen et al., 2013; Watanabe et al., 2013; Zhang et al., 2015; Papamichos-Chronakis et al., 2011). An alternative explanation of the changes in H2A.Z levels observed in response to stress conditions would be an increased turnover of nucleosomes under transcriptional activation. It is known that passage of RNAPII causes a temporary loss of the H2A/H2B dimer (Kireeva et al., 2002; Workman, 2006). We recognize this explanation as less likely because our H2A.Z enrichment is normalized to nucleosome occupancy as analyzed by MNase-seq.
We found that H2A.Z levels in gene bodies correlate well with gene responsiveness (Figure 2). This is similar to observations made by Coleman-Derr and Zilberman (2012) and Latorre et al. (2015). We further analyzed this phenomenon and observed that the levels of H2A.Z in the chromatin of responsive genes remain high even if changes in H2A.Z enrichment are considered. Consistent with this, the distribution profile of H2A.Z along the gene remains unchanged, independently of whether the gene is induced or repressed during stress (Figures 3A to 3E). This indicates that the nucleosomal H2A.Z pattern can be considered as a permanent property of genes. We hypothesize that high levels of H2A.Z are important to counteract unwanted transcription driven by strong promoters of responsive genes in noninductive conditions. At the same time, a weak negative correlation between H2A.Z level and gene responsiveness was observed at TSS, likely reflecting a different non-repressive role of H2A.Z in +1 nucleosomes on transcription (Figure 2D), which was further investigated by our analysis of the arp6 mutant (see below).
Interestingly, we observed that in genes upregulated in drought, the +1 nucleosome is more “fuzzy” and is relatively poor in H2A.Z, which is opposite to the case in drought repressed genes, where it is well positioned with high H2A.Z level (Figures 3A and 3B). This indicates that localization of H2A.Z at TSS is important for transcription initiation in normal conditions, where it facilitates RNA polymerase transit through the +1 nucleosome (Weber et al., 2014). In stress conditions, however, stress-induced genes require another way to overcome the +1 nucleosome transcriptional barrier. We hypothesize that this role is undertaken by longer NDRs, which we found to be typical for stress-induced genes (Figures 3C and 3D). The longer NDRs may serve to bind specific transcription activators and enable the RNA polymerase to pass the +1 nucleosome barrier even without high H2A.Z levels. Similar broader NDRs for stress-induced genes were reported in fission yeast (Sansó et al., 2011; García et al., 2014). Moreover, it has been shown that responsive genes are associated with highly accessible chromatin in Arabidopsis (Sullivan et al., 2014) and that transcription factors for stress-induced genes are often permanently associated with NDRs enabling rapid transcriptional activation of genes upon exposure to a stress signal (Liu et al., 2015; Zhang et al., 2012).
It has been widely accepted that in pie1 and arp6 mutants, which lack specific and crucial subunits for the SWR1 complex, nucleosomal H2A.Z level is significantly reduced (March-Díaz et al., 2008; Deal et al., 2007, 2005; Choi et al., 2005). Studies on pie1 and arp6 mutants of Arabidopsis demonstrated a uniform distribution of remaining H2A.Z across gene bodies of three genes involved in flowering time regulation without the prominent peaks observed in wild-type plants (Deal et al., 2005; Zhang et al., 2015). To confirm that this is true also for other genes, including those misregulated in arp6, we performed an extensive analysis of H2A.Z enrichment in 78 different genic regions. This showed that the majority of tested regions significantly lose H2A.Z in the arp6 background, and this is largely independent of their location within a gene (TSS, gene body, or TTS; Figure 6A) or the transcriptional behavior of the gene in arp6 (hyperactive, hypoactive, or unaffected; Figure 6B). It is important to emphasize that we did not find evidence for a redistribution of H2A.Z, which could occur via a hypothetical arp6-independent H2A.Z deposition pathway (Jeronimo et al., 2015) (Figure 6C; Supplemental Figure 10) or passive H2A.Z incorporation during nucleosome reassembly (no correlation with expression rate was observed) (Hardy and Robert, 2010). This indicates that the SWR1 complex is the major factor controlling spatial distribution of H2A.Z in chromatin in plants. Therefore, we assume that the arp6 mutant represents a chromatin state in which nucleosomal H2A.Z level is evenly decreased throughout the genome.
Wild-type H2A.Z occupancy of genes that are hyperactive in arp6 background is characterized by dramatically increased levels of the variant in gene bodies when compared with other genes (Figure 4). As we observed no difference in H2A.Z level at the +1 nucleosome (Figures 4A and 4E), it is tempting to speculate that the increase in the transcriptional activity in the arp6 mutant is directly linked to H2A.Z deficiency in gene bodies. This indicates a different role of H2A.Z in the first nucleosome from the TSS than in nucleosomes located farther downstream. We found that the arp6-hyperactive genes belong primarily to stress response-related genes when GO classification is applied. This is consistent with studies that suggest a role of H2A.Z in transcription control of responsive genes in plants (March-Díaz et al., 2008; Coleman-Derr and Zilberman, 2012; Kumar and Wigge, 2010; Choi et al., 2016; Latorre et al., 2015). Moreover, our data obtained from the arp6 mutant analysis strongly suggest that H2A.Z adopts a repressive role in transcription at the stage of elongation and termination, as its loss in gene bodies leads to an increase in their transcriptional activity, as estimated by RNA-seq. The molecular basis of this repressive function is not clear. H2A.Z may contribute to differential stability of nucleosomes (Ishibashi et al., 2009; Jin et al., 2009; Jin and Felsenfeld, 2007; Zhang et al., 2005) or specifically interact with elongation factors (Santisteban et al., 2011). However, in yeast, H2A.Z stimulates transcription elongation by affecting the assembly of the RNAPII elongation complex (Santisteban et al., 2011); hence, its repressive effect on transcription elongation in plants needs to be studied further.
In contrast to the conspicuous relationship described above, the pattern of H2A.Z distribution in arp6-hypoactive genes as measured by ChAP-seq in wild-type plants is relatively similar to the pattern in “all expressed” genes (Figure 4). GO analysis indicates that many genes from this group fall into a category that could be linked with the well-established role of H2A.Z and the SWR1 complex in DNA repair (Choi et al., 2013; Xu et al., 2012; Morillo-Huesca et al., 2010; Van et al., 2015; Horigome et al., 2014; Rosa et al., 2013). This may suggest existence of a feedback between the ARP6/SWR1 complex or nucleosomal H2A.Z and DNA repair pathways, which is currently unknown. On the other hand, the observation that genes hypoactive in arp6 fall into this GO category may be coincidental, and the effect could be due to disturbed activity of transcription activators or a signaling pathway controlling their expression (Pecinka and Mittelsten Scheid, 2012; Mirouze and Paszkowski, 2011). Regardless of the causes, our study clearly demonstrates that in many cases reduced transcriptional activity of genes in the arp6 mutant is not directly linked to changes in H2A.Z levels.
To investigate whether there are any genes in which transcriptional activation depends directly on H2A.Z, we focused on genes which are both drought responsive and exhibit altered expression in arp6. When the pattern of H2A.Z occupancy was compared between genes hypoactive in arp6 and all genes up- or downregulated in drought, we observed lower H2A.Z levels in the +1 nucleosome for genes hypoactive in arp6, regardless of their transcriptional behavior in stress (Figures 5C and 5D). In addition, these genes show no or opposite change in H2A.Z at TSS during their transcriptional activation when compared with genes in their control groups (compared with solid and dashed lines in the middle panels of Figures 5C and 5D). These results indicate that, indeed, there are genes that require H2A.Z for activation, at least in the +1 nucleosome (Figure 7). However, based on our experimental setups, where some residual H2A.Z levels could be observed, it is difficult to tell how universal this mechanism is.
Figure 7.
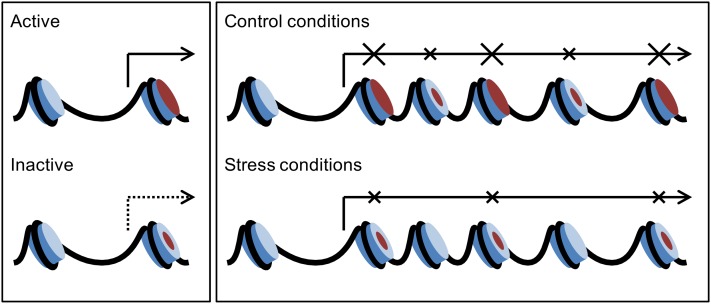
Role of H2A.Z in the Regulation of Gene Activity in Arabidopsis.
Some genes require H2A.Z in TSS to maintain their transcriptional activity (left panel). These genes stay hypoactive in the arp6 mutant. As the arp6 mutant exhibits some residual evenly distributed H2A.Z, it is difficult to speculate how general this mechanism is. On the other hand, H2A.Z across the whole gene length adopts a repressive role in transcription in most genes that change their expression under stress conditions (right panel). These genes stay hyperactive in the arp6 mutant. We hypothesize that the biological role of H2A.Z-repressive influence on transcription is to counteract unwanted expression under noninductive conditions. H2A.Z is partially removed from nucleosomes during transcriptional activation. The model assumes that in +1 nucleosomes, H2A.Z may adopt both a promoting and repressive role, depending on additional factors such as nucleosome composition or histone modifications.
The two opposite roles of H2A.Z in transcriptional regulation could be explained by the differences in the biochemical properties of H2A.Z-containing nucleosomes. This could result from posttranscriptional modifications of this histone variant, such as acetylation or ubiquitination. Although specific antibodies for investigation of these modifications have not yet been developed in plants, they have been studied in other organisms (Hu et al., 2013; Ku et al., 2012; Bruce et al., 2005; Valdes-Mora et al., 2012; Sarcinella et al., 2007; Millar et al., 2006). H2A.Z acetylation is generally considered as an important activating mark localized primarily in the +1 nucleosome (Hu et al., 2013; Ku et al., 2012; Bruce et al., 2005; Valdes-Mora et al., 2012; Millar et al., 2006), whereas H2A.Z ubiquitination is usually linked with its repressive role (Ku et al., 2012; Sarcinella et al., 2007). Specific properties of promoter-proximal nucleosomes may also result from different histone variant composition, e.g., it was suggested that nucleosomes containing both H2A.Z and H3.3 histone variants are much less stable than nucleosomes of other composition (Jin and Felsenfeld, 2007).
Taken together, our findings suggest that in Arabidopsis, H2A.Z may have either a promoting or repressive effect on transcription, depending on the location of H2A.Z-containing nucleosomes within the gene (Figure 7). Specifically, in +1 nucleosomes, H2A.Z is required for transcriptional activity. In nucleosomes located farther within gene bodies, it enhances the inhibitory effect of nucleosomes on transcriptional elongation and termination, thus acting as a repressive mark. This effect seems to be important for all the genes containing H2A.Z in their gene bodies. We show that the occupancy of H2A.Z across gene bodies decreases upon stress-dependent transcriptional activation, and these changes negatively correlate with changes in expression level. At the same time, levels of H2A.Z in gene bodies are positively correlated with gene responsiveness, suggesting that this histone variant may counteract unwanted transcription in noninductive conditions.
METHODS
Germination Assay
To ensure the same quality of wild-type (Col-0), arp6 (Choi et al., 2005), pie1-5 (SALK_096434), and hta9-1 hta11-1 (March-Díaz et al., 2008) seeds used for germination experiments, the seeds were harvested from the plants growing side-by-side in the same conditions. To remove potential differences in seed dormancy between the lines, harvested seeds were stored for at least 4 weeks before the germination assay. Seeds were surface-sterilized and sown on 0.5× Murashige and Skoog medium containing 0.8% (w/v) agar and 2.3 mM MES. For stress conditions, the medium was additionally supplemented with 150 mM NaCl or 300 mM sorbitol. All four lines were sown on each plate. Eleven biological replicates were performed, each containing 17 to 18 seeds per line. After sowing, seeds were stratified for 3.5 d at 4°C and then transferred to a plant culture room (22°C, 16/8 h light/dark, 150 µmol m−2 s−1). Germination rates were scored daily as radicle tip emergence or green cotyledons emergence. A Student’s t test was used to assess statistical significance of observed differences.
Construction of H2A.Z-Tagged Lines
To obtain H2A.Z-tagged lines, the in vivo biotinylation system (Mito et al., 2005) was adapted. The gene HTA11 (AT3G54560) encoding Arabidopsis thaliana H2A.Z histone was cloned under the control of its endogenous promoter and tagged at the 5′-end with BLRP. The BLRP-HTA11 cassette was further cloned into the pMOA34 binary vector (Barrell and Conner, 2006), modified by addition of the BirA gene encoding prokaryotic biotin ligase under the control of the Act2 gene promoter. Arabidopsis plants (Col-0) were transformed as previously described (Zhang et al., 2006). After initial selection on hygromycin-enriched media, expression of tagged H2A.Z gene was analyzed in ∼20 transformants by RT-qPCR, and expression of the cassette was confirmed at the protein level by immunoblotting using streptavidin-HRP conjugate (Sigma-Aldrich). Several transgenic lines were tested along with lines transformed with empty vector containing the Act2:BirA cassette, which was further used as a mock.
Complementation Assay and Analysis of H2A.Z Enrichment in the arp6 Background
To test the effect of our tagged HTA.Z expression on early flowering phenotype observed in the hta9-1 hta11-1 double mutant (March-Díaz et al., 2008), we performed a complementation assay. The hta9-1 hta11-1 line was crossed with a 5D line expressing BLRP-HTA11 and BirA cassettes, and plants homozygous for both mutations were selected in the F2 generation. The progeny of those plants were sown in long-day conditions alongside wild-type plants (Col) and hta9 hta11 plants, and flowering time was scored as the number of leaves at bolting. To measure H2A.Z enrichment in the arp6 mutant, the arp6 line was crossed with the 5D line expressing BLRP-HTA11 and BirA cassettes. The plants homozygous for the arp6 mutation carrying the tagged HTA11 gene were selected in the F2 generation, and the leaf material for ChAP and RNA extraction was collected from long-day conditions. The amplicons for ChAP-qPCR were selected from genes misregulated in arp6 (Supplemental Data Sets 1A and 1B) based on location of H2A.Z-containing nucleosomes as revealed by our ChAP-seq analysis. The full list of amplicons along with the primers used for qPCR is shown in Supplemental Table 3. For statistical analysis, the Real Statistics Resource Pack was used (www.real-statistics.com).
Plant Growth Conditions and Drought Stress
Arabidopsis seeds were sown in moistened peat pellets (Jiffy Products), stratified at 4°C for 3 d, and then transferred to a Sanyo growth chamber, where they were grown at 22°C and 70% humidity under long days (16/8 h light/dark, 130 μmol m−2 s−1, fluorescent lamps). Drought stress was applied to 4-week-old plants by withholding watering for 8 d. To eliminate a potential effect of early flowering of the arp6 mutant compared with wild-type plants, for the arp6 RNA-seq experiment, we used short-day conditions (8/16 h light/dark). Drought stress was applied to 3-week-old plants by withholding watering for 10 d. At least three biological replicates were performed per each experiment. The recovery test indicated that all the plants were able to survive these conditions.
Determination of RWC
RWC is the percentage water content in tissue relative to the water content at full turgor (Nishiyama et al., 2011). To determine RWC, leaves were collected and immediately weighed to quantify fresh weight (FW). Turgid weight (TW) was obtained after overnight immersion in distilled water and dry weight (DW) after subsequent overnight drying at 50 to 60°C. RWC was calculated from the following formula: RWC = [(FW − DW)/(TW − DW)].
ChAP
Approximately 4 g of leaves (2 g for stressed plants) was fixed for 10 min under vacuum at room temperature in buffer containing 1% formaldehyde (0.4 M sucrose, 10 mM Tris-HCl, pH 8.0, 1 mM PMSF, 1 mM EDTA, and 1% formaldehyde). The reaction was quenched by adding glycine to a final concentration of 100 mM and applying vacuum for another 5 min. Next, plant material was washed five times in water and ground in liquid nitrogen to fine powder. Material was stored at −80°C. The powder was resuspended in ice-cold Honda buffer (25 mM Tris-HCl, pH 7.5, 0.44 M sucrose, 10 mM MgCl2, 0.5% Triton X-100, 10 mM β-mercaptoethanol, and 2 mM spermine) and incubated for 30 min on ice with shaking. Then, the homogenate was filtered through two layers of Miracloth and spun down, and the pellet washed three times with Honda buffer (last time with no spermine). The nuclei pellet was resuspended in ice-cold TNE buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, and 1 mM EDTA) and after addition of CaCl2 to a final concentration of 4 mM MNase (16 units/mL) digested for 20 min at 37°C. The reaction was terminated by addition of EGTA to a final concentration of 25 mM. After centrifugation, the supernatant was stored in liquid nitrogen until ChAP. Chromatin was diluted 10-fold with dilution buffer (16.7 mM Tris-HCl, pH 8.0, 1.2 mM EDTA, 167 mM NaCl, 1.1% Triton X-100, 1.1 mM PMSF, and 1.1% Protease Inhibitor Cocktail [Sigma-Aldrich]) and incubated with Dynabeads M-280 Streptavidin (Life Technologies) overnight at 4°C with gentle agitation. The slurry was washed with wash buffer (20 mM Tris-HCl, pH 8.0, 2 mM EDTA, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and 1 mM PMSF).
For ChAP-qPCR, DNA was extracted with 10% Chelex (Bio-Rad) as described previously (Wierzbicki et al., 2008). ChAP-qPCR was performed using Applied Biosystems 7900HT Fast real-time PCR System and Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific). Three biological replicates were used for each experiment. Percentage of input was calculated as follows:
and all the results were normalized using the ACTIN7 gene. For high-throughput sequencing, DNA was eluted and purified with the use of the IPure kit (Diagenode) according to the manufacturer’s instructions. For both ChAP-qPCR and ChAP-seq, DNA from MNase-treated chromatin was used as input. DNA library preparation and subsequent DNA sequencing on the Illumina HiSeq 2000 platform was performed by BGI (Hong Kong).
ChAP-Seq Data Analysis
MNase-seq and ChAP-seq reads were mapped to the TAIR10 genome using Bowtie2 software (Langmead and Salzberg, 2012). H2A.Z enrichment was established by subtracting MNase-seq (input) reads from ChAP-seq reads (after normalization to library size). DANPOS2 (Chen et al., 2013) software was used for signal normalization, drawing profiles, and differential analysis of ChIP-seq and MNase-seq data. Differential binding of the H2AZ between stress and control samples was performed using the Dpeak algorithm, while the Dpos algorithm was used to analyze different types of nucleosome changes (occupancy change, fuzziness change, and position shift), as described in DANPOS documentation. For all analyzes, the false discovery rate threshold of 0.01 was used. The HOMER package (Heinz et al., 2010) was used for the annotation of detected differential binding regions and nucleosomes, as well as for the analysis of GO terms, molecular pathways, and protein domains overrepresented in affected sets of genes. A custom genome browser containing the processed experimental data was created using JBrowse software (Skinner et al., 2009).
RNA Isolation for RT-qPCR Analysis and RNA-Seq
RNA for each biological repeat was extracted from 100 mg of leaves (from at least eight plants) with Trizol and rounds of phenol-chloroform and chloroform extractions followed by isopropanol precipitation. RNA was treated with DNase (Promega) and then extracted with phenol-chloroform and precipitated with ethanol. Pure RNA water solution was sent to BGI Hong Kong, where libraries and sequencing were performed using Illumina chemistry with single-end protocol. After quality inspection (FastQC) and trimming (fastx; http://hannonlab.cshl.edu/fastx_toolkit/index.html), we obtained at least 12 million reads representing each sample. The sequences were mapped onto reference TAIR10 genome using TopHat2 aligner (Kim et al., 2013) with default parameters. The counts corresponding to each of the genes were scored using HTseq (Anders et al., 2015) tool using the “union” option. Subsequently, the counts representing each gene were used in estimation of differentially expressed genes by DESeq2 (Love et al., 2014) from R Bioconductor package. The significant differences in expression were selected based on padj value (P value adjusted with the Benjamini-Hochberg procedure) lower than 0.05 unless otherwise indicated. For GO annotations, we used VirtualPlant 1.3 platform run with TAIR10 GO classification (Katari et al., 2010). A Fischer exact test was used to estimate P values for functional categories. For RT-qPCR analysis, cDNA was synthesized using RevertAid first-strand cDNA synthesis kit (Thermo Scientific) and analyzed in triplicate on an Applied Biosystems 7900HT Fast real-time PCR system using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ARP6, AT3G33520; and HTA11, AT3G54560. Sequence data are under accession numbers SAMN05504885 to SAMN05504902 in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra). For additional accession numbers, see Supplemental Table 2 and Supplemental Data Sets 1 and 3.
Supplemental Data
Supplemental Figure 1. Comparison of the RWC for control plants and plants subjected to drought stress in wild-type and arp6 plants used for RNA-seq and ChIP-seq analysis.
Supplemental Figure 2. Different SWR1C mutants exhibit similar changes in transcription when compared with wild-type plants.
Supplemental Figure 3. H2A.Z-tagged lines exhibit similar nucleosomal H2A.Z levels on the FLC gene and complement hta9 hta11 mutations.
Supplemental Figure 4. Comparison of the RWC for H2A.Z-tagged line 5D and lined K3F transformed with an empty vector in control and drought stress conditions.
Supplemental Figure 5. H2A.Z levels in gene bodies correlates with gene responsiveness.
Supplemental Figure 6. H2A.Z levels in control and stress conditions.
Supplemental Figure 7. H2A.Z-ChAP signal in control conditions relative to TSS, ESS, TTS, and across the gene body.
Supplemental Figure 8. ChAP-qPCR validation of changes in H2A.Z occupancy in drought stress at selected genes.
Supplemental Figure 9. Genes hyperactive in arp6 have significantly higher levels of H2A.Z across their bodies.
Supplemental Figure 10. ARP6-dependent pathway is a major determinant of H2A.Z enrichment within Arabidopsis genes.
Supplemental Table 1. Germination assay in wild-type (Col), arp6, hta9 hta11, and pie1-5 plants in osmotic stress.
Supplemental Table 2. ChAP-qPCR analysis of H2A.Z enrichment for 5D and 5D-arp6 plants.
Supplemental Table 3. List of primers used for ChAP-qPCR and RT-qPCR analyses.
Supplemental Data Set 1. Genes that change their expression in arp6 mutant when compared with wild-type plants in control and drought conditions.
Supplemental Data Set 2. Significantly enriched GO classes for genes hypoactive in arp6 for control and drought conditions and genes hyperactive in arp6 in control conditions.
Supplemental Data Set 3. Genes that change their expression in drought stress conditions in wild-type plants.
Supplemental Data Set 4. Significantly enriched GO classes for genes upregulated and downregulated in drought conditions.
Supplemental Data Set 5. Chromosomal regions that lost or gained H2A.Z levels in drought when compared with control conditions.
Supplemental Data Set 6. Significantly enriched GO classes for genes corresponding to regions, which lost H2A.Z levels in drought when compared with control conditions.
Supplementary Material
Acknowledgments
This work was supported by National Science Center grants (2016/21/B/NZ2/01757 to P.A.Z., 2015/17/N/NZ1/00028 to W.S., and 2011/01/B/NZ2/01691 to J.S.) and Polish Ministry of Science and Higher Education grants (N/N303/313437 to P.A.Z., DI/2011/028641 to W.S., and 01/KNOW2/2014 to KNOW RNA Research Centre in Poznan). Hanna Korcz-Szatkowska is acknowledged for technical assistance. We thank Władysław Polcyn for physiological consultations and Kyuha Choi for helpful discussion on the manuscript. Emma J. Lawrence and Charles J. Underwood are acknowledged for critical reading of the manuscript.
AUTHOR CONTRIBUTIONS
W.S., M.K., W.M.K., T.B., M.K.-S., Ł.P., J.S., and P.A.Z. designed the research. W.S., T.B., M.K.-S., Ł.P., and P.A.Z. performed research. W.S., M.K., W.M.K., T.B., and P.A.Z. analyzed data. W.S., M.K., W.M.K., T.B., and P.A.Z. wrote the article.
Glossary
- TSS
transcription start site
- RWC
relative water content
- GO
Gene Ontology
- MNase
micrococcal nuclease
- ChAP
chromatin affinity purification
- TTS
transcription termination site
- ESS
exon start site
- NDR
nucleosome-depleted region
Footnotes
Articles can be viewed without a subscription.
References
- Altaf M., Auger A., Monnet-Saksouk J., Brodeur J., Piquet S., Cramet M., Bouchard N., Lacoste N., Utley R.T., Gaudreau L., Côté J. (2010). NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J. Biol. Chem. 285: 15966–15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P.T., Huber W. (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell P.J., Conner A.J. (2006). Minimal T-DNA vectors suitable for agricultural deployment of transgenic plants. Biotechniques 41: 708–710. [DOI] [PubMed] [Google Scholar]
- Berriri S., Gangappa S.N., Kumar S.V. (2016). SWR1 chromatin-remodelling complex subunits and H2A.Z have non-overlapping functions in immunity and gene regulation in Arabidopsis. Mol. Plant 9: 1051–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieluszewski T., Galganski L., Sura W., Bieluszewska A., Abram M., Ludwikow A., Ziolkowski P.A., Sadowski J. (2015). AtEAF1 is a potential platform protein for Arabidopsis NuA4 acetyltransferase complex. BMC Plant Biol. 15: 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon P., Côté J. (2013). Precise deposition of histone H2A.Z in chromatin for genome expression and maintenance. Biochim. Biophys. Acta 1819: 290–302. [DOI] [PubMed] [Google Scholar]
- Bruce K., Myers F.A., Mantouvalou E., Lefevre P., Greaves I., Bonifer C., Tremethick D.J., Thorne A.W., Crane-Robinson C. (2005). The replacement histone H2A.Z in a hyperacetylated form is a feature of active genes in the chicken. Nucleic Acids Res. 33: 5633–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Xi Y., Pan X., Li Z., Kaestner K., Tyler J., Dent S., He X., Li W. (2013). DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 23: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Kim J., Müller S.Y., Oh M., Underwood C., Henderson I., Lee I. (2016). Regulation of microRNA-mediated developmental changes by the SWR1 chromatin remodeling complex in Arabidopsis thaliana. Plant Physiol. 171: 1128–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Kim S., Kim S.Y., Kim M., Hyun Y., Lee H., Choe S., Kim S.-G., Michaels S., Lee I. (2005). SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell 17: 2647–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Zhao X., Kelly K.A., Venn O., Higgins J.D., Yelina N.E., Hardcastle T.J., Ziolkowski P.A., Copenhaver G.P., Franklin F.C., McVean G., Henderson I.R. (2013). Arabidopsis meiotic crossover hot spots overlap with H2A.Z nucleosomes at gene promoters. Nat. Genet. 45: 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman-Derr D., Zilberman D. (2012). Deposition of histone variant H2A.Z within gene bodies regulates responsive genes. PLoS Genet. 8: e1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal R.B., Kandasamy M.K., McKinney E.C., Meagher R.B. (2005). The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17: 2633–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal R.B., Topp C.N., McKinney E.C., Meagher R.B. (2007). Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Jacobsen S.E., Reik W. (2010). Epigenetic reprogramming in plant and animal development. Science 330: 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P., Paulo E., Gao J., Wahls W.P., Ayté J., Lowy E., Hidalgo E. (2014). Binding of the transcription factor Atf1 to promoters serves as a barrier to phase nucleosome arrays and avoid cryptic transcription. Nucleic Acids Res. 42: 10351–10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette B., Bataille A.R., Gévry N., Adam M., Blanchette M., Robert F., Gaudreau L. (2005). Variant histone H2A.Z is globally localized to the promoters of inactive yeast genes and regulates nucleosome positioning. PLoS Biol. 3: e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Jacques P.-E., Gévry N., Forest A., Fortin M.-E., Laflamme L., Gaudreau L., Robert F. (2009). The euchromatic and heterochromatic landscapes are shaped by antagonizing effects of transcription on H2A.Z deposition. PLoS Genet. 5: e1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Robert F. (2010). Random deposition of histone variants: A cellular mistake or a novel regulatory mechanism? Epigenetics 5: 368–372. [DOI] [PubMed] [Google Scholar]
- Heinz S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., Glass C.K. (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38: 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigome C., Oma Y., Konishi T., Schmid R., Marcomini I., Hauer M.H., Dion V., Harata M., Gasser S.M. (2014). SWR1 and INO80 chromatin remodelers contribute to DNA double-strand break perinuclear anchorage site choice. Mol. Cell 55: 626–639. [DOI] [PubMed] [Google Scholar]
- Hu G., Cui K., Northrup D., Liu C., Wang C., Tang Q., Ge K., Levens D., Crane-Robinson C., Zhao K. (2013). H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 12: 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Dryhurst D., Rose K.L., Shabanowitz J., Hunt D.F., Ausió J. (2009). Acetylation of vertebrate H2A.Z and its effect on the structure of the nucleosome. Biochemistry 48: 5007–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo J.A., Piñeiro M. (2015). H2A.Z mediates different aspects of chromatin function and modulates flowering responses in Arabidopsis. Plant J. 83: 96–109. [DOI] [PubMed] [Google Scholar]
- Jeronimo C., Watanabe S., Kaplan C.D., Peterson C.L., Robert F. (2015). The histone chaperones FACT and Spt6 restrict H2A.Z from intragenic locations. Mol. Cell 58: 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Felsenfeld G. (2007). Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 21: 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Zang C., Wei G., Cui K., Peng W., Zhao K., Felsenfeld G. (2009). H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat. Genet. 41: 941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katari M.S., Nowicki S.D., Aceituno F.F., Nero D., Kelfer J., Thompson L.P., Cabello J.M., Davidson R.S., Goldberg A.P., Shasha D.E., Coruzzi G.M., Gutiérrez R.A. (2010). VirtualPlant: a software platform to support systems biology research. Plant Physiol. 152: 500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. (2013). TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-M., Sasaki T., Ueda M., Sako K., Seki M. (2015). Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-M., To T.K., Ishida J., Morosawa T., Kawashima M., Matsui A., Toyoda T., Kimura H., Shinozaki K., Seki M. (2008). Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol. 49: 1580–1588. [DOI] [PubMed] [Google Scholar]
- Kireeva M.L., Walter W., Tchernajenko V., Bondarenko V., Kashlev M., Studitsky V.M. (2002). Nucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcription. Mol. Cell 9: 541–552. [DOI] [PubMed] [Google Scholar]
- Ku M., Jaffe J.D., Koche R.P., Rheinbay E., Endoh M., Koseki H., Carr S.A., Bernstein B.E. (2012). H2A.Z landscapes and dual modifications in pluripotent and multipotent stem cells underlie complex genome regulatory functions. Genome Biol. 13: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.V., Wigge P.A. (2010). H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140: 136–147. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre I., Chesney M.A., Garrigues J.M., Stempor P., Appert A., Francesconi M., Strome S., Ahringer J. (2015). The DREAM complex promotes gene body H2A.Z for target repression. Genes Dev. 29: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Seddon A.E., Tsai Z.T., Major I.T., Floer M., Howe G.A., Shiu S. (2015). Determinants of nucleosome positioning and their influence on plant gene expression. Genome Res. 25: 1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P.Y.T., Lévesque N., Kobor M.S. (2009). NuA4 and SWR1-C: two chromatin-modifying complexes with overlapping functions and components. Biochem. Cell Biol. 87: 799–815. [DOI] [PubMed] [Google Scholar]
- Luk E., Ranjan A., Fitzgerald P.C., Mizuguchi G., Huang Y., Wei D., Wu C. (2010). Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell 143: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Díaz R., García-Domínguez M., Florencio F.J., Reyes J.C. (2007). SEF, a new protein required for flowering repression in Arabidopsis, interacts with PIE1 and ARP6. Plant Physiol. 143: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Díaz R., García-Domínguez M., Lozano-Juste J., León J., Florencio F.J., Reyes J.C. (2008). Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J. 53: 475–487. [DOI] [PubMed] [Google Scholar]
- March-Díaz R., Reyes J.C. (2009). The beauty of being a variant: H2A.Z and the SWR1 complex in plants. Mol. Plant 2: 565–577. [DOI] [PubMed] [Google Scholar]
- Millar C.B., Xu F., Zhang K., Grunstein M. (2006). Acetylation of H2AZ Lys 14 is associated with genome-wide gene activity in yeast. Genes Dev. 20: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze M., Paszkowski J. (2011). Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 14: 267–274. [DOI] [PubMed] [Google Scholar]
- Mito Y., Henikoff J.G., Henikoff S. (2005). Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 37: 1090–1097. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G., Shen X., Landry J., Wu W.-H., Sen S., Wu C. (2004). ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303: 343–348. [DOI] [PubMed] [Google Scholar]
- Morillo-Huesca M., Clemente-Ruiz M., Andújar E., Prado F. (2010). The SWR1 histone replacement complex causes genetic instability and genome-wide transcription misregulation in the absence of H2A.Z. PLoS One 5: e12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23: 2169–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh Y.S., Amasino R.M. (2003). PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. Plant Cell 15: 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M., Watanabe S., Rando O.J., Peterson C.L. (2011). Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144: 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecinka A., Mittelsten Scheid O. (2012). Stress-induced chromatin changes: a critical view on their heritability. Plant Cell Physiol. 53: 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A.V., Mittelsten Scheid O. (2015). Stress-induced structural changes in plant chromatin. Curr. Opin. Plant Biol. 27: 8–16. [DOI] [PubMed] [Google Scholar]
- Rhee H.S., Bataille A.R., Zhang L., Pugh B.F. (2014). Subnucleosomal structures and nucleosome asymmetry across a genome. Cell 159: 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H.S., Pugh B.F. (2012). Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M., Von Harder M., Cigliano R.A., Schlögelhofer P., Mittelsten Scheid O. (2013). The Arabidopsis SWR1 chromatin-remodeling complex is important for DNA repair, somatic recombination, and meiosis. Plant Cell 25: 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansó M., Vargas-Pérez I., Quintales L., Antequera F., Ayté J., Hidalgo E. (2011). Gcn5 facilitates Pol II progression, rather than recruitment to nucleosome-depleted stress promoters, in Schizosaccharomyces pombe. Nucleic Acids Res. 39: 6369–6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban M.S., Hang M., Smith M.M. (2011). Histone variant H2A.Z and RNA polymerase II transcription elongation. Mol. Cell. Biol. 31: 1848–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarcinella E., Zuzarte P.C., Lau P.N.I., Draker R., Cheung P. (2007). Monoubiquitylation of H2A.Z distinguishes its association with euchromatin or facultative heterochromatin. Mol. Cell. Biol. 27: 6457–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.E., Uzilov A.V., Stein L.D., Mungall C.J., Holmes I.H. (2009). JBrowse: a next-generation genome browser. Genome Res. 19: 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.P., Jain A., Deal R.B., Nagarajan V.K., Poling M.D., Raghothama K.G., Meagher R.B. (2010). Histone H2A.Z regulates the expression of several classes of phosphate starvation response genes but not as a transcriptional activator. Plant Physiol. 152: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G., Polo S.E., Almouzni G. (2012). Prime, repair, restore: the active role of chromatin in the DNA damage response. Mol. Cell 46: 722–734. [DOI] [PubMed] [Google Scholar]
- Sullivan A.M., et al. (2014). Mapping and dynamics of regulatory DNA and transcription factor networks in A. thaliana. Cell Reports 8: 2015–2030. [DOI] [PubMed] [Google Scholar]
- Valdés-Mora F., Song J.Z., Statham A.L., Strbenac D., Robinson M.D., Nair S.S., Patterson K.I., Tremethick D.J., Stirzaker C., Clark S.J. (2012). Acetylation of H2A.Z is a key epigenetic modification associated with gene deregulation and epigenetic remodeling in cancer. Genome Res. 22: 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van C., Williams J.S., Kunkel T.A., Peterson C.L. (2015). Deposition of histone H2A.Z by the SWR-C remodeling enzyme prevents genome instability. DNA Repair (Amst.) 25: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk K., Ding Y., Malkaram S., Riethoven J.-J.M., Liu R., Yang J., Laczko P., Chen H., Xia Y., Ladunga I., Avramova Z., Fromm M. (2010). Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol. 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Radman-Livaja M., Rando O.J., Peterson C.L. (2013). A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 340: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C.M., Ramachandran S., Henikoff S. (2014). Nucleosomes are context-specific, H2A.Z-modulated barriers to RNA polymerase. Mol. Cell 53: 819–830. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A.T., Haag J.R., Pikaard C.S. (2008). Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman J.L. (2006). Nucleosome displacement in transcription. Genes Dev. 20: 2009–2017. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ayrapetov M.K., Xu C., Gursoy-Yuzugullu O., Hu Y., Price B.D. (2012). Histone H2A.Z controls a critical chromatin remodeling step required for DNA double-strand break repair. Mol. Cell 48: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K., Vinayachandran V., Pugh B.F. (2013). SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near +1 nucleosomes. Cell 154: 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Cao L., Rong L., An Z., Zhou W., Ma J., Shen W.H., Zhu Y., Dong A. (2015). The chromatin-remodeling factor AtINO80 plays crucial roles in genome stability maintenance and in plant development. Plant J. 82: 655–668. [DOI] [PubMed] [Google Scholar]
- Zhang H., Roberts D.N., Cairns B.R. (2005). Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123: 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang T., Wu Y., Jiang J. (2012). Genome-wide identification of regulatory DNA elements and protein-binding footprints using signatures of open chromatin in Arabidopsis. Plant Cell 24: 2719–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S.-S., Niu Q.-W., Chua N.-H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1: 641–646. [DOI] [PubMed] [Google Scholar]
- Zhu J., et al. (2013). Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell 152: 642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Dong A., Shen W.-H. (2013). Histone variants and chromatin assembly in plant abiotic stress responses. Biochim. Biophys. Acta 1819: 343–348. [DOI] [PubMed] [Google Scholar]
- Zilberman D., Coleman-Derr D., Ballinger T., Henikoff S. (2008). Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D., Gehring M., Tran R.K., Ballinger T., Henikoff S. (2007). Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 39: 61–69. [DOI] [PubMed] [Google Scholar]
- Zlatanova J., Thakar A. (2008). H2A.Z: view from the top. Structure 16: 166–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.