Introduction
Intracranial dural arteriovenous fistulae (dAVF) are typified by pathological anastomoses between meningeal arteries and dural venous sinuses and/or cortical veins. These fistulae frequently reside within the dural leaflets surrounding a venous sinus, characteristically at the transverse-sigmoid junction but also at the cavernous sinus, superior sagittal sinus, anterior cranial fossa, tentorium, and other locations. dAVF are distinguished from their pial counterparts (e.g., arteriovenous malformations, or AVM) by their arterial supply from vessels that perfuse the dura mater and lack of a parenchymal nidus.1
dAVF are rare lesions, accounting for 10–15% of all intracranial vascular malformations: 6% of supratentorial and 35% of infratentorial vascular malformations.2 Most frequently, dAVF affect patients in their middle-to-later years of life (e.g., 50 to 60 years of age).3 Less commonly, dAVF occur in younger age groups including children. There is neither a clear sex predilection nor genetic component to these lesions. Clinical manifestations vary widely and depend on both the hemodynamic properties and location of the fistula. Multiple classification systems have been proposed in an effort to predict which dAVF are at high risk for new neurological events. Most of these schemes stratify risk based on angiographic appearance of the dAVF, in particular involvement of a venous sinus and presence or absence of retrograde cortical venous drainage (CVD). Others schemes combine angiographic appearance with mode of presentation, given the impact symptoms related to intracerebral hemorrhage (ICH) and non-hemorrhagic neurological deficits (NHND) have on dAVF natural history.4–6
In the following review and synthesis, the authors describe the pathophysiology, classification, mode of presentation, imaging characteristics, natural history, and therapeutic armamentarium for intracranial dAVF. Particular attention will be given to recent evidence that helps stratify patient risk of future ICH and NHND in an effort to guide treatment decision-making as well as advances in the manner in which these lesions can be treated. The clinical characteristics and treatment options for this particular dAVF subtype will be considered separately. Given that carotid-cavernous fistulas (CCFs) have a variety of differentiating features related to their unique drainage pattern into the cavernous sinus, these lesions will be examined separately.
Pathophysiology
The pathological mechanisms underlying dAVF formation remain enigmatic. While the majority of dAVF are acquired in an idiopathic fashion, a smaller subset results from traumatic head injury, infection, previous craniotomy, tumors, or dural venous sinus thrombosis.3, 7–9 Most authorities believe that dAVF arise from progressive stenosis and/or occlusion of a dural venous sinus. The correlation between patients with dAVF and hereditary thrombotic diseases (e.g., Factor V Leiden, Protein C/S deficiency) supports this hypothesis.10, 11 As venous sinus pressure increases, meningeal arteries develop fistulous connections with the dural sinus and/or cortical veins. This may occur via enlargement of pre-existing physiological shunts or de novo fistula development from neoangiogenesis.1, 12, 13 The end result is a complex network of venous tributaries under arterial pressure. With worsening sinus outflow obstruction and venous hypertension, the normal antegrade pattern of venous flow is reversed, resulting in retrograde flow through cortical veins (termed cortical venous drainage, or CVD) that can produce venous hypertension within the surrounding brain.
dAVF formation is a dynamic process; fistula hemodynamics may change over time after recanalization of the thrombosed sinus, recruitment of additional ECA feeders (with potential upconversion from a dAVF without CVD to a higher risk dAVF with CVD), or spontaneous thrombosis (with complete angiographic resolution of the dAVF). Ultimately, this pathological entity is capable of producing hemorrhage or NHND that can lead to substantial neurological morbidity and mortality. Intracerebral hemorrhage is thought to occur from rupture of fragile arterialized parenchymal veins as a result of unrelenting venous reflux and cortical venous hypertension.3, 14 While the precise underlying mechanisms governing NHND are not well established, the resulting venous congestion from dAVFs with CVD likely prevents the adequate arterial delivery of oxygen to vital tissues with the inability to remove toxic waste products.15, 16 This leads to a collapse of normal cellular metabolism and produces a functional impairment of the involved cerebral tissues. As a result of this pathological cascade, CVD is an ominous radiographic feature that portends a higher likelihood of intracerebral hemorrhage and/or NHND.10, 17
CCFs comprise a unique subset of intracranial dAVF that involve an abnormal communication between the ICA and/or ECA and the CS.18 These lesions may be further defined based upon their underlying etiology (spontaneous vs. traumatic), angioarchitecture (direct shunt from cavernous ICA, branches of cavernous ICA, meningeal branches of ECA, or combinations thereof), and hemodynamics (high-flow vs. low-flow). Most commonly, direct CCFs occur at the proximal horizontal intracavernous ICA segment due to trauma involving the vessel wall (e.g., skull base fracture or iatrogenic) or rupture of an aneurysm involving the cavernous ICA segment. This results in a high-flow fistula between the ICA and CS. Less frequently, direct fistulas occur spontaneously via conditions that predispose to weakness of the cavernous ICA wall (e.g., connective tissue disorders). Indirect CCFs are typically low-flow lesions that occur spontaneously. A variety of medical comorbidities—including post-menopausal state, pregnancy, diabetes, collagen vascular disease, and hypertension—may predispose to the formation of indirect CCF.
It should be noted that while CCFs have long been considered a distinct pathological entity from dAVFs, the two lesions share many similar pathophysiological features. While direct CCFs— for the reasons outlined above—indeed encompass their own unique category, indirect CCFs represent dAVFs of the parasellar region with a relationship to the cavernous sinus. For this reason, many authorities advocate changing the nomenclature of the “indirect” CCF in favor of the more accurate description of parasellar dAVF.
Classification
The simplest and most commonly used scheme for differentiating dAVF is the Borden-Shucart classification.17 Using this system, dAVF are distinguished based on venous drainage characteristics—especially the absence of CVD (Type I) or presence of CVD (Types II and III) (Table 1). Borden-Shucart Type I fistulae harbor dural arteries that drain exclusively into a dural sinus with antegrade venous flow (Figure 1A). Borden-Shucart Type II fistulae drain into a dural sinus with venous flow that is both antegrade into the dural sinus and retrograde into cortical veins (i.e. CVD) (Figure 1B). Borden-Shucart Type III fistulae drain exclusively into cortical veins in a retrograde fashion (i.e. CVD) (Figure 1C). Type II and III fistulae are considered high-grade due to the presence of CVD and the impact this venous drainage pattern has on natural history (see below). These lesions may be further subcategorized as having a single fistula (subtype a) or multiple fistulae (subtype b).17
Table 1.
Various Classification Schemes of Intracranial dAVFs*
| Zipfel | Borden-Shucart Type | Cognard Type | Venous Drainage | CVD | CVH | Presents with ICH or NHND | ICH Risk (%) | Death Risk (5%) | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | I | I, IIa | Dural sinus | No | No | No | <1.0 | 0.0 | Elective for intractable symptoms |
| 2A | II | IIb, IIa+b | Dural sinus | Yes | No | No | 1.4–1.5 | 0.0 | Elective to prevent ICH/NHND |
| 2S | II | IIb, IIa+b | Dural sinus | Yes | Yes | Yes | 7.4–7.6 | 3.8 | Early to prevent ICH/NHND |
| 3A | III | III, IV, V | Cerebral vein | Yes | No | No | 1.4–1.5 | 0.0 | Elective to prevent ICH/NHND |
| 3S | III | III, IV, V | Cerebral vein | Yes | Yes | Yes | 7.4–7.6 | 3.8 | Early to prevent ICH/NHND |
Modified with permission from Zipfel et al., Neurosurg Focus 26(5):E14, 2009.
CVD = Cortical venous Drainage; CVH = Cortical venous hypertension; ICH = Intracranial hemorrhage; NHND = Non-hemorrhagic neurological deficits
Figure 1.
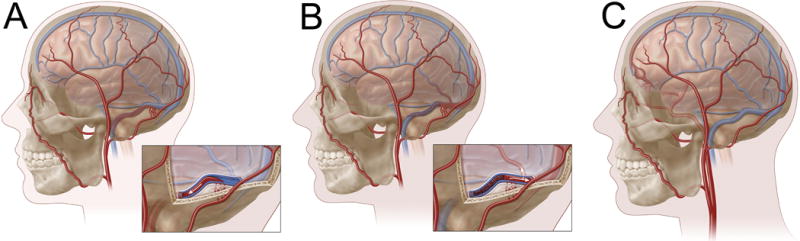
Borden-Shucart classification of intracranial dAVF. In Type I fistulae (A), progressive venous sinus stenosis and/or occlusion results in venous congestion and anastomoses between meningeal arteries (e.g., trans-osseous feeders from the occipital artery, as depicted here) and a dural venous sinus (e.g., transverse-sigmoid junction, as depicted here). Venous flow is entirely anterograde and cortical venous drainage (CVD) is absent. In Type II fistulae (B), progressively worsening venous hypertension results in both anterograde (into sinus) and retrograde (CVD) venous outflow. In Type III lesions (C), a meningeal artery shunts directly into a cortical vein (with CVD). Venous outflow, by definition, is entirely retrograde. In this example, ethmoidal branches from the ophthalmic artery shunt directly into a cortical frontal vein resulting in a venous varix.
The Cognard classification (Table 1) is predicated on shunt location, venous drainage characteristics, and venous outflow angioarchitecture.10 Cognard type I and IIa dAVFs are analogous to Borden-Shucart type I fistulae (no CVD), with Type I lesions draining antegrade into a dural sinus and Type II lesions draining retrograde into a venous sinus. Type IIb fistulae drain antegrade into a venous sinus and have venous reflux into cortical veins (CVD). Type II a+b fistulae drain retrograde into a dural sinus and have venous reflux into cortical veins (CVD). Type III dAVF drain directly into cortical veins (CVD). Type IV fistulae drain directly into cortical veins (CVD), but also display venous ectasia. Type V fistulae drain exclusively into spinal perimedullary veins. Type IIb through Type V fistulae are considered high-grade given that all contain CVD and therefore had more aggressive natural history (see below).
Zipfel and colleagues6 proposed a modification to the aforementioned Borden-Shucart classification scheme based on new natural history data indicating mode of presentation markedly impacts future risk of ICH and NHND (Table 1). This new system divides Borden-Shucart Type II and III dAVF into two categories: (1) lesions presenting with asymptomatic CVD (i.e. no symptoms or symptoms related to increased sinus flow including tinnitus or ophthalmologic phenomena)—Type 2A and 3A; and (2) lesions presenting with symptomatic CVD (i.e. symptoms related to cortical venous hypertension including ICH and NHND)—Type 2S and 3S.6, 19 By integrating angiographic appearance (presence or absence of CVD) and mode of presentation (asymptomatic vs. symptomatic CVD) into a single scheme, this classification system more accurately predicts risk of future hemorrhagic and non-hemorrhagic neurological events and therefore affords improved risk stratification to guide appropriate timing and manner of dAVF treatment.
While the Borden-Shucart, Cognard, and Zipfel classification systems can be applied to CCFs, the most frequently used scheme for these particular dAVFs is the Barrow classification.18 In this 4-type angiographic classification system, CCFs are differentiated based on whether a direct, high-flow connection occurs between the cavernous ICA and cavernous sinus (CS) (as opposed to an indirect, low-flow dural shunt) and whether the fistula is supplied by the ICA, ECA, or both (Supplementary Table I). Type A CCFs are direct, high-flow connections between the intracavernous ICA and the CS (Figure 2A). Type B CCFs involve a dural shunt between intracavernous ICA branches and the CS (Figure 2B). Type C and D CCFs are similar to the Type B designation, but comprise either a dural shunt supplied by meningeal braches of the ECA (Type C) (Figure 2C) or both meningeal ECA branches and intracavernous ICA branches (Type D) (Figure 2D).
Figure 2.
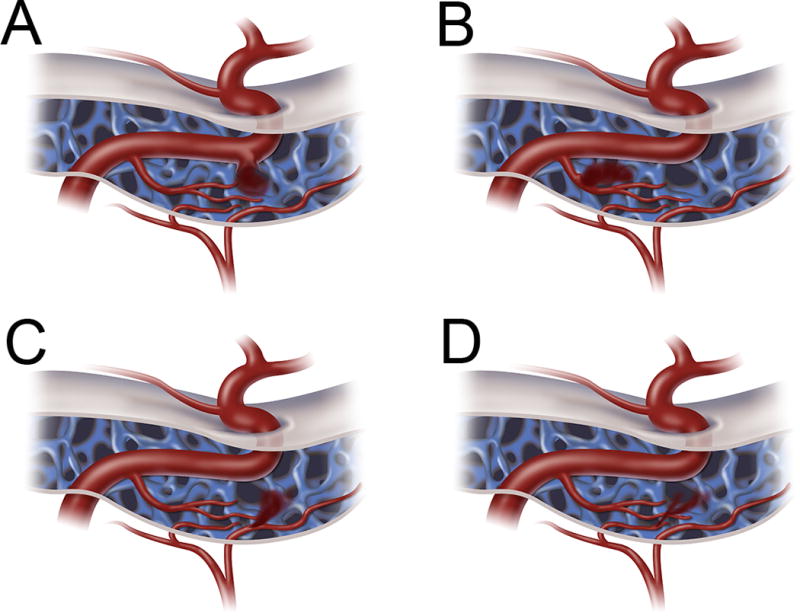
Barrow classification of CCFs. Type A (A) CCFs are direct fistulas between the intracavernous ICA and the CS (most common). Type B (B) CCFs are indirect fistulas between meningeal branches of the intracavernous ICA and the CS. Type C (C) CCFs are indirect fistulas between meningeal branches of the ECA and the CS. Type D (D) CCFs are indirect fistulas between both meningeal branches of the intracavernous ICA and meningeal braches of the ECA and the CS.
Mode of Presentation
All patients suspected of harboring a dAVF require a full neurological examination including an ophthalmological evaluation (especially if a CCF/parasellar dAVF is suspected) and auscultation for a flow-related intracerebral bruit. The clinical findings combined with a thorough history and physical form the basis for the efficient use of diagnostic imaging studies to definitively identify the underlying dAVF. Patients with dAVFs present in three primary ways: (1) aggressive symptoms such as ICH and NHND related to cortical venous hypertension, which only develop in dAVFs with CVD (e.g., Borden-Shucart Type II and III); (2) more benign symptoms such as tinnitus and ophthalmologic phenomenon related to increased sinus drainage, which can occur in any dAVF having venous sinus drainage; and (3) incidental.
ICH from a dAVF often causes sudden-onset headache with varying degrees of neurological disability related to the location and size of the ICH. These events are the result of rupture of delicate, arterialized leptomeningeal veins or hemorrhagic transformation of cortical venous congestion. NHNDs usually develop more gradually over days to weeks and result from focal or global cortical venous hypertension that produces varying degrees of cerebral ischemia.15, 16 Reported NHNDs related to dAVFs include seizures, focal cortical deficits, cranial nerve palsies, trigeminal neuralgia, thalamic or cortical dementia, Parkinsonism, cerebellar dysfunction, dysmetria, paresthesias, myelopathy, quadriparesis, aphasia or dysphasia, apraxia, apathy, failure to thrive, and symptoms related to increased pressure including headache, nausea, and vomiting.1, 17, 19–23
More benign symptoms caused by increased venous sinus drainage are dependent on dAVF location. For instance, dAVFs located in the anterior cranial fossa are often fed by ethmoidal branches off the ophthalmic artery and drain into the cavernous sinus venous system.21, 24 Ophthalmological manifestations including visual deterioration, proptosis, chemosis, cranial nerve palsies, and cavernous sinus syndrome are common presenting features of these dAVFs due to their intimate association with the orbit.14.21, 25 dAVFs in the middle cranial fossa are typified by drainage into the transverse and/or sigmoid venous sinuses.21, 26 Given their close proximity to the auditory apparatus, pulsatile tinnitus is a common symptom from these fistulae.21 dAVFs that drain directly into the superior sagittal sinus or deep venous system (e.g., vein of Galen) may manifest with symptoms related to global venous congestion/hypertension such as seizures, CSF obstruction, papilledema, or dementia.14, 27 Brainstem dAVFs, while uncommon, may cause symptoms related to cranial neuropathies or quadriparesis.14, 28
Imaging
Initial radiographic evaluation typically includes cross-sectional studies from non-contrast head computed tomography (CT) and magnetic resonance imaging (MRI). While head CT alone cannot make the diagnosis of dAVF, this modality can detect dAVF-related ICH and vasogenic edema due to venous congestion. Since venous varices are often the source of hemorrhage, the hemorrhage can be remote from the actual location of the AV shunt.29
MRI is helpful for delineating dAVF anatomy given its superior resolution and ability to define surrounding neurological structures. Flow voids from large arterialized draining veins and varices may be visualized on T2-weighted imaging (Figure 3). Fistula-related microhemorrhages are often more pronounced on T2*/gradient echo MR sequences. Post-gadolinium T1-weighted imaging may show dilated leptomeningeal and medullary vessels, venous ectasia, parenchymal enhancement, and venous sinus occlusion and/or thrombosis.30 Hyperintensities on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences suggests vasogenic edema due to venous hypertension. As an example, Holekamp and colleagues recently identified a series of patients with rapidly-progressive thalamic dementia likely resulting from dAVFs with deep venous drainage into the vein of Galen who demonstrated pronounced bi-thalamic edema on T2/FLAIR sequences (Figure 4).19 In all cases, treatment of the fistula resulted in radiographic resolution of these MRI abnormalities. In general, any serpiginous arrangement of vessels surrounding a dural sinus warrants further evaluation with dynamic vascular imaging.
Figure 3.
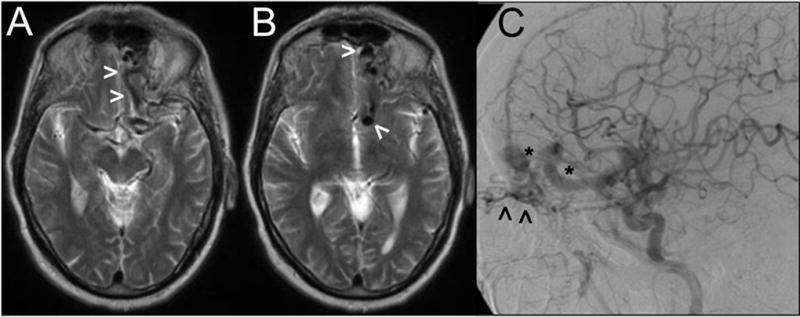
Axial sections from a T2-weighted MRI study (A, B) showing large vascular flow voids (arrowheads) near the left anterior fossa floor. Digital subtraction angiography (lateral view following a left CCA contrast injection) demonstrates a Borden-Shucart Type III dAVF fed by ethmoidal branches from the ophthalmic artery (arrowheads) and draining into enlarged, arterialized cortical frontal veins with an associated venous ectasia and varices (asterisks).
Figure 4.
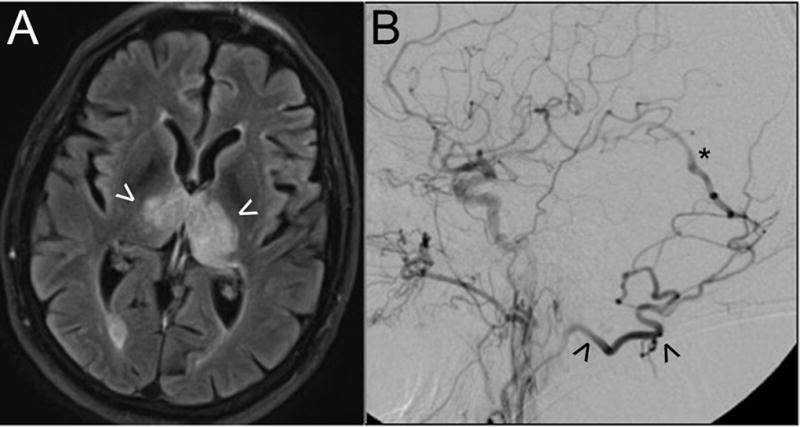
This patient presented with rapidly-progressive dementia. Axial MR imaging with FLAIR sequences (A) demonstrates bi-thalamic edema (arrowheads) as a result of venous hypertension. Digital subtraction angiography (B; lateral view following a left CCA contrast injection) shows a high-grade dAVF fed by the occipital artery (arrowhead) with early arteriovenous shunting into the deep venous system (e.g., vein of Galen, asterisk). Endovascular embolization resulted in complete fistula obliteration and resolution of clinical and radiographic abnormalities.
Catheter-based cerebral angiography (e.g., digital subtraction angiography, or DSA) remains the gold standard for dAVF diagnosis. DSA is essential for delineating fistula angioarchitecture, location and number of fistulae, anatomy of the external carotid arteries and their dural branches, presence or absence of CVD, degree of dural sinus stenosis/occlusion, and presence of venous ectasia (Figure 5).31 Venous congestion may be angiographically quantified not only by the presence of CVD, but also by the number and pattern of tortuous, engorged leptomeningeal veins (“pseudo-phlebitic pattern”)32 seen in the venous phase. Superselective angiography may also be required to identify the area of convergence of the feeding dural arteries and the origin of the draining vein.
Figure 5.
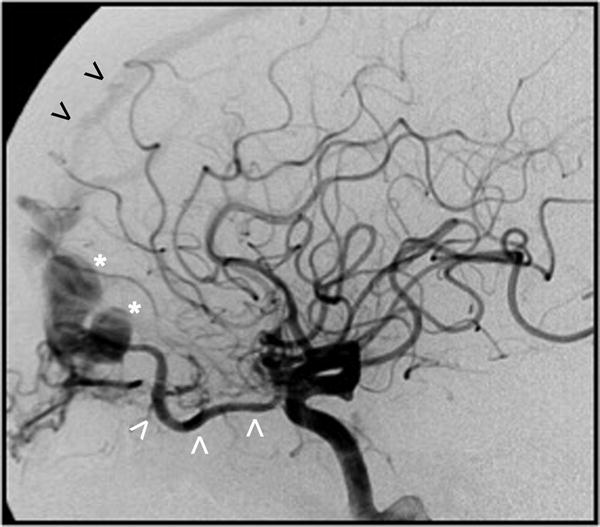
Digital subtraction angiogram (lateral view after a left CCA contrast injection) shows a Borden-Shucart Type III dAVF supplied via ethmoidal branches from a hypertrophic ophthalmic artery (white arrowheads) and draining into a cortical frontal vein causing venous ectasia and a large venous varix (asterisk). Early filling of the anterior third of the superior sagittal sinus can also be appreciated (black arrowheads).
Natural History
Low-grade dAVFs (i.e. fistulae without CVD)
Several studies have documented that dAVFs without CVD including Borden-Shucart Type I, Zipfel Type 1, and Cognard Type I and IIa have a benign natural history and rarely cause ICH or NHND.33–36 The first to document this clinical course was Satomi et al.33 who retrospectively examined 112 patients with Borden-Shucart Type I dAVFs that were either treated conservatively or with palliative endovascular therapy (i.e. dAVF flow was reduced but not eliminated). They observed one symptomatic ICH and no NHND over a mean follow-up of 2.3 years, and calculated an annual neurological event rate of 0.6% and a mortality rate of 0.0%. Shah et al.34 retrospectively examined 23 Borden-Shucart Type I dAVFs that were treated conservatively or with palliative endovascular therapy. They observed no ICH or NHND over a mean follow-up of 5.6 years. They calculated an annual rate of ICH or NHND of 0.0% and an annual mortality rate of 0.0%. Gross and Du retrospectively examined 24 Borden-Shucart Type I dAVF. They reported no cases of ICH, NHND, or death during 409 lesion-years of follow-up.35 Based on these three studies, the annual rate of new neurological events for dAVFs without CVD is 0.0–0.6% and the annual mortality rate for these lesions is 0.0%.
Notably, dAVFs without CVD (e.g., Borden-Shucart Type I) can convert to dAVFs with CVD (e.g., Borden-Shucart Type II and III) over time10, 33, 34—a phenomenon caused by progressive stenosis or thrombosis of venous outlets, increased arterial flow, and/or recruitment or extension of the fistulous connection. Cognard et al.10 documented this “upconversion” in 7 patients with dAVFs who were treated conservatively or with palliative endovascular therapy. Importantly, they noted that each of these dAVF transformations occurred in the setting of new or recurrent neurological symptoms. Satomi et al.33 documented upconversion in 2 patients who were treated conservatively—both of which were discovered at the time of change in patient symptoms (one symptomatic ICH and one spontaneous resolution of tinnitus). Shah et al.34 documented upconversion of 2 patients who were treated with palliative endovascular therapy—both of which were discovered at the time of recurrent tinnitus. They calculated an annual rate of upconversion from dAVF without CVD to dAVF with CVD of 0.8%. Based on these reports, it should be emphasized that prompt angiographic re-evaluation is warranted when any change in a patient’s symptoms (either deterioration or improvement) occurs.
High-grade dAVFs (i.e. fistulae with CVD)
dAVFs with CVD including Borden-Shucart Type II and III; Zipfel Type 2A, 2S, 3A, and 3S; and Cognard Types IIb, IIa+b, III-V have an aggressive natural history and frequently develop ICH or NHND if untreated.3, 6, 10, 17, 37 This was first suggested by Duffau et al.38 who retrospectively examined 20 patients with Borden-Shucart Type II and III dAVFs and observed a re-bleeding rate of 35% after an average follow-up of only 20 days. Van Dijk et al.25 retrospectively examined 20 patients with Borden-Shucart Type II and III dAVFs that either refused treatment or underwent therapy that did not eliminate the CVD. They observed an annual neurological event rate of 15.0% and an annual mortality rate of 10.4%. Based on these important early studies, it became clear that patients diagnosed with dAVFs with CVD are at substantial risk of ICH, NHND, and death, and that the majority of these patients should strongly consider treatment of their dAVF to eliminate this risk.
Notably, the majority of patients in the Duffau et al.38 and Van Dijk et al.25 cohorts presented with aggressive symptoms of ICH or NHND (100% and 80%, respectively), raising the question of whether dAVFs with CVD presenting without such symptoms (i.e. incidentally or with more benign symptoms of increased venous sinus flow such as tinnitus) carried similar risk for ICH or NHND. Soderman et al.4 retrospectively examined 81 patients with Borden-Shucart Type II and III dAVFs with time at risk of 49.6 patient-years. They observed a significant difference in the annual rate of new neurological events between patients presenting ICH vs. patients presenting without ICH – 7.4% vs. 1.5%, respectively. Strom et al.5 retrospectively examined 28 patients with Borden-Shucart Type II and III dAVFs that either refused treatment, underwent therapy that did not eliminate the CVD, or underwent delayed treatment that eliminated the CVD. They observed a significant difference in the annual rate of new neurological events between patients presenting with ICH or NHND (median follow-up of 9.7 months) vs. patients presenting without ICH or NHND (median follow-up of 31.4 months)—19.0% vs. 1.4%, respectively. They also reported a significant difference in annual mortality rates between the two cohorts—3.8% vs. 0.0%, respectively. Gross and Du35 pooled their cohort of Borden-Shucart Type II and III dAVFs with other available case series from the literature and reported on their clinical course after ~400 patient-years of follow-up. They observed a significant difference in the annual rate of ICH between patients presenting with ICH vs. patients presenting with NHND vs. patients presenting incidentally or with more benign symptoms of increased sinus drainage—46% vs. 10% vs. 2%, respectively. From these reports, an association between the mode of presentation and natural history of dAVFs with CVD has become apparent. Specifically, patients harboring dAVFs with CVD that present with symptoms of either ICH or NHND (i.e. symptomatic CVD) have a greater likelihood of suffering hemorrhagic or non-hemorrhagic neurological injury compared to those presenting incidentally or with symptoms of increased sinus drainage (i.e. asymptomatic CVD).
Finally, two studies have recently examined an angiographic factor that also impacts risk of new neurological events in patients having dAVFs with CVD. Bulters et al. retrospectively examined 75 dAVFs with CVD and assessed their clinical course from time of diagnosis to definitive treatment (71 patients) or last follow-up (4 patients) with an average follow-up of 1.2 years. They observed a non-significant trend towards increased annual risk of hemorrhage in patients presenting with aggressive symptoms of ICH or NHND vs. patients presenting incidentally or with more benign symptoms of increased sinus drainage—20% and 22% vs. 4.3%, respectively. Interestingly, they noted a statistically significant increase in annual risk of hemorrhage between patients with dAVFs having venous ectasia vs. those with dAVFs without venous ectasia – 19.0% vs. 1.4%, respectively. The pooled analysis by Gross and Du also examined the impact of venous ectasia on dAVF natural history. They found a statistically significant increase in hemorrhagic risk in patients with dAVFs having venous ectasia by both random effects (Incidence Risk Ratio of 6.07; 95% CI: 2.12–10.0) and fixed-effects (Incidence Risk Ratio of 6.07; 95% CI: 2.12–10.0) models.
Taken together, these data on the natural history of dAVFs with CVD underscores the necessity of treating these higher risk vascular lesions in most patients. As compared to other well-known cerebrovascular lesions such as intracerebral aneurysms (e.g., annual risk of rupture for < 7 mm of ~1–2% per year)39 and pial arteriovenous malformations (e.g., annual risk of rupture ~1–4% per year)40, dAVFs with CVD likely comprise the most dangerous group of cerebrovascular lesions (from a natural history perspective) that neurointerventional surgeons treat.
Treatment Options
The natural history of dAVFs with CVD that present with aggressive symptoms such as ICH and NHND is poor—annual rate of ICH and NHND is 7.4–19.0% and the annual rate of mortality is 3.8%.6 For this reason, urgent treatment of these lesions by modalities expected to immediately eliminate this risk (endovascular, surgical, or combination) is recommended for most patients. The natural history of dAVFs with CVD presenting incidentally or with more benign symptoms such as tinnitus or ophthalmologic phenomena is less aggressive but still significant—annual rate of ICH and NHND is 1.4–1.5% and the annual rate of mortality is 0.0%.6 For this reason, treatment is often recommended in properly selected patients (i.e. patients without major co-morbidities with prolonged life expectancy); however, treatment timing can be more elective and the modalities considered can be expanded to include radiosurgery even though this treatment typically takes 1–3 years to achieve complete dAVF obliteration.41 In contradistinction, the natural history of dAVFs without CVD is quite benign—annual rate of ICH and NHND is 0.0–0.6% and the annual rate of mortality is 0.0%.6 Patients harboring these lesions should therefore be treated primarily based on their presenting symptoms. Those presenting incidentally should be managed conservatively with repeat vascular imaging studies performed if new symptoms develop (e.g. tinnitus, ophthalmologic symptoms, seizure, focal neurological deficit) or if pre-existing symptoms change (e.g. resolution of a bruit in a patient with pulsatile tinnitus), as the onset of new symptoms referable to the dAVF, or resolution of previous symptoms, could represent upconversion to a lesion with CVD and a more aggressive clinical course. Those presenting with symptoms of tinnitus or ophthalmologic phenomena should be treated based on the severity of their symptoms and the impact of these symptoms on the patients’ quality of life. When treated, the primary goal of therapy is to reduce dAVF flow enough to eliminate the intolerable symptoms rather than to achieve complete dAVF obliteration, though the latter if achieved provides the additional benefit of eliminating the risk of recurrent symptoms and the possibility of upconversion to a dAVF with CVD. Given the complexity of these lesions and the many therapeutic options available, dAVF patients are likely best treated in centers where therapeutic decisions are made in a collaborative fashion by an experienced team of neurosurgeons, endovascular specialists, and radiosurgeons. In general, options for dAVF treatment include conservative therapy, endovascular embolization (Supplemental Figure I), microsurgical disconnection of the CVD (Supplemental Figure II), stereotactic radiosurgery (SRS), or combinations thereof. These treatment options—with their attendant risks and outcomes—are discussed in the Online-Only Supplementary Material.
Conclusions
Intracranial dAVFs are a heterogeneous group of vascular malformations with their own distinct pathobiology, symptomatology, angioarchitecture, natural history, and treatment options. While many classification schemes exist to stratify risk of hemorrhage and ischemic neurological deficits, the presence of CVD is an ominous sign that portends a high risk of adverse neurological sequelae. More recently, patients with dAVFs and CVD presenting with aggressive symptoms have been shown to be at particularly high risk for ICH and NHND, particularly if venous ectasia is present. As such, these patients should be treated urgently with endovascular and/or microsurgical therapy, with the primary goal of eliminating the CVD and a secondary goal of completely obliterating the dAVF. In contrast, patients with dAVFs and CVD without aggressive symptoms or venous ectasia have a lower risk of ICH and NHNDs. Though treatment is advisable for most of these patients, endovascular or microsurgical therapy can be pursued in a more elective fashion. In a subset of patients harboring dAVFs with CVD but without high-risk features, the best management may be SRS or close observation (e.g., patients with major co-morbidities, short life expectancy, and/or complex vascular anatomy that increases the risk of dAVF treatment). Finally, patients having dAVFs without CVD have a very benign natural history and are therefore only treated if they develop intolerable symptoms such as tinnitus or orbital phenomena. In patients with dAVFs without CVD who are managed without neurosurgical intervention (e.g., no embolization, stereotactic radiosurgery, microsurgery, or combinations thereof), close clinical follow-up is appropriate as any change in symptoms (including resolution of previous symptoms) should warrant prompt angiographic re-evaluation to determine if the lesion has converted to a dAVF with CVD—an upconversion that should prompt strong consideration for dAVF intervention to eliminate the risk associated with the newly-acquired CVD.
Supplementary Material
Abbreviations
- AVM
Arteriovenous Malformation
- CCF
Carotid-Cavernous Fistula
- dAVF
Dural Arteriovenous Fistula
- CS
Cavernous Sinus
- CVD
Cortical Venous Drainage
- DSA
Digital Subtraction Angiography
- ECA
External Carotid Artery
- FLAIR
Fluid-Attenuated Inversion Recovery
- ICA
Internal Carotid Artery
- ICH
Intracerebral Hemorrhage
- MRI
Magnetic Resonance Imaging
- mRS
Modified Rankin Scale
- NHND
Non-Hemorrhagic Neurological Deficit
- SRS
Stereotactic Radiosurgery
- TAE
Trans-Arterial Embolization
- TVE
Trans-Venous Embolization.
Footnotes
Disclosures:
None.
References
- 1.Gandhi D, Chen J, Pearl M, Huang J, Gemmete J, Kathuria S. Intracranial dural arteriovenous fistulas: Classification, imaging findings, and treatment. American Journal of Neuroradiology. 2012;33:1007–1013. doi: 10.3174/ajnr.A2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations 1. Radiology. 1969;93:1071–1078. doi: 10.1148/93.5.1071. [DOI] [PubMed] [Google Scholar]
- 3.Brown RD, Jr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: Angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. Journal of neurosurgery. 1994;81:531–538. doi: 10.3171/jns.1994.81.4.0531. [DOI] [PubMed] [Google Scholar]
- 4.Söderman M, Pavic L, Edner G, Holmin S, Andersson T. Natural history of dural arteriovenous shunts. Stroke. 2008;39:1735–1739. doi: 10.1161/STROKEAHA.107.506485. [DOI] [PubMed] [Google Scholar]
- 5.Strom RG, Botros JA, Refai D, Moran CJ, Cross DT, III, Chicoine MR, et al. Cranial dural arteriovenous fistulae: Asymptomatic cortical venous drainage portends less aggressive clinical course. Neurosurgery. 2009;64:241–248. doi: 10.1227/01.NEU.0000338066.30665.B2. [DOI] [PubMed] [Google Scholar]
- 6.Zipfel GJ, Shah MN, Refai D, Dacey RG, Jr, Derdeyn CP. Cranial dural arteriovenous fistulas: Modification of angiographic classification scales based on new natural history data. Neurosurgical focus. 2009;26:E14. doi: 10.3171/2009.2.FOCUS0928. [DOI] [PubMed] [Google Scholar]
- 7.Brown RD, Flemming KD, Meyer FB, Cloft HJ, Pollock BE, Link MJ. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clinic Proceedings. 2005;80:269–281. doi: 10.4065/80.2.269. [DOI] [PubMed] [Google Scholar]
- 8.Bulters DO, Mathad N, Culliford D, Millar J, Sparrow OC. The natural history of cranial dural arteriovenous fistulae with cortical venous reflux—the significance of venous ectasia. Neurosurgery. 2012;70:312–319. doi: 10.1227/NEU.0b013e318230966f. [DOI] [PubMed] [Google Scholar]
- 9.Vellimana AK, Daniels DJ, Shah MN, Zipfel GJ, Lanzino G. Dural arteriovenous fistulas associated with benign meningeal tumors. Acta neurochirurgica. 2014;156:535–544. doi: 10.1007/s00701-013-1946-z. [DOI] [PubMed] [Google Scholar]
- 10.Cognard C, Gobin YP, Pierot L, Bailly A-L, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 11.Fujita A, Kuwamura K, Saitoh M, Sakagami Y, Takaishi Y, Suzuki S, et al. Cerebral sinus thrombosis in a patient with protein s deficiency: A case report No shinkei geka. Neurological surgery. 1997;25:467–472. [PubMed] [Google Scholar]
- 12.Kojima T, Miyachi S, Sahara Y, Nakai K, Okamoto T, Hattori K, et al. The relationship between venous hypertension and expression of vascular endothelial growth factor: Hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surgical neurology. 2007;68:277–284. doi: 10.1016/j.surneu.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 13.Chung SJ, Kim JS, Kim JC, Lee SK, Kwon SU, Lee MC, et al. Intracranial dural arteriovenous fistulas: Analysis of 60 patients. Cerebrovascular Diseases. 2002;13:79–88. doi: 10.1159/000047755. [DOI] [PubMed] [Google Scholar]
- 14.Lasjaunias P, Chiu M, Ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. Journal of neurosurgery. 1986;64:724–730. doi: 10.3171/jns.1986.64.5.0724. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda S, Furukawa K, Shiga T, Ushikoshi S, Katoh C, Aoki T, et al. Pretreatment and posttreatment evaluation of hemodynamic and metabolic parameters in intracranial dural arteriovenous fistulae with cortical venous reflux. Neurosurgery. 2004;54:585–592. doi: 10.1227/01.neu.0000108863.30871.fd. [DOI] [PubMed] [Google Scholar]
- 16.Iwama T, Hashimoto N, Takagi Y, Tanaka M, Yamamoto S, Nishi S, et al. Hemodynamic and metabolic disturbances in patients with intracranial dural arteriovenous fistulas: Positron emission tomography evaluation before and after treatment. Journal of neurosurgery. 1997;86:806–811. doi: 10.3171/jns.1997.86.5.0806. [DOI] [PubMed] [Google Scholar]
- 17.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. Journal of neurosurgery. 1995;82:166–179. doi: 10.3171/jns.1995.82.2.0166. [DOI] [PubMed] [Google Scholar]
- 18.Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. Journal of neurosurgery. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 19.Holekamp TF, Mollman ME, Murphy RK, Kolar GR, Kramer NM, Derdeyn CP, et al. Dural arteriovenous fistula-induced thalamic dementia: Report of 4 cases. Journal of neurosurgery. 2015:1–14. doi: 10.3171/2015.5.JNS15473. [DOI] [PubMed] [Google Scholar]
- 20.Hirono N, Yamadori A, Komiyama M. Dural arteriovenous fistula: A cause of hypoperfusion-lnduced intellectual impairment. European neurology. 1993;33:5–8. doi: 10.1159/000116889. [DOI] [PubMed] [Google Scholar]
- 21.Kim MS, Han DH, Kwon O-K, Oh C-W, Han MH. Clinical characteristics of dural arteriovenous fistula. Journal of clinical neuroscience. 2002;9:147–155. doi: 10.1054/jocn.2001.1029. [DOI] [PubMed] [Google Scholar]
- 22.Hurst RW, Bagley LJ, Galetta S, Glosser G, Lieberman AP, Trojanowski J, et al. Dementia resulting from dural arteriovenous fistulas: The pathologic findings of venous hypertensive encephalopathy. American Journal of Neuroradiology. 1998;19:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 23.Wachter D, Hans F, Psychogios M-N, Knauth M, Rohde V. Microsurgery can cure most intracranial dural arteriovenous fistulae of the sinus and non-sinus type. Neurosurgical review. 2011;34:337–345. doi: 10.1007/s10143-011-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito J, Imamura H, Kobayashi K, Tsuchida T, Sato S. Dural arteriovenous malformations of the base of the anterior cranial fossa. Neuroradiology. 1983;24:149–154. doi: 10.1007/BF00347832. [DOI] [PubMed] [Google Scholar]
- 25.van Dijk JMC, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233–1236. doi: 10.1161/01.str.0000014772.02908.44. [DOI] [PubMed] [Google Scholar]
- 26.Davies MA, TerBrugge K, Willinsky R, Coyne T, Saleh J, Wallace MC. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. Journal of neurosurgery. 1996;85:830–837. doi: 10.3171/jns.1996.85.5.0830. [DOI] [PubMed] [Google Scholar]
- 27.Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland J-J. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. Journal of Neurology, Neurosurgery & Psychiatry. 1998;65:308–316. doi: 10.1136/jnnp.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagares A, Perez-Nunez A, Alday R, Ramos A, Campollo J, Lobato R. Dural arteriovenous fistula presenting as brainstem ischaemia. Acta neurochirurgica. 2007;149:965–967. doi: 10.1007/s00701-007-1250-x. [DOI] [PubMed] [Google Scholar]
- 29.Daniels DJ, Vellimana AK, Zipfel GJ, Lanzino G. Intracranial hemorrhage from dural arteriovenous fistulas: Clinical features and outcome. Neurosurgical focus. 2013;34:E15. doi: 10.3171/2013.4.FOCUS1335. [DOI] [PubMed] [Google Scholar]
- 30.Letourneau-Guillon L, Cruz JP, Krings T. Ct and mr imaging of non-cavernous cranial dural arteriovenous fistulas: Findings associated with cortical venous reflux. European journal of radiology. 2015;84:1555–1563. doi: 10.1016/j.ejrad.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Van Rooij W, Sluzewski M, Beute G. Intracranial dural fistulas with exclusive perimedullary drainage: The need for complete cerebral angiography for diagnosis and treatment planning. American journal of neuroradiology. 2007;28:348–351. [PMC free article] [PubMed] [Google Scholar]
- 32.Willinsky R, Goyal M, Montanera W. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: Correlations with presentation, location, and mr findings in 122 patients. American Journal of Neuroradiology. 1999;20:1031–1036. [PMC free article] [PubMed] [Google Scholar]
- 33.Satomi J, van Dijk JMC, Terbrugge KG, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: Outcome of conservative management based on the natural history of the lesion. Journal of neurosurgery. 2002;97:767–770. doi: 10.3171/jns.2002.97.4.0767. [DOI] [PubMed] [Google Scholar]
- 34.Shah MN, Botros JA, Pilgram TK, Moran CJ, Cross DT, III, Chicoine MR, et al. Borden-shucart type i dural arteriovenous fistulas: Clinical course including risk of conversion to higher-grade fistulas: Clinical article. Journal of neurosurgery. 2012;117:539–545. doi: 10.3171/2012.5.JNS111257. [DOI] [PubMed] [Google Scholar]
- 35.Gross BA, Du R. The natural history of cerebral dural arteriovenous fistulae. Neurosurgery. 2012;71:594–603. doi: 10.1227/NEU.0b013e31825eabdb. [DOI] [PubMed] [Google Scholar]
- 36.Davies M, Saleh J, Ter Brugge K, Willinsky R, Wallace M. The natural history and management of intracranial dural arteriovenous fistulae part 1: Benign lesions. Interventional Neuroradiology. 1997;3:295–302. doi: 10.1177/159101999700300404. [DOI] [PubMed] [Google Scholar]
- 37.Awad IA, Little JR, Akrawi WP, Ahl J. Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. Journal of neurosurgery. 1990;72:839–850. doi: 10.3171/jns.1990.72.6.0839. [DOI] [PubMed] [Google Scholar]
- 38.Duffau H, Lopes M, Janosevic V, Sichez J-P, Faillot T, Capelle L, et al. Early rebleeding from intracranial dural arteriovenous fistulas: Report of 20 cases and review of the literature. Journal of neurosurgery. 1999;90:78–84. doi: 10.3171/jns.1999.90.1.0078. [DOI] [PubMed] [Google Scholar]
- 39.Wiebers D, Whisnant J, Huston J, 3rd, Meissner I, Brown R, Jr, Piepgras D, et al. International study of unruptured intracranial aneurysms investigators Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 40.Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. Journal of neurosurgery. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 41.Söderman M, Edner G, Ericson K, Karlsson B, Rähn T, Ulfarsson E, et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. Journal of neurosurgery. 2006;104:867–875. doi: 10.3171/jns.2006.104.6.867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


