INTRODUCTION
In many patients with chronic pain disorders, pain becomes a persistent experience with little or no resolution despite treatment. Despite this chronicity, symptoms often continue to change across different time-scales (days, weeks, months) in an individual even in the chronic state [14; 42; 47]. If the biological factors that predict these symptom changes could be identified, especially those that predict symptom reduction, novel treatments directed toward these factors may have enhanced potential to promote more sustained symptom abatement.
While changes in brain function and structure are believed to play a role in the initial development of persistent pain symptoms (pain chronification) [4], it is not known whether specific brain factors are associated with symptom change during the chronic state. Here, we address this question in the context of a prevalent but poorly understood condition termed urologic chronic pelvic pain syndrome (UCPPS). UCPPS, which is comprised of interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), is estimated to affect the lives of millions of women and men in the US and around the world [2; 5]. UCPPS is associated with pain referred to the pelvic region and varied symptoms related to urinary function [7]. These symptoms initiate for unknown reasons and can be sustained across years and even decades. Despite commonalities of symptom presentation, no generally effective and standard-of-care treatments for UCPPS have been identified [7].
Recently, several changes in brain structure and function have been identified in patients with UCPPS compared to healthy individuals [3; 10; 17; 19; 22; 27; 46], but it is unknown whether there are brain features that predict longitudinal symptom change in symptoms of UCPPS. Here, we begin the process of identifying such brain features by examining the ability of functional connectivity measures derived from resting state functional MRI (rs-fMRI). rs-fMRI captures the strength of functional interaction among brain regions during rest, and has been used to predict future disease symptoms in several clinical conditions including early stages of chronic low back pain [4] and autism [29]. Although rs-fMRI has not yet demonstrated the predictive accuracy necessary for clinical use [28], it has an important advantage over non-neuroimaging questionnaire-based predictors commonly used in chronic pain in that it can point to specific brain networks for further study in animal models or as potential treatment targets.
The goal of the present study was to examine a cohort of UCPPS patients with many years of symptom history (who are already in a “chronified” state), and to determine if rs-fMRI measures obtained at the beginning of the study can predict trends in symptom change over multiple months following the scan. The results described below support the novel finding that rs-fMRI measures predict reduction in UCPPS pain symptoms in the initial months following the scan. These results represent an important step forward in understanding predictive factors underlying UCPPS and may also inform the study of other chronic pain conditions.
METHODS
Participant population
Participants from the multi-site Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network study were studied [7; 24]. At each site, the Institutional Review Board approved the study. All participants provided informed consent. We selected patients for analysis according to three criteria. First, we selected individuals for whom neuroimaging data met quality standards described previously [1]. We then selected only UCPPS patients, and did not analyze data from other groups of participants studied in MAPP (healthy controls and patient-controls without UCPPS). Finally, we divided the UCPPS patients into two cohorts. The primary UCPPS cohort contained patients who had neuroimaging procedures within 4 weeks of study enrollment and that completed bi-weekly symptom assessments for 12 months following enrollment (the full duration of possible study participation), from which we derived measures of longitudinal symptom change (see below). These selection criteria yielded a primary cohort of 52 UCPPS patients. The secondary UCPPS cohort had neuroimaging procedures but did not meet the criteria of neuroimaging followed by 12 months of symptom data. These selection criteria yielded a secondary cohort of 60 UCPPS patients, who were used along with the primary cohort to control for potential site effects in this multi-site neuroimaging study as described below [1], but were not used for any further longitudinal prediction analysis. The UCPPS patients described here have been included in previously-published cross-sectional analyses [20; 22; 27], but this is the first analysis of this neuroimaging data with respect to longitudinal symptom change.
Outcomes for prediction: UCPPS longitudinal symptom trends
We first calculated summary scores of pain symptoms and urinary symptoms, the primary dimensions of UCPPS [13], at each time-point of the bi-weekly internet-based symptom assessments. UCPPS pain and urinary symptom scores were developed and published by the MAPP Research Network [13]. The pain symptom score combines the pain domain score from the Genitourinary Pain Index (GUPI) questionnaire (0–23), and the response to the Interstitial Cystitis Symptom Index (ICSI) question “Have you experienced pain or burning in your bladder?” (0–5), into a single index that ranges 0–28. The urinary symptom score combines the urinary domain score from the GUPI questionnaire (0–10), and the responses to the ICSI questions “How often have you felt the strong need to urinate with little or no warning?” (0–5), “Have you had to urinate less than two hours after you finished urinating?” (0–5), and “How often did you most typically get up at night to urinate?” (0–5), into a single index that ranges 0–25. Figure 1 shows pain symptoms in two example UCPPS patients to illustrate the analysis of longitudinal symptom data we used to derive outcome measures for our predictive modeling. We used a linear mixed effects model with random intercept and random slope to estimate the participant-specific slopes for pain score over three time periods post-enrollment: the first 3 months, first 6 months, and first 12 months. Previous work on MAPP symptom assessments has shown a strong early symptom regression effect (regression to the mean) in the first 4 weeks after enrollment [40]; in this study, we therefore started analysis of longitudinal symptoms after a 4-week run-in period. The participant-specific slopes are estimated by their posterior means, also termed best linear unbiased predictors (BLUPs). We will refer to these participant-specific slopes as symptom change trends over the associated time period.
Figure 1.
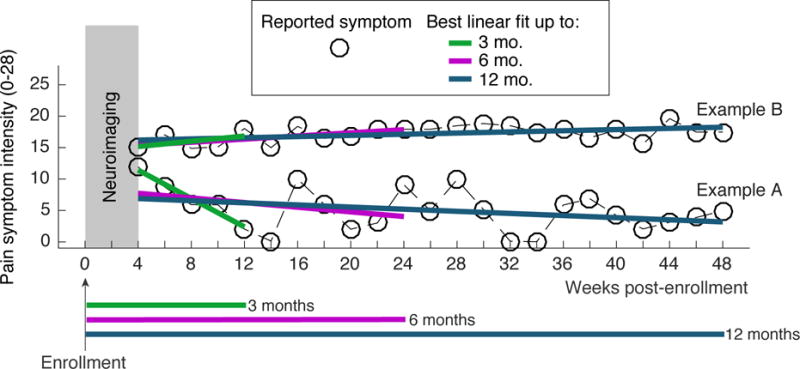
Data from two example UCPPS patients illustrating the design of the study and outcome measures. Within 4 weeks of enrollment, each UCPPS patient underwent neuroimaging procedures that captured the resting state fMRI (rs-fMRI) data. UCPPS symptoms, both pain symptom severity and urinary symptom severity, were assessed every 2 weeks for 48 weeks (12 4-week months). Symptoms during the first 4 weeks of the study were not analyzed to allow for a run-in period. Linear regression was used to quantify the trend in symptoms over a 3-month (12 week) period, a 6 month (24 week) period, and a 12 month (48 week) period. rs-fMRI measures were used to predict symptom change across the population of UCPPS patients studied. Example patient A exhibited a rapid pain symptom decrease in the first 3-months after neuroimaging and will be part of a group termed “improvers” for the 3-month time period. Example patient B does not exhibit 3-month symptom change and will be part of a group termed “non-improvers” for the 3-month time period.
The goal of the present work is to determine if baseline rs-fMRI data contains information about future symptom trends in UCPPS patients. Previous neuroimaging-based longitudinal symptom prediction papers in other conditions (low back pain, autism) have been able to report classification accuracy as a primary outcome because pre-determined thresholds were available for dividing participants into a “better” and “worse” class based on a continuous outcome [4; 29]. In practice, these thresholds were close to the median of the distribution of the continuous outcome variable. As these previous papers, we wanted to report classification accuracy as a measure of information in the baseline resting state scan about future change in symptoms. However, the UCPPS condition does not have a pre-determined threshold for symptom change. Therefore, we divided symptom improvement trends in each time period across the population median to classify UCPPS patients as either “improvers” or “non-improvers”. The distribution of symptom trends for improvers and non-improvers were tested for an average of 0 (no improvement) using a t-test. As a sensitivity analysis, we also present results based on dichotomizing the symptom trends according to the 25th and 75th percentile. As an additional analysis, we also use a continuous regression approach to associate the rs-fMRI predictors directly with the continuous symptom change trends.
Predictors: strength of functional connections in the brain
We sought to determine if functional connectivity measures derived from rs-fMRI could predict UCPPS patient classification as improver or non-improver in the 3, 6, or 12 month time period. MRI acquisition parameters in the MAPP study have been described previously [1]. The MAPP cohort was imaged using 3 Tesla scanners according to the following procedures. A high resolution structural image was acquired from each subject with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, with repetition time (TR) = 2200 ms, echo time (TE) = 3.26 ms, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 13 mm voxel size. Resting state scans were acquired while subjects rested with eyes closed for 10 min in 40-slice whole brain volumes, with slice thickness = 4 mm, TR = 2000 ms, TE = 28 ms, and flip angle = 77°. MAPP neuroimaging data were collected, quality controlled (independently of the authors) and archived according to multi-site imaging procedures (PAINrepository.org).
We processed the rs-fMRI images from each patient according to the following procedures: slice time correction, motion correction, spatial smoothing using a Gaussian kernel of full-width half-maximum of 5 mm and nonlinear high-pass temporal filtering (150 s). We registered processed images to the Montreal Neurological Institute (MNI) standard template using the flirt function in the FSL software package. We then extracted an average signal from each region of 165 anatomically-defined regions of the Destrieux atlas [9]. We calculated the functional connectivity between each pair of regions using a general linear model that controlled for 9 confounds of no interest: the global signal, CSF signal, white matter signal, and the 6 parameters of rigid body head motion. For each participant, this analysis produced 13,530 unique connectivity values. Connectivity values were site-corrected by subtracting the mean of all UCPPS patients (both primary and secondary UCPPS cohort) at that site and adding the global (across site) mean, as described previously [1]; once site correction was performed, no additional analysis was performed on the secondary UCPPS cohort.
We then attempted to classify improvers versus non-improvers based on connectivity vectors. We used a support vector machine (SVM) classification with leave-one-out cross-validation (LOOCV). LOOCV is appropriate as a preliminary estimate classifier generalizability [3], and has been commonly used in longitudinally predictive neuroimaging studies where sample sizes may not be adequate for a separate training and validation set [29]. We assessed the predictive ability of the SVM classifier in each time period using the classification accuracy, the sensitivity, and the precision. Accuracy is the percent of correctly classified individuals. Sensitivity can be understood as the ability of the SVM to correctly identify improvers. Precision, a complementary measure, can be understood as the ability of the SVM to not incorrectly classify a non-improver as a improver. Furthermore, we calculated a p-value as the probability that random chance would classify as accurately as the SVM (permutation test with 50,000 iterations).
Some previous longitudinal prediction studies perform correction of functional connectivity data for head motion only at the individual-participant level [4; 29], but several previous studies have established that head motion is an important group-level confound to consider in functional connectivity studies [30; 31; 37; 38; 43], especially when there may be group differences in head motion between the groups of interest [35]. To control for this confound, we performed the following group-level analyses beyond standard head motion correction at the individual-level. First, we performed simple t-tests in each translation and rotation variable to compare improvers and non-improvers. Second, we used head motion as predictor data (without neuroimaging data) for the symptom trends using the same SVM approach with LOOCV described above to determine if any significant prediction could be obtained from head motion alone. Third, we used linear regression to remove the effect of motion from the symptom trend before performing prediction analysis. In each case, head motion was described by reducing the x-rotation, y-rotation, z-rotation, x-translation, y-translation, and z-translation time series to either a maximum change in a single time-step (repetition time, TR) or a maximum deviation from baseline.
We provided a visualization of SVM weights that were most important to the classification. After the LOOCV procedure, we trained a single SVM on all participants. The SVM weight indicates the importance of each functional connection to the classification; connections of high positive weight indicate connections that are important to the prediction of improvers and connections of high negative weight indicate connections that are important to the prediction of non-improvers. As a function of N, we examined brain locations (centroids of regions in the Destrieux structural atlas) of the N connections with most positive and most negative SVM weight. To determine if the identified connections preferentially aligned with known resting brain networks, we also determined the fraction of these N connections for which both regions of the connection were contained in the same published resting state network as determined by independent components analysis (ICA). We examined ICA templates from 10 common resting state networks available online as defined previously [39]. We used the following 10 networks: 3 visual networks, default mode network, cerebellar network, sensorimotor network, salience network, frontal network, right frontoparietal network, left frontoparietal network.
To examine the robustness of our classification algorithm, we used LOOCV to examine classification accuracy as we defined improver versus non-improver according to different percentile splits. In addition to the median (50th percentile) described above, we also examined splitting the data at the 25th and 75th percentiles. After LOOCV, we trained a single SVM for each of these splits. We rank-ordered the weights according to the SVM trained for the median split, ordered the weights for the SVMs trained for the other two percentile splits according to the rank determined for the median, and finally calculated the Spearman rank correlation coefficient for the SVM weights of the 25th and 75th percentile splits with the SVM weights for the median split. This procedure allowed us to quantify the disturbance to the SVM weights associated with choosing a different split (other than the median) to define improvers versus non-improvers.
Finally, as an additional test of the association between rs-fMRI and symptom change trends, we used the symptom change trends as continuous variables and performed a regression analysis to identify significant predictors in the baseline rs-fMRI data. A number of different high-dimensional continuous regression approaches for neuroimaging data have been previously described, including LASSO regression (least absolute shrinkage and selection operator) on voxel-based data [44] and ridge regression on atlas-based functional connectivity data [29]. We implemented a ridge regression algorithm since the structure of our data was atlas-based rather than voxel-based functional connectivity data. We only implemented the continuous analysis to verify the prediction of symptom change trends that showed promise in the classification approach described above. We implemented the continuous analysis as a high-dimensional linear regression model (fitrlinear in MATLAB). The regression model was used to associate the neuroimaging data in a 52 × 13530 matrix X (identical to the SVM input) with the 52 × 1 vector Y containing the symptom change trends. LOOCV was used with the actual Y to determine the mean squared error (MSE) and the distribution of regression coefficients. To assess the overall significance of the prediction, a bootstrapping procedure was used to estimate the distribution of the MSE obtained by randomly permuting Y in 1000 iterations as in previous studies [29; 44], and we calculated the number of bootstrap iterations in which the MSE was better than the MSE in the unpermuted data.
In the classification and regression techniques described above, it is important to note that predictions are made based on the full set of weights across all connections [44], which provides that some connections may be individually variable across participants while their combination can form a valid prediction. Hence, our first visualization of important connections simply rank-ordered the connections according to weight in the prediction without thresholding. None-the-less, previous publications have used a bootstrapping procedure (with 1000 bootstrap samples) to estimate the significance of individual predictors (voxels or connections) against the null distribution generated by randomly permuting the outcome to be predicted [29; 44]. We implemented the same procedure here both for the classification and continuous regression approaches. We repeated the LOOCV procedure for each bootstrap sample, and calculated the fraction of samples for which the particular connections had SVM weight or continuous regression coefficient (calculated on the un-permuted data) outside of the distribution of the bootstrap samples. This fraction provided a p-value for each functional connection, which we then corrected for multiple comparisons using a false discovery rate of 0.05.
Analysis of phenotypic and treatment effects on prediction modeling
To further characterize our patient population and to examine phenotypic and treatment factors that could potentially confound the prediction results, we examined additional baseline questionnaire data. We examined sex, age, the body mass index (BMI), the UCPPS symptom duration, the number of painful body regions from a 45-region body map of pain on the Brief Pain Inventory (BPI) [6], and the Hospital Anxiety and Depression Scale (HADS). These variables were compared between improvers and non-improvers to examine any clear association between non-neuroimaging data and symptom change trend.
Although prediction of symptom change trends from baseline neuroimaging data was the sole goal of this study, we also explored possible psychological correlates of the identified predictive neuroimaging patterns. We explored the univariate correlation between the cross-validated output of the neuroimaging-based SVM classifier or continuous regression and the following phenotypic/psychological variables: sex, age in years, symptom duration in years (dursym-yrs), educational level (edu, range 1 - Less than high schoolto 5 - Graduate or professional school), income (income, range 1 - $10,000 or less to 5 – more than $100,000), anxiety (HADS-anx) and depression (HADS-dep), Positive and Negative Affect Schedule (PANAS-positive and PANAS-negative), Multiple Abilities Self-Report Questionnaire for language (MASQ-language) verbal memory(MASQ-VM) visual spatial memory (MASQ-VSM) and attention (MASQ-Attention), Self-Esteem And Relationship for sexual relations (SEAR-SexRel) confidence (SEAR-Confi) self-esteem (SEAR-SlfEsteem) overall relationship (SEAR-OvalRel) and total (SEAR-tot), International Personality Item Pool for neuroticism (IPIP-N) extraversion (IPIP-E) openness (IPIP-O) agreeableness (IPIP-A) conscientiousness (IPIP-C), Beliefs in Pain Control for internal locus of pain control (BPCQ-I) powerful others/doctors (BPCQ-D) and chance happenings (BPCQ-C), perceived stress scale (PSS), genitourinary pain index at baseline for pain symptoms (GUPI-pain) urinary symptoms (GUPI-urin) and quality-of-life (GUPI-qol), Coping Strategies Questionnaire for ability to control pain with coping (CSQ-C) and ability to decrease pain with coping (CSQ-D), Childhood Trust Events Survey for events before age of 17 (CTES-17) and events in the last 3 years (CTES-3yrs), Complex Multi-Symptom Inventory for co-morbid symptoms during the lifetime (CMSI-life) and during the past year (CMSI-year), and the SF-12 scale for physical function (SF12-PCS) and mental function (SF12-MCS). A p-value for the linear regression of the neuroimaging output as the dependent variable and the phenotypic/psychological factor as the independent variable was calculated to screen for associations between the variables, and an uncorrected threshold of p<0.05 was used to define variables of potential interest.
Treatment data were obtained from bimonthly open-ended text responses on the BPI to the question “What treatments or medications are you receiving for your pain?”. Reported interventions were classified according to the following categories: alpha-blockers, benzodiazepines, anticholinergic agents, oral elmiron, gabapentinoids, antihistamines, bladder instillations, anti-inflammatories, opioids, physical therapy, selective serotonin reuptake inhibitors (SSRI), serotonin–norepinephrine reuptake inhibitors (SNRI), tricyclic agents, antibiotics/antifungals, or none-of-the-above. The proportion of patients using interventions in each category at specific time points was compared for between improvers and non-improvers using Fisher’s Exact test.
RESULTS
Our sample of UCPPS patients with longitudinal symptom data and baseline neuroimaging in the primary cohort included 52 adults (34 Female, 18 Male). The participants in the primary UCPPS cohort had an average age of 38.8 years (± 11.9, standard deviation), and an average age of UCPPS symptom onset of 32.2 years (± 12.9). Participants in the secondary UCPPS cohort (45 female, 15 male) had an average age of 40.4 (± 13.5) and average age of UCPPS symptom onset of 27.8 (± 12.2). See Table 1 for clinical characteristics of the sample. No significant differences in age, sex, or age of UCPPS symptom onset were observed between the primary and secondary UCPPS cohort.
Table 1.
Clinical characteristics
| Clinical Characteristics (mean ± std) | Primary UCPPS cohort (n = 52) | Secondary UCPPS cohort (n = 60) | p-value |
|---|---|---|---|
| Age (years) | 38.8 ± 11.9 | 40.4 ± 13.5 | 0.5037 |
| Sex | 34 F, 18 M | 45 F, 15 M | 0.2656 |
| Age (years) of UCPPS symptom onset | 32.2 ± 12.9 | 27.8 ± 12.2 | 0.0715 |
| Body Mass Index | 25.3 ± 5.7 | 26.7 ± 5.8 | 0.2056 |
| Race and ethnicity | |||
| Native American | 2 | 0 | |
| Asian | 2 | 2 | |
| African American | 5 | 2 | |
| Native Hawaiian | 0 | 0 | |
| Caucasian | 44 | 54 | |
| Other Race | 2 | 3 | |
| Hispanic or latino | 5 | 2 |
We observed considerable interindividual differences in the trends (slopes) of pain and urinary symptoms (Figure 2). Interindividual differences in symptom trends were especially pronounced for pain and urinary symptoms for the 3-month time period. To demonstrate that the ordering of patients according to symptom trends could be very different for the different time intervals, we show that patients dichotomized according to the median symptom slope for the 3-month time period did not maintain that dichotomization at the 6-month or 12-month time period (Figure 2). Thus, the neuroimaging data would have the potential to predict symptom trends for certain time periods but not others, as we did not observe a strong association in how patients would be labeled improvers versus non-improvers for the different time periods, especially for 3-month pain trends compared to longer-term pain trends. Spearman rank correlation (ρ) of symptom trends within the same individuals for different time periods were as follows. For pain symptoms: ρ3,6 = 0.38, ρ3,12 = 0.31, and ρ6,12 = 0.64; for urinary symptoms: ρ3,6 = 0.50, ρ3,12 = 0.26, and ρ6,12 = 0.60. Thus, individuals showing the greatest short term improvement may not be the same individuals showing the greatest longer term improvement, and thus neuroimaging data may be able to predict trends in certain time periods but not others.
Figure 2.
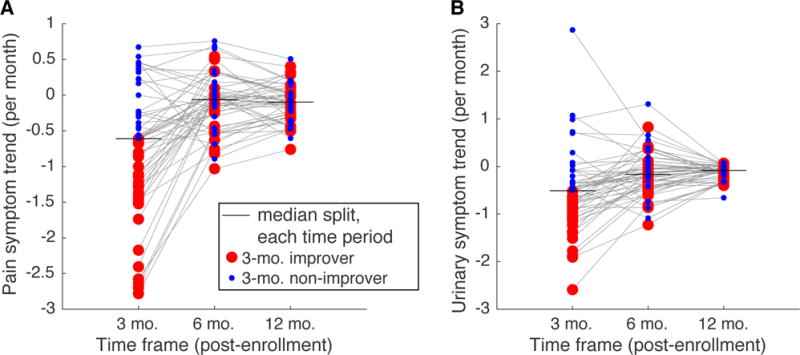
Distribution of trends in symptom change across the cohort of UCPPS patients examined in the present study. Each dot represents the symptom trend (slope) of a single UCPPS patient from the end of the 4 week run-in period through the end of the specified time frame (3, 6, or 12 months). Horizontal lines show median splits of the trends at each time frame. Dots are color coded as “improvers” or “non-improvers” according to a median split of the trends in the 3 month time period, thin gray lines connect the same patient. A. Pain symptoms. B. Urinary symptoms. We used the median split at each time period to dichotomize the participants for classification; the carry-over of the 3 month dichotomization is shown here simply to illustrate that the dichotomization was not preserved across different time periods.
Most importantly, we found that rs-fMRI data were able to predict 3-month pain trends with significant accuracy (Table 2). Using LOOCV, we observed that rs-fMRI data correctly identified 73.1% of patients correctly as improvers or non-improvers (p=0.0012), with 69.2% sensitivity and 75.0% precision. We also observed that rs-fMRI data showed a trend toward significant prediction of 12-month pain symptom trends (p=0.07), but was much less significant than the prediction of 3-month pain symptom trends. We examined both pain and urinary symptom trends for the 3, 6, and 12-month time periods, and observed that rs-fMRI data was predictive of 3-month pain symptom trends.
Table 2.
SVM-based neuroimaging classification of symptom slope performance - accuracy (p-value), sensitivity, precision - based on leave-one-out cross-validation. The p-value is calculated by estimating the probability that random assignment of an improver vs. non-improver label (assuming equal numbers) could achieve this accuracy by chance.
| Symptom | ||
|---|---|---|
| Pain | Urinary | |
| Time Frame | ||
| 3 Months | 73.1% (0.0012), 69.2%, 75.0% | 48.1% (0.6086), 53.8%, 48.3% |
| 6 Months | 53.8% (0.3905), 46.2%, 54.5% | 42.3% (0.9187), 46.2%, 42.9% |
| 12 Months | 59.6% (0.0790), 57.7%, 60.0% | 40.4% (0.9188), 42.3%, 40.7% |
We found that head motion did not differ between the improvers and non-improvers either in the maximum change in a single time-step (p-values for t-test for x-rotation, y-rotation, z-rotation, x-translation, y-translation, and z-translation were 0.67, 0.93, 0.37, 0.24, 0.73, 0.97, respectively) or in the maximum deviation from baseline (p-values for t-test were 0.67, 0.48, 0.84, 0.98, 0.71, and 0.74, respectively). Using head motion as predictor data for symptom trend class, we observed that head motion data alone could not make significant predictions: neither maximum change in a single time-step (51.9% accuracy) nor maximum deviation from baseline (46.2% accuracy) were significant. Regressing head motion estimates from longitudinal symptom trends before dichotomization and prediction from neuroimaging data, we found that the neuroimaging prediction remained significant even after adjusting for either maximum change in a single time-step (71.2% accuracy, p=0.001) or after adjusting for maximum deviation from baseline (67.3% accuracy, p=0.006).
We then studied the internal structure of the predictive SVM to identify the brain features contributing most to the prediction of 3-month pain trends. We initially ranked connections according to SVM weight (Figure 3A-1, 3B-1). This analysis revealed that the 100 connections with most positive weight and the 100 connections with most negative weight, together representing only 1.48% of the 13,530 pair-wise connections, accounted for 44% of the range of the SVM weight values. We observed that the 100 connections with most positive weight, associated with connectivity being larger in improvers compared to non-improvers, were strongly concentrated in the left hemisphere and that a disproportionate percentage of these connections involved the left frontal and left parietal lobes (Figure 3A-2). Upon examining how the connections with the most positive SVM weights would align with common resting state networks, we observed a preferential alignment with the left frontoparietal network (Figure 3A-3). A connectogram of the 100 connections with most positive SVM weight can be used to visualize the brain regions associated with each of these connections (Figure 3A-4). We performed identical analyses for the 100 connections with most negative weight, but we did not observe any obvious spatial pattern or alignment with known networks for these connections (Figure 3B).
Figure 3.

Brain functional connections that contribute most to the prediction of 3-month improvement in pain. A. Functional connections with most positive weight in the SVM (improvers > non-improvers). A1. We initially examined 100 connections as there appeared to be diminishing change in weight with the addition of new connections beyond these. A2. These 100 connections, when visualized on the brain, appeared to be concentrated in the frontal and parietal cortices on the left side of the brain (each sphere represents a region making one of these 100 connections, and the size of the sphere codes the number of these 100 connections made by that region). Regions are colored according to their associated lobe in the connectogram in A4. A3. Compared to templates of 10 common resting state networks based on independent components analysis (3 visual networks, default mode network, cerebellar network, sensorimotor network, salience network, frontal network, right frontoparietal network, left frontoparietal network) [39], the left frontoparietal network (L-FPN) appeared to contain pairs of locations with the greatest percentage of the altered connections. A4. Diagram of connections (connectogram) showing the 100 connections in A1 (see supplemental information for region abbreviations). B. Identical figures for connections with most negative weight in the SVM (non-improvers > improvers). Notice that these regions do not tend to align preferentially with any common resting state networks.
To ensure that our prediction results were not overly sensitive to the choice of dichotomization (median) to determine improvers and non-improvers, we also performed a sensitivity analysis of this dichotomization. For comparison, we dichotomized the 3-month symptom trend according to the 25th percentile (creating more non-improvers) and the 75th percentile (creating more improvers). We found that the accuracy increased for the 25th percentile split (75% accuracy, p=0.01), whereas the accuracy decreased for the 75th percentile and became non-significant (63.5% accuracy, p=0.41) (Figure 4A). The rank order of the SVM weights for the median split and 25th percentile split exhibited similar structure (ρ=0.67) (Figure 4B). These sensitivity analysis results suggest that rs-fMRI features predictive of patients with the greatest downward trends in 3-month pain symptoms are very likely to be distinct from patients with more modest trends, but that differences among patients with the smallest downward trends are least likely to be predicted by rs-fMRI data.
Figure 4.
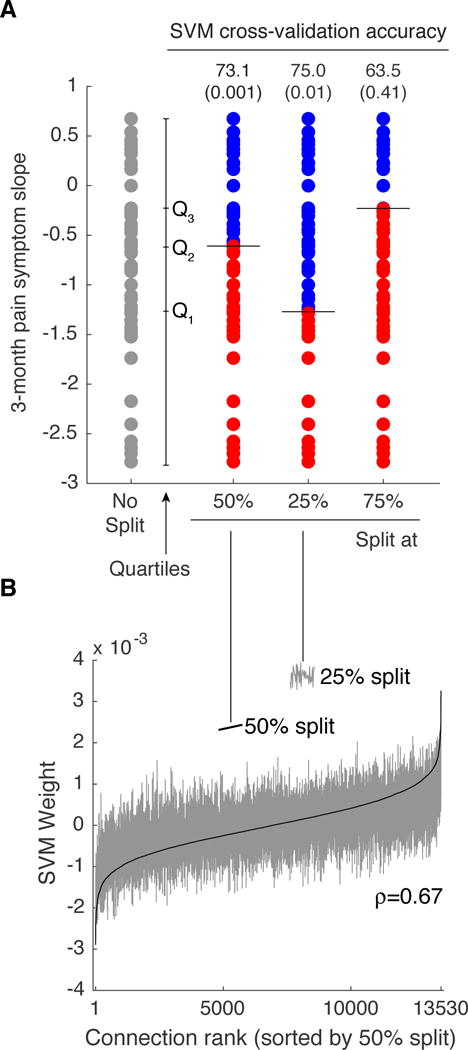
Analysis of SVM classification sensitivity to categorization of pain trends (slopes) into improvers and non-improvers. A. Original distribution of 3-month pain symptom trend (no split), as well as data split across the median (Q2), the 25th percentile (Q1) and the 75th percentile (Q3). Classification accuracy for the 25th percentile split increased to 75% (p=0.01), but decreased for the 75th percentile split to 63.5% (p=0.41). B. SVM weights ordered according to increasing value for the 50th percentile split (solid black line) largely retained the same ordering for the SVM trained on the 25th percentile split (Spearman rank correlation = 0.67).
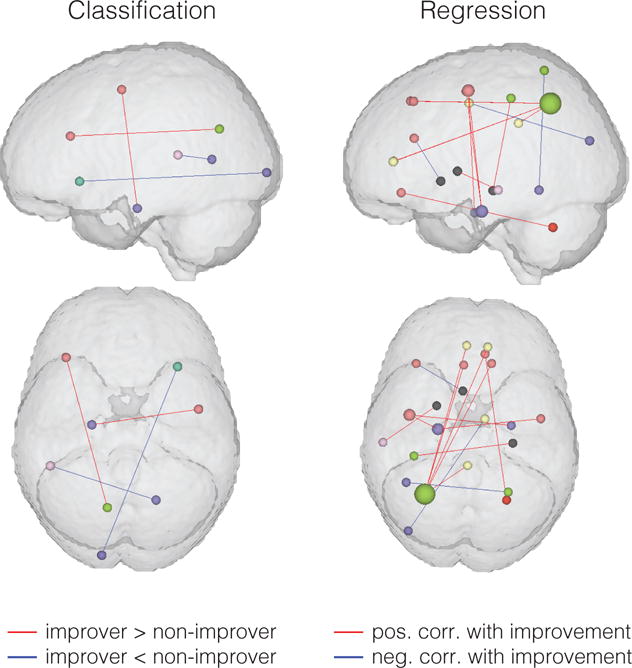
Continuous regression analysis revealed that the rs-fMRI data could make a significant prediction of 3-month pain symptom change trends (p=0.045). Displaying the 100 connections with most important SVM weights from the classification findings along with the 100 connections with most important continuous regression coefficients showed a general similarity in the findings of these two techniques (Figure 5), particularly with frontoparietal connections in the left hemisphere important in both classification and regression analyses.
Figure 5.
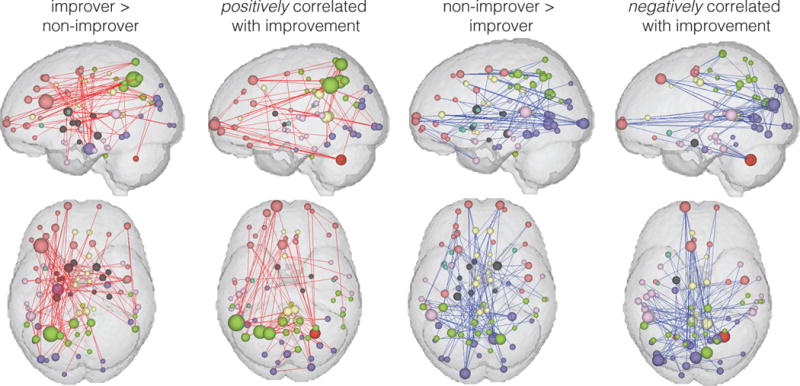
100 most important connections for classification analysis (improver > non-improver and non-improver > improver) and for continuous regression analysis (positive correlation with improvement and negative correlation with improvement).
Using both SVM classification and continuous regression, we observed evidence of individual significant connections that repeatedly contributed more to the prediction of symptom trends than would be expected by chance (Figure 6). The SVM classifier showed two significant connections that were stronger in improvers compared to non-improvers – one left frontoparietal connection and a connection between precentral gyrus (motor cortex) and the parahippocampal gyrus. The SVM classifier showed two additional significant connections that were stronger in non-improvers compared to improvers. The continuous regression showed 11 connections which were positively correlated with improvement – including 5 connections that involved the left intraparietal sulcus, as well as 3 connections involving bilateral precentral gyri and parahippocampal gyri. The continuous regression had 2 significant connections that were negatively correlated with improvement. All significant connections are detailed in Table 3.
Figure 6.
Functional connections that were found to be altered significantly more than would be expected by chance (p<0.05, FDR-corrected).
Table 3.
Significant connections associated with 3-month pain symptom change trends
| Abbreviation | Description | Freesurfer Code |
|---|---|---|
| Continous, positively correlated with better symptom change trend | ||
| L_IntPS/TrPS | L Intraparietal sulcus (interparietal sulcus) and transverse parietal sulci | ctx_lh_S_intrapariet_and_P_trans |
| R_SupFG | R Superior frontal gyrus | ctx_rh_G_front_sup |
| L_SupF G | L Superior frontal gyrus | ctx_lh_S_intrapariet_and_P_trans |
| R_ACgG/S | R Anterior part of the cingulate gyrus and sulcus (ACC) | ctx_rh_G_and_S_cingul-Ant |
| L_ACgG/S | L Anterior part of the cingulate gyrus and sulcus (ACC) | ctx_lh_S_intrapariet_and_P_trans |
| L_PosDCgG | L Posterior-dorsal part of the cingulate gyrus (dPCC) | ctx_lh_G_cingul-Post-dorsal |
| L_PaHipG | L Parahippocampal gyrus, parahippocampal part of the medial occipito-temporal gyrus | ctx_lh_G_precentral |
| R_PRCG | R Precentral gyrus | ctx_rh_G_precentral |
| L_PRCG | L Precentral gyrus | ctx_lh_G_precentral |
| L_PaHipG | L Parahippocampal gyrus, parahippocampal part of the medial occipito-temporal gyrus | ctx_lh_G_precentral |
| R_PaHipG | R Parahippocampal gyrus, parahippocampal part of the medial occipito-temporal gyrus | ctx_rh_G_oc-temp_med-Parahip |
| L_Pu | L Putamen | Left-Putamen |
| L_MTG | L Middle temporal gyrus | ctx_lh_G_temporal_middle |
| R_CeB | R Cerebellum | Right-Cerebellum-Cortex |
| R_SbOrS | R Suborbital sulcus (sulcus rostrales, supraorbital sulcus) | ctx_rh_S_suborbital |
| R_Hip | R Hippocampus | Right-Hippocampus |
| L_PosCS | L Postcentral sulcus | ctx_lh_S_postcentral |
| Continous, negatively correlated with better symptom change trend | ||
| L_Nacc | L Nucleus Accumbens | Left-Accumbens-area |
| L_InfFS | L Inferior frontal sulcus | ctx_lh_S_front_inf |
| L_MOcG | L Middle occipital gyrus | ctx_lh_G_occipital_middle |
| R_MPosCgG/S | R Middle-posterior part of the cingulate gyrus and sulcus (pMCC) | ctx_rh_G_and_S_cingul-Mid-Post |
| L_LOcTS | L Lateral occipito-temporal sulcus | ctx_lh_S_oc-temp_lat |
| R_SupPL | R Superior parietal lobule | ctx_rh_G_parietal_sup |
| Classification, higher in improvers | ||
| L_InfFS | L Inferior frontal sulcus | ctx_lh_S_front_inf |
| L_POcS | L Parieto-occipital sulcus (or fissure) | ctx_lh_S_parieto_occipital |
| L_PaHipG | L Parahippocampal gyrus, parahippocampal part of the medial occipito-temporal gyrus | ctx_lh_G_oc-temp_med-Parahip |
| R_PRCG | R Precentral gyrus | ctx_rh_G_precentral |
| Classification, lower in improvers | ||
| R_ACirInS | R Anterior segment of the circular sulcus of the insula | ctx_rh_S_circular_insula_ant |
| L_OcPo | L Occipital pole | ctx_lh_Pole_occipital |
| L_SupTS | L Superior temporal sulcus (parallel sulcus) | ctx_lh_S_temporal_sup |
| R_CcS | R Calcarine sulcus | ctx_rh_S_calcarine |
Additional questionnaire data were used to assess any obvious differences between 3-month improvers and non-improvers (based on the original median split) in pain symptoms (Table 4). Patients in the improver group were no different on average from the non-improver group in sex, age, BMI, symptom duration, anxiety, depression, number of painful body locations, or initial pain or urinary symptom severity. Identical distribution of sexes and nearly identical distribution of ages in the improver and non-improver groups emerged by chance and were not part of the study design. Univariate associations with the predictive classification-based neuroimaging patterns revealed belief in pain control (BPCQ-P), education and duration of symptoms as potentially important factors (Figure 7). Univariate associations with predictive regression-based neuroimaging results revealed only BPCQ-P as a potentially important factor (Figure 7). The regression coefficient for BPCQ-P had the same sign in both classification and regression analyses, and suggested that increases in the neuroimaging pattern predictive of improvement were associated with decreases in the belief that powerful others control pain. No significant univariate associations were found with age or sex. Finally, none of the treatment categories were preferentially endorsed by improvers compared to non-improvers at either the baseline assessment or at an 8 week assessment (all p-values greater than 0.25). Table 5 shows the distribution of treatment use in the improver and non-improver group at the baseline and 8 week time point. Very few patients in the study reported using many of the treatments of interest (no more than 10/52 patients reported actively the same treatment for their pelvic pain).
Table 4.
Patient characteristics according to 3-month pain symptom change trend improvers and non-improvers.
| UCPPS Patient Group | |||
|---|---|---|---|
| Variable | 3 month Greater-Improvers |
3 month Lesser-Improvers |
p-value |
| Sex | 9 M,17 F | 9 M,17 F | – |
| Age (years) | 38.39 ± 10.54 | 39.23 ± 13.37 | 0.804 |
| BMI | 25.67 ± 4.63 | 24.86 ± 6.77 | 0.616 |
| Symptom duration (years) | 6.16 ± 5.69 | 7.39 ± 10.48 | 0.604 |
| Age at symptom onset (years) | 32.23 ± 11.50 | 32.29 ± 14.67 | 0.987 |
| HADS Anxiety | 7.42 ± 3.88 | 8.00 ± 4.31 | 0.617 |
| HADS Depression | 5.09 ± 3.58 | 6.47 ± 4.83 | 0.252 |
| # painful body sites (0–45) | 6.88 ± 7.40 | 6.92 ± 7.50 | 0.987 |
| Initial Pain Severity (0–28) | 12.82 ± 4.60 | 12.70 ± 5.16 | 0.927 |
| Initial Urinary Severity (0–25) | 10.18 ± 5.85 | 10.97 ± 5.68 | 0.627 |
Figure 7.
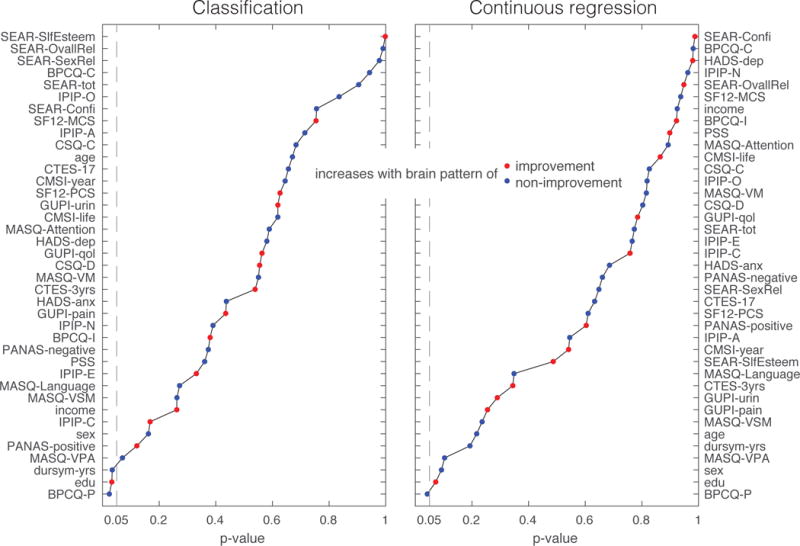
Univariate associations between neuroimaging patterns predictive of 3-month pain symptom change trends and non-neuroimaging questionnaire data. Associations are ranked according to p-value, red dots indicate that the questionnaire variable tends to increase when the brain pattern moves toward values associated with improvers, blue dots indicate that the questionnaire variable tends to increase when the brain pattern moves toward values associated with non-improvers.
Table 5.
Medication use by category for 3-month pain symptom change trend improvers and non-improvers at the baseline and 8-week assessment.
| Baseline Assessment | 8-week Assessment | |||||
|---|---|---|---|---|---|---|
| Treatment | 3 month Improvers N=26 |
3 month Non-Improvers N=26 |
p-value | 3 month Improvers N=26 |
3 month Non-Improvers N=26 |
p-value |
| Alphablockers | 3 | 1 | 0.60 | 0 | 0 | 1.0 |
| Benzodiazepines | 1 | 1 | 1.0 | 0 | 0 | 1.0 |
| Anticholinergics | 1 | 2 | 1.0 | 0 | 1 | 1.0 |
| Elmiron | 1 | 2 | 1.0 | 0 | 0 | 1.0 |
| Gabapentinoids | 1 | 1 | 1.0 | 1 | 1 | 1.0 |
| Antihistamines | 0 | 1 | 1.0 | 0 | 0 | 1.0 |
| Bladder instillation | 1 | 1 | 1.0 | 0 | 0 | 1.0 |
| NSAIDs | 6 | 4 | 0.24 | 3 | 1 | 0.68 |
| Opioids | 2 | 4 | 0.66 | 1 | 0 | 1.0 |
| Pelvic floor physical therapy | 4 | 6 | 0.72 | 2 | 2 | 1.0 |
| NSRIs | 0 | 0 | 1.0 | 1 | 1 | 1.0 |
| SSRIs | 0 | 0 | 1.0 | 0 | 0 | 1.0 |
| Tricyclic antidepressants | 2 | 5 | 0.41 | 2 | 3 | 1.0 |
| Antibiotics/antifungals | 0 | 1 | 1.0 | 0 | 0 | 1.0 |
DISCUSSION
This is the first study to examine the ability of rs-fMRI to predict short-term longitudinal change in pain symptoms for patients in a state of chronic pain. The results not only demonstrate that such prediction is possible, but also point to functional connectivity in specific brain networks as a potential biological factor associated with this predictive ability. The results of the study have broad implications for understanding biological mechanisms that may contribute to symptom change on multi-month timescales in UCPPS and other chronic pain conditions, and ultimately designing and testing treatment approaches that aim to enhance symptom reduction and long-term symptom abatement by targeting these mechanisms. Here we discuss preliminary interpretations of the brain functional connectivity patterns that we identified, how such functional connectivity patterns might be influenced to test causality, and critical future studies to refine and test the predictions of the new hypotheses that emerge from this work.
Several intriguing observations and questions arise from our findings. First, why does the rs-fMRI baseline data most readily predict 3 month symptoms but not perform as well on 6 or 12 month symptom change trends? Based on previous literature, UCPPS is understood as a relatively sustained chronic condition, with a gradual change in symptoms over several years averaged across patients, but without lasting “remission” periods [32; 33]. However, at the level of individual UCPPS patients, longitudinal symptom profiles exhibit substantial variability [40]. Therefore, we hypothesize that the population of longitudinal symptom profiles for pain and urinary symptoms in UCPPS patients across many months can be conceptualized as signals varying around a relatively stable average. Thus, there may be less meaningful differences among patients across the 6 or 12 month time period, making prediction difficult. Variations on shorter time-scales may be considered “random noise”, but here we open the possibility that they are informative of disease mechanisms. One such source of these variations is early symptom regression also referred to as “regression to the mean” (RTM) [40]. The RTM effect has been observed in previous studies of UCPPS over the first few months [32; 33; 40]. RTM may arise from two primary sources – recruitment bias and an enrollment effect. RTM due to recruitment bias may occur because recruitment is shifted toward patients whose symptoms are higher than their own average, and consequently symptoms return to a more typical level after repeated longitudinal measurement. If RTM is due to recruitment bias, symptom changes in individual patients are still “natural” in that they may have occurred even if the study was not conducted. If RTM is due to an enrollment effect, symptom changes in individual patients may be more related to individual differences in placebo response. RTM is a large problem in treatment studies because it can produce the appearance of a treatment effect when no such effect exists. However, in a longitudinal observational study such as MAPP, identifying biological factors (including brain connectivity) associated with those patients who had the largest short-term symptom reductions compared to those that did not may provide insight into dynamic factors that mediate UCPPS symptoms. Future longitudinal studies in UCPPS will be necessary to disambiguate RTM due to recruitment bias from RTM due to enrollment. For example, longitudinal symptom assessment with multiple imaging time-points in the same participants (e.g. baseline and 6-months) could be used to determine if rs-fMRI can predict symptom change trends across 3-month periods regardless of whether this period follows enrollment or follows the 6-month scan. If RTM is due to recruitment bias and reflects natural symptom changes, we would expect that the brain networks identified in this study would be predictive of future symptom progression following either the baseline or 6-month scan.
Second, why is connectivity predictive of pain symptom change trends distributed across the brain in the observed pattern? The most striking features in the pattern of brain functional connectivity associated with greater pain improvement is its composition (relatively large contribution from the frontal and parietal cortices) and its laterality. Frontoparietal networks have been traditionally associated with attentional modulation [41], specifically the selection of sensory information by attention [34]. It integrates feature information elaborated in sensory cortex and top-down representations of behavioral goals and expectations originating in the dorsolateral prefrontal and premotor cortex. The network has also been referred to as the frontoparietal control system which regulates distributed sensory, emotional and motor systems according to current task goals and expectations, and alterations in this control system have been implicated in the pathophysiology of several psychiatric disorders [8]. Previous studies in experimentally-induced pain in healthy individuals have identified that frontoparietal networks play an important role in expectancy-induced modulation of pain [21; 45] including the explanation of inter-individual differences in placebo response [44]. It is currently not known if these findings directly apply to a population of individuals with chronic pain, but the current study points to the exciting possibility that frontoparietal networks may play an important role in chronic pain even in a highly chronified state. We showed preliminary evidence that the frontoparietal pattern we identified may relate to beliefs about pain control. These beliefs may be coupled to the natural increases and decreases of symptoms in these patients in the chronic state (RTM due to recruitment bias), or induced by enrollment in the study (RTM due to enrollment). It is well-known that belief plays a critical role in pain modulation [45], but it has been difficult to exploit this fact to enhance treatment effectiveness [44]. Given the association between placebo response and expectation of pain relief [44], expectation of pain change may be important to measure in the UCPPS population in future studies even if these studies do not involve treatment.
Third, why does the rs-fMRI baseline data most readily predict pain but not urinary symptom change trends? One possibility is that biological processes that control changes in urinary symptoms do not reside in the brain, unlike those that control changes in pain. Information about processes that may act to change urinary symptoms, such as spinal-level control of urination [11] or bladder biomarkers [2], may not be manifest as changes in functional interactions in the resting brain. Another possibility is that trends in changing urinary symptoms do arise from brain processes but on a faster time-scale than pain processes and were therefore not captured in this study. Future studies linking bladder biomarkers and brain imaging and examining prediction of urinary symptom changes over different time scales will be necessary.
Previous work that has considered neuroimaging-based symptom prediction in chronic pain identified stronger functional connectivity between the nucleus accumbens and frontal cortex as a key predictive factor in transitioning from acute to chronic pain [4]. Interestingly, our analysis indicated that the stronger functional connectivity between the nucleus accumbens and a frontal cortex region was correlated with worse 3-month pain symptom trends. This fronto-striatal connection has been associated with aversive learning in chronic pain [4]. Dynamic changes in fronto-striatal connections may therefore continue to be associated with pain beyond the initial chronification phase into the fully chronic phase.
The current study is limited in that we could not directly predict long-term symptom trends, and the specificity and sensitivity in the prediction of short-term symptom trends do not reach levels of clinical utility. Nonetheless, the aim of the present study was simply to demonstrate that there is information in the resting brain of patients with UCPPS about future symptom trends and to point toward potential brain networks of interest, not to develop a neuroimaging-based clinical test to predict future symptom progression. Future studies with larger sample sizes, more refined analytic techniques, and separate validation groups may overcome these limitations by predicting longer-term symptom trends with better sensitivity and specificity. The current study is also limited in that it did not test the causal role of brain functional connectivity in mediating pain symptom reduction. However, if brain functional connectivity does influence symptom reduction in a causal way, our study points to brain regions and connections that might be most likely to play a role. A potential approach to test this causality in future studies would be a randomized controlled study in which frontoparietal connectivity was manipulated in a group of UCPPS patients and symptom reductions were compared to a group of UCPPS patients in which no such manipulation was performed. Importantly, several candidate approaches exist for manipulating frontoparietal network function. For example, transcranial direct current stimulation (tDCS) applied to left dorsolateral prefrontal cortex has been shown to induce distant changes in functional connectivity within the left frontoparietal network [18]. Also, transcranial magnetic stimulation (TMS) applied to the left dorsolateral prefrontal cortex can reduce symptoms of depression [26] and can modulate brain connectivity [12]. Finally, attentional modulation through meditation may also be a promising approach for manipulating brain network function [20; 25]. All of these may be potential approaches in future studies to test causal influences on the brain connections we identified to be predictive.
The goal of our study was to demonstrate the feasibility of predicting longitudinal change in chronic pain observed in UCPPS. rs-fMRI was chosen as a starting modality because we expected that predictors of symptom change should, at the very least, have correlates in brain function. Furthermore, pair-wise connectivity among regions in an anatomically-parcellated brain was chosen because we have previously applied this approach to analyze brain data from both rs-fMRI [22] and structural imaging [23] for chronic pain conditions. SVM was used as a starting approach because we have previously applied SVM to distinguish UCPPS patients from healthy controls based on neuroimaging data [3]. Although our approach demonstrated that baseline rs-fMRI data in UCPPS patients contains more information about 3-month pain symptom change trend than would be expected due to chance, our sensitivity and specificity did not yet reach levels of clinical utility. Therefore, future studies can investigate optimization of the prediction algorithm by comparing numerous machine learning techniques that could be applied to this prediction problem. Future studies can expand to optimize the prediction of longitudinal symptom change in UCPPS by testing multiple brain imaging modalities (e.g. gray and white matter structure), testing multiple classifier input data (e.g. anatomical parcellation and independent components analysis), and testing different classifiers (e.g. SVM and partial least squares with discriminant analysis). Care must be used when interpreting multivariate classifiers applied to neuroimaging data, as some features may appear to be significant in order to compensate for noise in other truly predictive features [15]. Therefore, the classification approach with optimal performance using LOOCV on the dataset described here must first be tested with other cross-validation approaches (due to the limitations of LOOCV [15]) and then ultimately tested on an independent validation data set to ensure that the likelihood of identifying spurious features is minimized. Predictive power of neuroimaging should then be compared with prediction based on non-neuroimaging data (e.g. questionnaires). Nonetheless, the current study demonstrated the feasibility of predicting longitudinal pain symptom change in the chronic pain of UCPPS, and provided preliminary insight into the functional brain connections involved. Therefore, the current work presents critical groundwork for future studies of the pathophysiology of UCPPS and other chronic pain states and may aid in conceptualizing strategies for improved clinical management.
Supplementary Material
Acknowledgments
We thank all of the volunteers who participated in the study. We would like to thank Bruce Naliboff for helpful discussions. Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316).
Footnotes
Conflicts of interest: We declare the following interests: financial interest and/or other relationship with National Institutes of Health (CM).
References
- 1.Alger JR, Ellingson BM, Ashe-McNalley C, Woodworth DC, Labus JS, Farmer M, Huang L, Apkarian AV, Johnson KA, Mackey SC. Multisite, multimodal neuroimaging of chronic urological pelvic pain: Methodology of the MAPP research network. NeuroImage: Clinical. 2016 doi: 10.1016/j.nicl.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allsop SA, Erstad DJ, Brook K, Bhai SF, Cohen JM, Dimitrakoff JD. The DABBEC Phenotyping System: towards a mechanistic understanding of CP/CPPS. Nature Reviews Urology. 2011 doi: 10.1038/nrurol.2010.227. [DOI] [PubMed] [Google Scholar]
- 3.Bagarinao E, Johnson K, Martucci K, Ichesco E, Farmer M, Labus J, Ness T, Harris R, Deutsch G, Apkarian A, Mayer E, Clauw D, Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. PAIN. 2014;155(12):2502–2509. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. The Journal of urology. 2011;186(2):540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleeland C, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 7.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodríguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC urology. 2014;14(1):57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole MW, Repovš G, Anticevic A. The Frontoparietal Control System A Central Role in Mental Health. The Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer M, Huang L, Martucci K, Yang C, Maravilla K, Harris R, Clauw D, Mackey S, Ellingson B, Mayer E, Schaeffer A, Apkarian A. Brain White Matter Abnormalities in Female Interstitial Cystitis/Bladder Pain Syndrome: A MAPP Network Neuroimaging Study. The Journal of urology. 2015;194(1):118–126. doi: 10.1016/j.juro.2015.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature Reviews Neuroscience. 2008;9(6):453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) NeuroImage. 2012;62(4):2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith JW, Stephens-Shields AJ, Hou X, Naliboff BD, Pontari M, Edwards TC, Williams DA, Clemens JQ, Afari N, Tu F, Lloyd RB, Patrick DL, Mullins C, Kusek JW, Sutcliffe S, Hong BA, Lai HH, Krieger JN, Bradley CS, Kim J, Landis JR. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. The Journal of urology. 2016;195(4, Part 1):949–954. doi: 10.1016/j.juro.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis & Rheumatism. 2005;52(11):3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 15.Haufe S, Meinecke F, Görgen K, Dähne S, Haynes J-D, Blankertz B, Bießmann F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage. 2014;87:96–110. doi: 10.1016/j.neuroimage.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 16.Kabat-Zinn J, Lipworth L, Burncy R, Sellers W. Four-Year Follow-Up of a Meditation-Based Program for the Self-Regulation of Chronic Pain: Treatment Outcomes and Compliance. The Clinical Journal of Pain. 1986;2(3):159–774. [Google Scholar]
- 17.Kairys A, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus J, Martucci K, Farmer M, Ness T, Deutsch G, Mayer E, Mackey S, Apkarian A, Maravilla K, Clauw D, Harris R. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2015;193(1):131–137. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Brunelin J, Möller H-J, Reiser M, Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. The Journal of Neuroscience. 2011;31(43):15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey SC, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. The Journal of urology. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, Tillisch K, Mayer EA, Naliboff BD. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage. 2011;56(1):290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong J, Jensen K, Loiotile R, Cheetham A, Wey H-Y, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. PAIN®. 2013;154(3):459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP Research Network Neuroimaging Study. NeuroImage: Clinical. 2015;8(0):493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labus JS, Van Horn JD, Gupta A, Alaverdyan M, Torgerson C, Ashe-McNalley C, Irimia A, Hong J-Y, Naliboff B, Tillisch K. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain. 2015;156(8):1545–1554. doi: 10.1097/j.pain.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV. The MAPP research network: design, patient characterization and operations. BMC urology. 2014;14(1):58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11(7):1581–1585. [PubMed] [Google Scholar]
- 26.Lefaucheur J-P, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, De Carvalho M, De Ridder D. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network. Pain. 2015;156(9):1755–1764. doi: 10.1097/j.pain.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plitt M, Barnes KA, Martin A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. NeuroImage: Clinical. 2015;7:359–366. doi: 10.1016/j.nicl.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(48):E6699–E6706. doi: 10.1073/pnas.1510098112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Propert KJ, McNaughton-Collins M, Leiby BE, O’Leary MP, Kusek JW, Litwin MS, Network CPCR A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. The Journal of urology. 2006;175(2):619–623. doi: 10.1016/S0022-5347(05)00233-8. [DOI] [PubMed] [Google Scholar]
- 33.PROPERT KJ, SCHAEFFER AJ, BRENSINGER CM, KUSEK JW, NYBERG LM, LANDIS JR, Group ICDBS A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. The Journal of urology. 2000;163(5):1434–1439. doi: 10.1016/s0022-5347(05)67637-9. [DOI] [PubMed] [Google Scholar]
- 34.Ptak R. The frontoparietal attention network of the human brain action, saliency, and a priority map of the environment. The Neuroscientist. 2012;18(5):502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- 35.Pujol J, Macià D, Blanco-Hinojo L, Martínez-Vilavella G, Sunyer J, de la Torre R, Caixàs A, Martín-Santos R, Deus J, Harrison BJ. Does motion-related brain functional connectivity reflect both artifacts and genuine neural activity? NeuroImage. 2014;101:87–95. doi: 10.1016/j.neuroimage.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 36.Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephens-Shields AJ, Clemens JQ, Jemielita T, Farrar J, Sutcliffe S, Hou X, Landis JR. Symptom Variability and Early Symptom Regression in the MAPP Study, a Prospective Study of Urologic Chronic Pelvic Pain Syndrome. The Journal of urology. 2016 doi: 10.1016/j.juro.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szczepanski SM, Kastner S. Shifting attentional priorities: control of spatial attention through hemispheric competition. The Journal of Neuroscience. 2013;33(12):5411–5421. doi: 10.1523/JNEUROSCI.4089-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tupper SM, Rosenberg AM, Pahwa P, Stinson JN. Pain Intensity Variability and Its Relationship With Quality of Life in Youths With Juvenile Idiopathic Arthritis. Arthritis Care & Research. 2013;65(4):563–570. doi: 10.1002/acr.21850. [DOI] [PubMed] [Google Scholar]
- 43.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. The Journal of neuroscience. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 46.Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, Tillisch K, Kutch JJ, Farmer MA, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Landis JR, Deutsch G, Harris RE, Clauw DJ, Mullins C, Ellingson BM, Network MR Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS ONE. 2015;10(10):e0140250. doi: 10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zakoscielna KM, Parmelee PA. Pain Variability and Its Predictors in Older Adults: Depression, Cognition, Functional Status, Health, and Pain. Journal of Aging and Health. 2013 doi: 10.1177/0898264313504457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


