Abstract
p65 is a member of the NF-κB family of transcriptional regulatory proteins that functions as the activating component of the p65–p50 heterodimer. Through its acidic transactivation domain (TAD), p65 has the capacity to form interactions with several different transcriptional regulatory proteins, including TFIIB, TFIIH, CREB-binding protein (CBP)/p300 and TAFII31. Like other acidic TADs, the p65 TAD contains two subdomains (p65TA1 and p65TA2) that interact with different regulatory factors depending on the target gene. Despite its role in controlling numerous NF-κB target genes, there are no high-resolution structures of p65TA1 bound to a target transcriptional regulatory factor. In this work, we characterize the interaction of p65TA1 with two factors, the Tfb1/p62 subunit of TFIIH and the KIX domain of CBP. In these complexes, p65TA1 transitions into a helical conformation that includes its characteristic ΦXXΦΦ motif (Φ = hydrophobic amino acid). Structural and functional studies demonstrate that the two binding interfaces are primarily stabilized by three hydrophobic amino acids within the ΦXXΦΦ motif and these residues are also crucial to its ability to activate transcription. Taken together, the results provide an atomic level description of how p65TA1 is able to bind different transcriptional regulatory factors needed to activate NF-κB target genes.
INTRODUCTION
The NF-κB proteins are a family of transcription regulatory factors that control genes involved in a number of processes including the immune response, cell proliferation and cell survival (1). The five proteins that constitute the NF-κB family in mammals include p50, p52, p65 (also known as RelA), RelB and c-Rel. Members of the NF-κB family form different combinations of homo- and hetero-dimers with each other, and these various dimer combinations constitute the biologically active forms of the proteins, with the p65–p50 heterodimer being the most abundant cellular form. In unstressed cells, the various NF-κB dimers are predominantly sequestered in the cytoplasm, where they are maintained in a latent state through interactions with inhibitor of κB (IκB) proteins (2). In the canonical pathway, in response to a wide variety of stimuli such as stress or viral infections, the IκB protein is phosphorylated and this results in the rapid ubiquitination of the IκBα protein and its subsequent degradation via the ubiquitin-proteasome system. Following the degradation of the IκBα protein, the NF-κB dimer is released from its latent cytoplasmic state and translocated into the nucleus, where it activates transcription through its ability to specifically bind regulatory regions within target genes (3).
The p65 protein is the most thoroughly investigated of the five members of the NF-κB family, in part, because it is the activating component of the p65–p50 heterodimer. The p65 protein is essential for cell survival as p65 knockout cells are known to survive however p65 knockout mice do not, whereas this is not to be the case for other NF-κB family members, where functional redundancy has been observed in genetic studies (4). In response to different cellular signals, the p65 protein is transformed into one of its several active forms following a sequence of posttranslational modifications that includes both phosphorylation and acetylation events (5). For example, it has been shown that phosphorylation of p65 on Ser276 results in a conformational change and this leads to enhanced binding to the histone acetyltransferase (HAT) CREB-binding protein(CBP)/p300 (6). The interaction with CBP leads to the acetylation of p65 at several positions and this acetylation is directly linked to enhanced transcriptional activity of p65 (7). In addition, several studies have identified other phosphorylation sites that appear to regulate the activity of p65 either by enhancing its acetylation or modifying its ability to interact with other transcriptional regulatory factors (6,8–12). Based on these combined results, it appears that acetylation is required for maximal activity of p65, which is consistent with results showing that overexpression of CBP/p300 enhances the activation potential of p65 (7,13), whereas overexpression of histone deacetylases (HDAC) represses p65 activity (14).
In order to activate transcription of NF-κB target genes, p65 binds specifically to κB-binding sites present in either the enhancer or promoter regions of target genes. Like all members of the NF-κB family, the p65 protein contains a Rel homology domain (RHD) located near the amino-terminal region of the protein, which is responsible for both its ability to recognize specific DNA sequences on NF-κB regulated genes as well as to form heterodimers with other NF-κB-family members (2). Several crystal structures of the RHD of p65:p50 heterodimers bound to DNA sequences containing various NF-κB binding sites clearly demonstrate how the p65:p50 heterodimer is able to regulate a wide variety of target genes (15–17). In addition to the RHD, the p65 protein contains an acidic transactivation domain (TAD) located within the carboxyl-terminal 124 amino acids (residues 428–551 in the human p65 protein). The TAD is essential for the regulation of p65 target genes (18) and it functions by participating in a series of protein–protein interactions with a number of transcriptional regulatory proteins including HATs such as CBP/p300 (19), general transcription factors (TAFII31, TFIIB, TFIID and TFIIH) (20–22) and chromatin remodeling complexes (23).
The TAD of p65 can be subdivided into two subdomains that are both capable of independently activating transcription of p65 regulated genes, and they are referred to as TA2, which is located between residues 428 and 521, and TA1, which is located between residues 521 and 551. Initial structural studies indicated that the full-length TAD of p65 (residues 428–551) was unstructured in the free form. This conclusion was based on the fact that homonuclear nuclear magnetic resonance (NMR) spectroscopy experiments demonstrated that the TAD of p65 displayed minimal chemical shift dispersion and was devoid of interproton nuclear Overhauser effects (NOEs) characteristic of helical structure. However, it was determined that addition of trifluoroethanol (TFE) to a fragment containing just the TA1 subdomain of the TAD of p65 induced changes characteristic of a helical conformation when analyzed using circular dichroism (CD) spectroscopy (24). Based on the presence of a TFE-induced helical conformation, it was suggested that the TAD of p65 adopts a helical conformation when bound to its partner proteins. This model is consistent with the fact that both the TA1 and TA2 subdomains of p65 contain consensus ΦXXΦΦ motifs (where Φ is a hydrophobic amino acid and X is any amino acid). This motif is present in numerous acidic TADs, including those of p53 and VP16, that transition from a predominantly disordered state to a helical conformation when bound to interacting domains of partner proteins such as CBP, TAFII31, TFIIH and TFIIB (25–31). A recent NMR structure of the TA2 subdomain of p65 (p65TA2, residues 425–490 of mouse p65) in complex with the TAZ1 domain of CBP demonstrated the importance of two ΦXXΦΦ motifs within TA2 for binding (32). In complex with TAZ1, four regions within p65TA2 transition from an unstructured state into a helical conformation. Two of these helical regions encompass the ΦXXΦΦ motifs, and both motifs make important contributions to the binding interface of the complex.
Previous studies have shown that deletion of the TA1 subdomain of p65 (p65TA1) results in the loss of ∼85% of its transactivation capacity (18). However, full transactivation activity is observed when p65TA1 is tethered to a segment of p65 containing only the DNA-binding domain. Despite the importance of this domain in regulating the transcriptional activity of p65, there is currently no high-resolution structure of p65TA1 bound to a transcriptional regulatory protein. Therefore, we have examined the interaction of p65TA1 with two well-characterized targets of several other acidic TADs, the pleckstrin homology (PH) domain of the p62/Tfb1 (human/yeast) subunit of TFIIH (p62PH/Tfb1PH) and the KIX domain of CBP (CBPKIX). We show that p65TA1 binds to both Tfb1PH and CBPKIX and adopts a similar α-helical conformation upon binding. In addition, we demonstrate that three hydrophobic residues within the ΦXXΦΦ motif of p65TA1 play important roles in forming the binding interface with both Tfb1PH and CBPKIX and that these residues are important to the ability of p65TA1 to activate transcription. Taken together, the results provide detailed information on how p65TA1 binds to two transcriptional regulatory factors that are required to help control the vast array of genes targeted by the NF-κB family.
MATERIALS AND METHODS
Cloning and purification of recombinant proteins
Tfb1PH (residues 1–115 of Tfb1) and p62PH (residues 1–108 of p62) were constructed as GST-fusion proteins and purified as previously described (33). CBPKIX (residues 586–672) was cloned into a pET21a vector with a His-Tag and purified as previously described (34). The F612A/D622A/R624A/K667E quadruple CBPKIX mutant (CBPKIX-ΔMLL) and the Y650A/A654Q/Y658V triple CBPKIX mutant (CBPKIX-Δc-Myb) cloned into the pET21a vector encoding a His-Tag were generously provided by Dr Steven Smith (Queens University) and purified as previously described for the wild-type CBPKIX. The p65TA1 (residues 521–551) was cloned into the pET-His-GST-TEV vector generating a GST-fusion protein with an L523Y mutation to allow determination of peptide concentration at 280 nm. Mutations of p65TA1 were carried out using the QuickChange II site-directed mutagenesis kit. GST-His–p65TA1 and related mutants were grown in Escherichia coli host strain BL21(DE3) at 30°C and induced for 4 h in the presence of 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG). The cells were lysed in 20 mM TRIS at pH 7.4 containing 1 M NaCl and 0.5 mM ethylenediaminetetraacetic acid, and bound to glutathione (GSH) resin (GE Healthcare). The GSH resin was washed first with lysis buffer and then with Tobacco Etch Virus (TEV) protease cleavage buffer. The p65TA1 peptide was cleaved in batch from the resin overnight at room temperature in the presence of TEV protease. The resulting supernatant was then further purified on a Q-sepharose High-Performance column (GE Healthcare) equilibrated in 20 mM phosphate buffer at pH 6.5 and protein was eluted using a gradient of 20 mM NaPO4 buffer at pH 6.5 containing 1 M NaCl. Purified protein was further dialyzed in suitable buffer for isothermal titration calorimetry (ITC) or NMR studies. For NMR experiments, Tfb1PH, CBPKIX and p65TA1 were isotopically labeled by growth in minimal media containing 15N-ammonium chloride (Sigma) and/or 13C-glucose (Sigma) as the sole nitrogen and carbon sources.
Isothermal titration calorimetry studies
ITC titrations were performed at 25°C in 50 mM Tris–HCl buffer at pH 7.4 using a MicroCal VP-ITC system. Concentrations of injected p65TA1 wild-type and mutant peptides in the syringe and Tfb1PH/p62PH/CBPKIX proteins in the cell varied respectively from 200 to 500 μM and from 20 to 50 μM, as determined by absorbance at 280 nm. Data were analyzed using MicroCal Origin Software and all experiments fit the single binding site model with 1:1 stoichiometry. Errors in KD values were estimated from triplicate measurements or more.
NMR spectroscopy
NMR experiments were recorded at 25°C on Varian Unity Inova 500 and 600 MHz spectrometers. All NMR samples were prepared in 20 mM NaPO4 buffer at pH 6.5 in either 10% D2O/90%H2O (v/v) or 100% D2O. The Tfb1PH–p65TA1 complex was prepared using either 0.8 mM of 15N-13C Tfb1PH with two molar equivalents of unlabeled p65TA1 or 0.8 mM of 15N/13C-p65TA1 with two molar equivalents of unlabeled Tfb1PH. The backbone and side chain resonances were assigned using 3D HNCO, HNCACB, HBCBCACONNH (35) and HCCH-TOCSY (36) experiments recorded on both complexes. Intramolecular distance restraints were extracted from 3D 15N-edited NOESY-HSQC (37) and 13C-edited HMQC-NOESY spectra (38), and intermolecular distance restraints were extracted from 3D 15N-13C F1-filtered, F3-edited NOESY experiments (39). All NOESY spectra for the complexes were recorded using a mixing time of 90 ms. Samples were exchanged three times in 100% D2O before recording the 13C-edited and the filtered NOESY spectra. In addition, HNCACB, HNCO and HCCONH experiments were recorded with 0.8 mM 15N/13C-p65TA1 either in the absence or presence of two molar equivalents of CBPKIX to extract chemical shifts for the secondary structure propensities (SSP) analyses. 13C-edited HMQC-NOESY spectra in 100% D2O were recorded at 25°C with a mixing time of 120 ms for the p65TA1–CBPKIX complex, and at 10°C with a mixing time of 350 ms for the free peptide. Resonance assignment of the CBPKIX–p65TA1 complex was performed using 3D HNCO, HNCACB, HBCBCACONNH and HCCH-TOCSY experiments on a 0.8 mM sample of 15N/13C-CBPKIX with two molar equivalents of unlabeled p65TA1. Intermolecular distance restraints for the p65TA1–CBPKIX complex were extracted from two 3D 15N-13C F1-filtered, F3-edited NOESY experiment, the first with 15N/13C-p65TA1 in the presence of two molar equivalents of unlabeled CBPKIX and the second with 15N/13C-CBPKIX in the presence of two molar equivalents of unlabeled p65TA1 in 100% D2O. NMR data were processed with NMRPIPE (40) and analyzed with Analysis from the CCPNMR suite (41).
SSP analysis
SSP were assessed using SSP software (42) in p65TA1 free form, bound to Tfb1PH and to CBPKIX. Chemical shifts from 1HN, 1Hα, 15NH, 13Cα and 13Cβ nuclei were used as input data and compared with random-coil chemical shift values from RefDB database (43).
Structural restraints and structure calculation
Backbone dihedral angles were obtained using either TALOSN for Tfb1PH (44) and TALOS+ (45), which gives more accurate predictions for p65TA1. NOESY spectra were manually peaked and partially assigned. The NOE assignment was completed using ARIA2.3 (46), concomitantly with structure calculation. The ARIA program was run with eight iterations including twenty structures and a final iteration including 100 structures. The 20 best structures were subsequently refined in explicit water using CNS (47). Sidechain geometry was further optimized with the software YASARA (48) using the force field YASARA2 (49) and the SCWALL method (50). The Protein Structure Validation Suite was used to assess the overall quality of the structures (51). The figures of the structures were prepared using PyMol software (Warren Delano, http://www.pymol.org).
Yeast activation assays
DNA constructs encoding for p65TA1 and its mutants were ligated into the PSH18–34 vector to produce fusion proteins with the DNA-binding domain of LexA. Yeast strains were transformed with the LexA operator-lacZ fusion proteins following a previously described protocol (52) and ß-galactosidase assays were performed as previously described (53). Results of the assays are presented as the mean of the percentage of ß-galactosidase units obtained for each of the tested LexA-fusion proteins. The activity of the LexA-GAL4 positive control is arbitrarily set to 100% for comparison with the other fusion proteins. The reported values are ± standard error of the mean obtained from a minimum of six independent experiments. Western blot analyses were performed with an anti-LexA antibody to verify equivalent expression of the LexA-fusion proteins.
RESULTS
TA1 from the p65 subunit of NF-κB binds to Tfb1PH/p62PH and CBPKIX
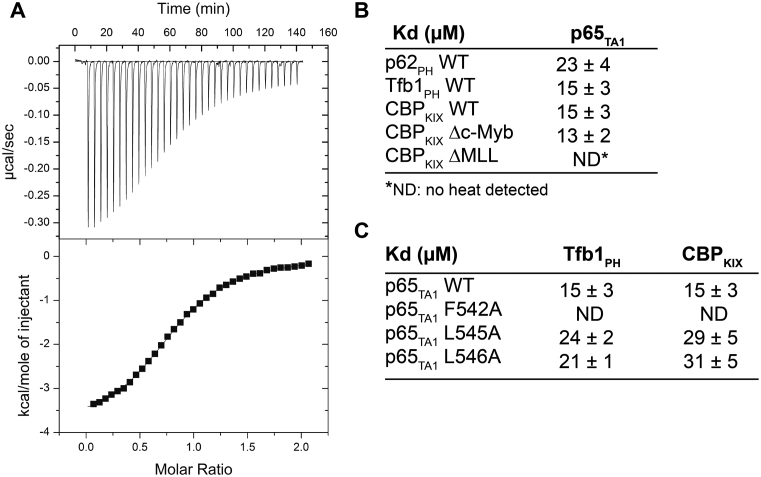
Based on amino acid sequence alignment (Supplementary Figure S1A and B), p65TA1 contains a highly conserved region between residues 542 and 546 homologous to the ΦXXΦΦ motif that forms the recognition interface of several acidic TADs bound to their target proteins (54). Given that several of these acidic TADs have been shown to target both the PH domain present at the N-terminal of p62/Tfb1 subunit of the general transcription factor TFIIH (p62PH/Tfb1PH) and the KIX domain present in the histone acetyl transferase CBP (CBPKIX), we attempted to examine the interaction of p65TA1 with these two common targets of acidic TADs. Initially, we examined p65TA1 binding to Tfb1PH and p62PH using ITC. The ITC experiments demonstrate that p65TA1 binds to either Tfb1PH or p62PH with similar dissociation constants (KD) of 15 ± 3 μM and 23 ± 4 μM, respectively, under the experimental conditions (Figure 1). Next, we examined whether or not p65TA1 also binds to CBPKIX using similar ITC experiments. Similar to Tfb1PH and p62PH, CBPKIX binds to p65TA1 with a KD of 15 ± 3 μM.
Figure 1.
Binding of p65TA1 to Tfb1PH/p62PH and CBPKIX characterized by ITC. (A) Representative ITC thermogram obtained by successive additions of p65TA1 to Tfb1PH. (B) Summary of dissociation constants (KD) values obtained from ITC experiments of p65TA1 with p62PH, Tfb1PH, CBPKIX and CBPKIX mutants. (C) Summary of KD values obtained from ITC experiments for Tfb1PH and CBPKIX with p65 TA1 mutants from the ΦXXΦΦ motif.
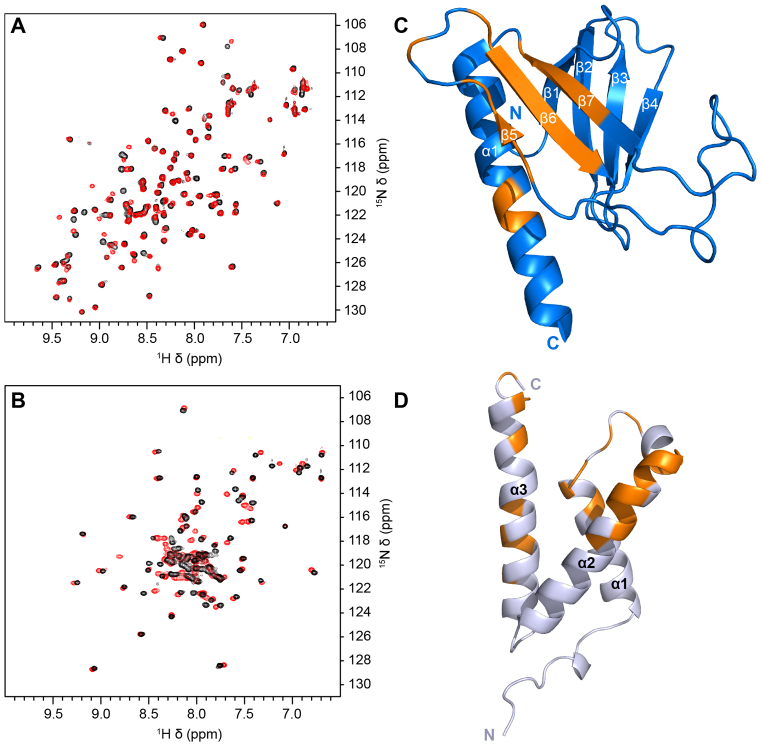
To further investigate these interactions, NMR chemical shift perturbation studies were performed to identify the binding site for p65TA1 on both Tfb1PH and CBPKIX. Addition of unlabeled p65TA1 to either 15N-labeled Tfb1PH or 15N-labeled CBPKIX results in significant changes in the 1H and 15N chemical shifts of the 2D 1H-15N HSQC spectrum and in both cases the binding of p65TA1 occurs in fast-intermediate exchange on the NMR time scale (Figure 2A and B). In the case of Tfb1PH, the signals that exhibited the most significant chemical shift changes in the HSQC spectrum following addition of p65TA1(Supplementary Figure S2A), correspond to residues within the β5, β6 and β7 strands of the PH domain fold (Figure 2C). When mapped on the structure of Tfb1PH, the binding site for p65TA1 overlaps with the binding site of several other acidic TADs including those from p53 (27) and VP16 (29). In the experiments with CBPKIX, the signals of CBPKIX that exhibit the most significant chemical shift changes in the HSQC spectrum following addition of p65TA1 (Supplementary Figure S2B), are associated with residues located in a groove formed by the C-terminal end of the α1 helix, the N-terminal end of the α2 helix and the C-terminal end of the α3 helix. When mapped on the structure of CBPKIX (Figure 2D), the binding site for p65TA1 appears to be similar to the binding site for the acidic TADs of the mixed lineage leukemia (MLL) protein (55) and the E-protein E2A (56).
Figure 2.
Nuclear magnetic resonance (NMR) spectra of p65TA1 binding to Tfb1PH and CBPKIX. (A and B) Overlay of the 2D 1H-15N-HSQC NMR spectra of 15N-Tfb1PH (A) and 15N-CBPKIX (B) either in the absence (black) or in the presence (red) of two equivalents of p65TA1. (C) Residues that undergo significant chemical shift perturbations (>0.05 ppm) in the 2D 1H-15N HSQC spectrum upon addition of p65TA1 to 15N-labeled Tfb1PH are mapped in orange onto the structure of free Tfb1PH (in blue, PDB ID: 1Y5O (33)). (D) Residues that undergo significant chemical shift perturbations (>0.1 ppm) in the 2D 1H-15N HSQC spectrum upon addition of p65TA1 to 15N-labeled CBPKIX are mapped in orange onto the structure of CBPKIX (in silver, PDB ID: 2AGH (55)).
NMR structure of the Tfb1PH–p65TA1 complex
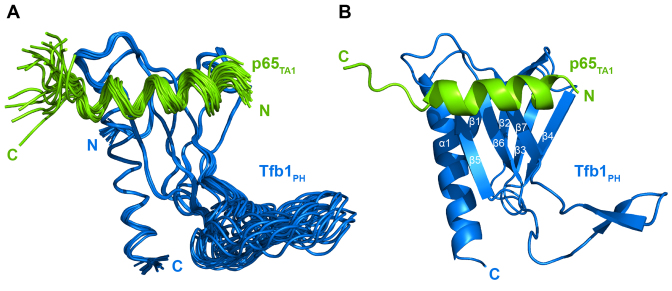
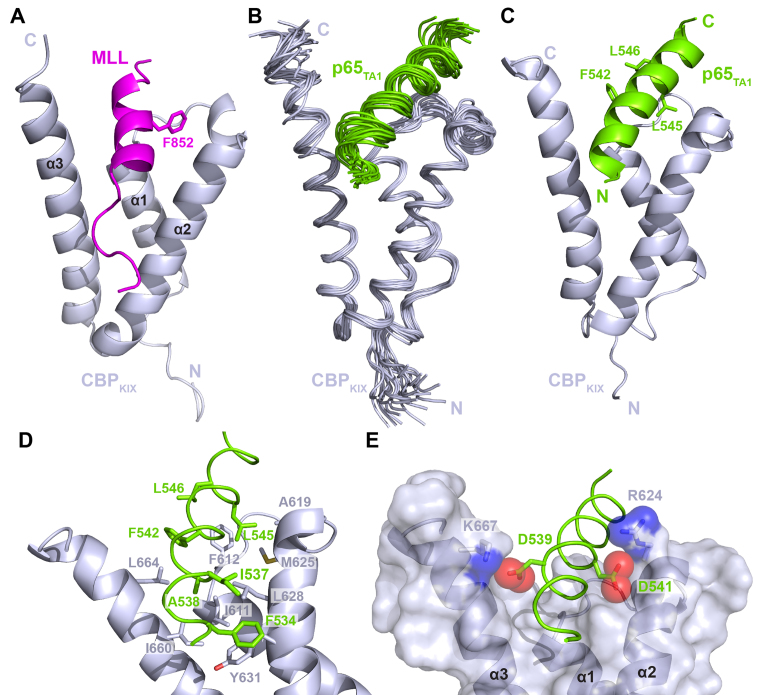
To determine its structure in the bound state, we examined p65TA1 in complex with Tfb1PH using NMR spectroscopy studies. The Tfb1PH was chosen for the NMR studies because it is considerable more stable than the p62PH protein over the long period of time required to collect the NMR data and it has served as a good model system for examining interactions with acidic TADs (27,29,33,34,57,58). The high-resolution NMR structure of the Tfb1PH–p65TA1 complex was calculated using 3447 NOE-derived distance restraints, 46 intermolecular NOEs and 211 dihedral angle restraints. The structure of the Tfb1PH–p65TA1 complex is well defined by the NMR data (Table 1). A total of 260 structures were calculated and the 20 lowest-energy structures are characterized by favorable backbone geometry, no significant violations of the experimental restraints and a pairwise root mean-square deviation (r.m.s.d.) of 0.3 Å for the backbone atoms and 0.8 Å for heavy atoms (Table 1). In complex with p65TA1, the structure of Tfb1PH (Figure 3) is similar to its structure in the unbound form (33), which consists of a PH-domain fold containing a seven-stranded β sandwich (β1–β7) followed by a single α-helix (α1). In complex with Tfb1PH, p65TA1 forms a 13-residue α-helix (Figure 3) between F534 and L546, and the helix forms the interface with Tfb1PH.
Table 1. NMR and refinement statistics for the Tfb1PH–p65TA1 complex (PDB ID: 5URN).
| NMR distance and dihedral constraints | Tfb1PH | p65TA1 |
|---|---|---|
| Number of distance constraints | ||
| Total intramolecular NOEs | 3066 | 381 |
| Intra-residue | 914 | 252 |
| Inter-residue | ||
| Sequential (|i-j| = 1) | 544 | 86 |
| Medium-range (2< = |i-j|< = 4) | 361 | 43 |
| Long-range (|i-j|>5) | 905 | 0 |
| Total intermolecular NOEs | 46 | |
| Total dihedral angle restraints | ||
| φ | 94 | 12 |
| ψ | 93 | 12 |
| Structure statistics | Tfb1PH–p65TA1 | |
| Global quality scores (raw/Z-score) | ||
| Verify 3D | 0.29/−2.73 | |
| ProsaII (-ve) | 0.43/−0.91 | |
| Procheck (φ/ψ only) | −0.53/−0.77 | |
| Procheck (all dihedral angles) | −0.27/−1.60 | |
| MolProbity clashscore | 20.77/−2.04 | |
| Violations (mean and s.d.) | ||
| Distance constraints (Å) | 0.041 ± 0.002 | |
| Dihedral angle constraints (°) | 1.56 ± 0.03 | |
| Deviations from idealized geometry | ||
| Bond lengths (Å) | 0.0080 ± 0.0001 | |
| Bond angles (°) | 0.78 ± 0.01 | |
| Atomic pairwise coordinate RMSD a (Å) | ||
| Heavy atoms | 0.8 ± 0.1 | |
| Backbone atoms | 0.3 ± 0.1 | |
| Ramachandran statistics a (%) | ||
| Residues in most favored regions | 92.7 | |
| Residues in additional allowed regions | 6.7 | |
| Residues in generously allowed regions | 0.6 | |
| Residues in disallowed regions | 0.0 | |
aPairwise r.m.s. deviation and Ramachandran statistics were calculated among 20 structures refined in water for residues 4–63 and 86–112 of Tfb1PH and residues 534–546 of p65TA1.
Figure 3.
NMR structure of the Tfb1PH–p65TA1 complex. (A) Overlay of the 20 NMR structures of the Tfb1PH–p65TA1 complex. The structures of Tfb1PH (blue) and p65TA1 (chartreuse) are displayed in the coil representation. For clarity, only residues 533–551 of p65TA1 are shown. (B) Ribbon representation of the lowest-energy structure of the Tfb1PH–p65TA1 complex with Tfb1PH and p65TA1 colored as in A. Secondary structure elements are indicated on Tfb1PH. The α-helix of p65TA1 includes residues F534 to L546.
Tfb1PH–p65TA1 binding interface
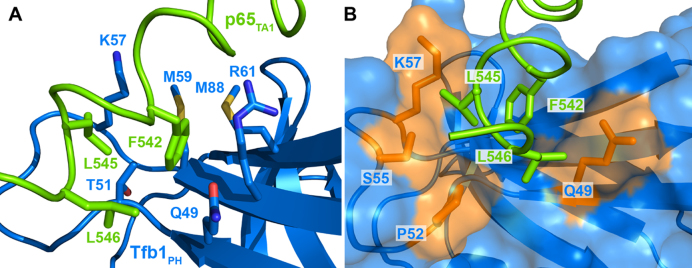
The key residues of p65TA1 at the interface with Tfb1PH are three hydrophobic residues (F542, L545 and L546) located within the ΦXXΦΦ motif. In the structure of the complex, the side chain of F542 from p65TA1 is fully buried in a pocket formed by Q49, A50, T51, K57, M59, R61 and M88 of Tfb1PH (Figure 4A). In this pocket, one side of the aromatic ring of F542 is in position to form an amine-π interaction with the side chain of Q49. In addition, the other side of the aromatic ring of F542 has the potential to form either a cation-π interactions with K57 of Tfb1PH or a sulfur–π interaction with M59 of Tfb1PH. This is based on the fact that the positive charge of K57 occurs at a distance between 5 and 9 Å from the center of the aromatic ring of F542 and the sulfur atom of M59 occurs at a distance between 5 and 7 Å from the center of the aromatic ring of F542 in the 20 NMR structures of the p65TA1–Tfb1PH complex. Although F542 of p65 seems to be the most crucial residue at the interface with Tfb1PH, L545 and L546 from the ΦXXΦΦ motif in p65TA1 also appear to contribute to the overall binding energy. These two leucine residues are located at the extreme C-terminal end of the p65TA1 helix, where L545 is inserted into a pocket formed by P52, S55 and K57, whereas L546 appears to form van der Waals contacts with the side chain of Q49 from Tfb1PH (Figure 4B). In addition to the interactions involving hydrophobic residues from the ΦXXΦΦ motif in p65TA1, the structures suggest two possible electrostatic interactions between negatively charged residues of p65TA1 and positively charged residues of Tfb1PH (Supplementary Figure S3). The first one occurs between D539 from p65TA1 and either R61 or R86 of Tfb1PH, and a second one occurs between D541 of p65TA1 and K57 of Tfb1PH.
Figure 4.
Close up of key residues forming the binding interface in the Tfb1PH–p65TA1 complex. (A) The structures of p65TA1 (in chartreuse) and Tfb1PH (in marine) are shown in ribbon representations with the side chains of the key residues at the interface in stick representations. F542 of p65TA1 is in position to form an amine–π interaction with Q49 as well as either a cation–π with K57 or a sulfur–π interaction with M59 of Tfb1PH. In addition, the methyl group of L545 from p65TA1 is in close proximity to the methyl group of T51 of Tfb1PH. (B) In this second orientation where Tfb1PH is shown as a surface representation, the methyl groups of L545 and L546 of p65TA1 are buried in hydrophobic pockets on the surface of Tfb1PH. The side chains of the key residues forming the interface from Tfb1PH in close proximity to L545 and L546 are colored in orange and include Q49, P52, S55 and K57.
p65TA1 α-helical propensity depends on substrate presence
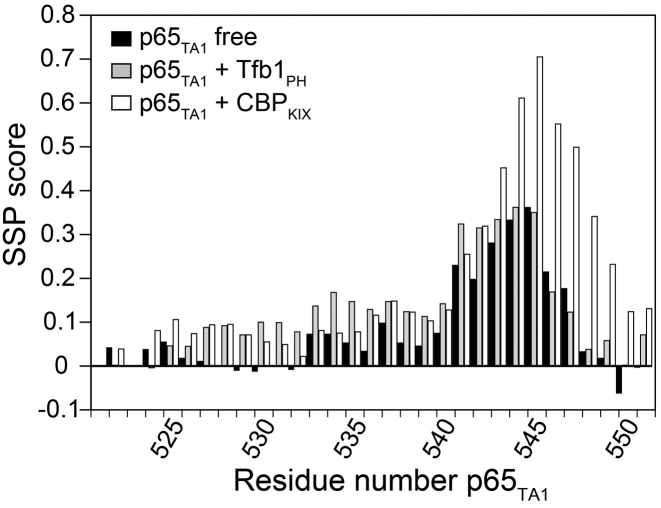
Our structure of the Tfb1PH–p65TA1 complex reveals that p65TA1 forms a 13-residue α-helix between residues 534 and 546 in complex with Tfb1PH and this suggests that a significant conformational change occurs between the unbound and the bound states based on earlier NMR studies with the free p65TA1. Given the recent advances in the identification of protein secondary structure elements based on NMR chemical shifts values (59), we decided to reinvestigate the α-helical propensity of p65TA1 in the free form as well as to determine if p65TA1 forms a similar α-helix in complex with CBPKIX. For these studies, we performed NMR experiments to obtain the complete 1H, 15N and 13C chemical shift assignment for 15N/13C-labeled p65TA1 in the free state and bound to CBPKIX and compared them with the values obtained for the complex with Tfb1PH (Supplementary Figure S4). The SSP of p65TA1 in the free state, bound to Tfb1PH as well as bound to CBPKIX were determined using the SSP software (42). For the SSP analysis, 1HN, 1Hα, 15NH, 13Cα and 13Cβ chemical shift values of p65TA1 were used as the input data. The SSP predictions indicate that p65TA1 displays helical propensity for the residues within the ΦXXΦΦ motif in the free form, and that this helical propensity is accentuated when it is bound to either Tfb1PH or CBPKIX (Figure 5).
Figure 5.
Helical propensity of p65TA1 in the free form as well as in complex with Tfb1PH and CBP KIX. The secondary structure propensities (SSP) were determined based on the 15N, 1HN, 13Cα, 13Cβ and 1Hα chemical shifts of p65TA1. The values were derived using chemical shifts determined from NMR experiments with p65TA1 in the unbound state (black) as well as in complex with either Tfb1PH (gray) or CBPKIX (white). Positive values represent a propensity to form α-helical secondary structure and negative values represent β-strand structure propensity.
To further investigate the helical propensity of p65TA1 in the unbound state, a series of 3D 13C-NOESY-HMQC experiments were conducted with 15N/13C-labeled p65TA1. The initial experiments were recorded at 25°C and no NOE signals were observed using mixing times of either 90 and 180 ms. In subsequent experiments, the temperature was lowered to 10°C to decrease the molecular tumbling rate of p65TA1 and the mixing time was increased to 350 ms. At 10°C, the chemical shift values of the free p65TA1 did not change significantly after taking into account the global chemical shift displacement due to the temperature change, and this suggests that the conformation of p65TA1 did not change dramatically as a result of the change in temperature. At this lower temperature, NOE signals indicative of a helical conformation are present between the Hα and the Hβi+3 for the Hα signals F542, S543 and A544 of p65TA1. The presence of these medium range NOE signals correlate with the SSP results and suggests there is a short transient helical conformation in the free form that involves residues within the ΦXXΦΦ motif of p65TA1.
Localization of the p65TA1-binding site on CBPKIX
CBPKIX contains two distinct binding sites that are recognized by acidic TADs and these two sites are referred to as the ‘MLL-binding site’ and the ‘c-Myb-binding site’ (60–62). The MLL-binding site is located at the interface where helices α1, α2 and α3 of CBPKIX converge, whereas the c-Myb-binding site is located on the opposite face in a hydrophobic groove created by the α3 helix. To verify that the MLL-binding site is in fact the preferred binding site as suggested by the NMR chemical shift perturbation studies, two previously described mutants of CBPKIX [CBPKIX-ΔMLL (F612A/D622A/R624A/K667E quadruple mutant) and CBPKIX-Δc-Myb (Y650A/A654Q/Y658V triple mutant)] were prepared and their binding to p65TA1 determined by ITC. The ITC experiments demonstrate that p65TA1 binds to CBPKIX-Δc-Myb mutant with a KD of 13 ± 2 μM, which is virtually identical to the binding observed with wild-type CBPKIX (Figure 1A and B). In contrast, no significant heat change is observed for p65TA1 binding to the CBPKIX-ΔMLL mutant and this indicates that the affinity for p65TA1 has decreased by at least two orders of magnitude. Thus, the ITC results with the CBPKIX binding site mutants are in agreement with the NMR chemical shift perturbation studies (Figure 2D) and support the fact that p65TA1 preferentially binds to the MLL-binding site of CBPKIX.
NMR structure of the CBPKIX–p65TA1 complex
The interaction of p65TA1 with CBPKIX was further investigated by high resolution NMR spectroscopy to determine a structure of the CBPKIX–p65TA1 complex. A total of 37 intermolecular NOE restraints were incorporated into the calculations to define the orientation of p65TA1 toward CBPKIX. The residues of CBPKIX involved in the intermolecular NOEs are all located either in the cleft formed at the junction of the three helices (I611, F612, A619) or on the surface of the α2 and α3 helices (R624, M625, N627, L628, Y631 on α2; I660 and L664 on α3). The structure of the CBPKIX–p65TA1 complex displays good structural statistics (Table 2) and it resembles the structures of several other acidic TADs bound to CBPKIX including MLL (55) and E2A (56) (Figure 6A–C). Consistent with the SSP results, p65TA1 forms a 17-residue helix between F534 and S550 when bound to CBPKIX. The predominant determinants for forming the p65TA1–CBPKIX interface come from contacts involving hydrophobic residues along almost the entire length of the p65TA1 helix (F534, I537, A538, F542, L545 and L546), which is anchored onto CBPKIX (Figure 6D). The first contacts occur between residues in the N-terminal part of the p65TA1 helix and the α2 and α3 helices of CBPKIX. In particular, the phenyl ring of F534 from p65TA1 is in position to form a π-π stacking interaction with the phenol ring of Y631 from CBPKIX, whereas the methyl group of A538 is positioned near the methyl groups of I611, L628 and I660. In addition, there are several interactions involving the three hydrophobic residues from the ΦXXΦΦ motif (F542, L545 and L546). These three residues of p65TA1 are buried in a deep hydrophobic cleft on the surface of CBPKIX formed by the three helices. In the structure, they are positioned to be in close contact with F612, A619, M625, L664 and R668 of CBPKIX. Although interactions involving the hydrophobic residues in the p65TA1 helix appear to be the driving force for binding to CBPKIX, the structure suggests that electrostatic interactions involving negatively charged amino acids of p65 and positively charged residues of CBP are also important for forming the binding interface (Figure 6E). More specifically, D539 and D541 of p65TA1 are in position to form an electrostatic interaction with the ammonium group of K667 and the guanidinium group of R624 of CBPKIX, respectively. Thus, the structure demonstrates that the binding of p65TA1 to CBPKIX involves a combination of hydrophobic and electrostatic interactions and this is consistent with what has been observed for other acidic TADs binding to CBPKIX.
Table 2. NMR and refinement statistics for the CBPKIX–p65TA1 complex (PDB ID: 5U4K).
| NMR distance and dihedral constraints | CBPKIX | p65TA1 |
|---|---|---|
| Number of distance constraints | ||
| Total intramolecular NOEs | 1006 | 333 |
| Intra-residue | 631 | 226 |
| Inter-residue | ||
| Sequential (|i-j| = 1) | 141 | 75 |
| Medium-range (2< = |i-j|< = 4) | 133 | 32 |
| Long-range (|i-j|>5) | 101 | 0 |
| Total intermolecular NOEs | 37 | |
| Total dihedral angle restraints | ||
| φ | 82 | 18 |
| ψ | 82 | 19 |
| Structure statistics | CBPKIX–p65TA1 | |
| Global quality scores (raw/Z-score) | ||
| Verify 3D | 0.30/−2.57 | |
| ProsaII (-ve) | 0.69/0.17 | |
| Procheck (φ/ψ only) | −0.10/−0.08 | |
| Procheck (all dihedral angles) | 0.01/0.06 | |
| MolProbity Clashscore | 11.25/−0.40 | |
| Violations (mean and s.d.) | ||
| Distance constraints (Å) | 0.060 ± 0.006 | |
| Dihedral angle constraints (°) | 0.49 ± 0.08 | |
| Deviations from idealized geometry | ||
| Bond lengths (Å) | 0.0078 ± 0.0001 | |
| Bond angles (°) | 0.73 ± 0.02 | |
| Atomic pairwise coordinate RMSD a (Å) | ||
| Heavy atoms | 1.1 ± 0.1 | |
| Backbone atoms | 0.6 ± 0.1 | |
| Ramachandran statistics a (%) | ||
| Residues in most favored regions | 99.3 | |
| Residues in additional allowed regions | 0.7 | |
| Residues in generously allowed regions | 0.0 | |
| Residues in disallowed regions | 0.0 | |
aPairwise r.m.s. deviation and Ramachandran statistics were calculated among 20 structures refined in water for residues 587–672 of CBPKIX and residues 534–550 of p65TA1.
Figure 6.
p65TA1 interacts with CBPKIX in the same binding-site as MLL. (A) Cartoon representation of CBPKIX (gray) in complex with the MLL peptide [PDB ID : 2LXS (61)]. The key residue F852 of MLL is shown as sticks. (B) Overlay of the 20 NMR structures of the CBPKIX–p65TA1 complex. The structures of CBPKIX (gray) and p65TA1 (chartreuse) are displayed in the coil representation. For clarity and for comparison with the Tfb1PH–p65TA1 complex, residues 533 to 551 of p65TA1 are shown. (C) Ribbon representation of the lowest-energy structure of the CBPKIX–p65TA1 complex. The three hydrophobic residues from the ΦXXΦΦ motif of p65TA1 (F542, L545 and L546) are represented in stick form. (D) Close-up view of key interactions at the interface of the CBPKIX–p65TA1 complex. Several residues in both domains contribute to anchor p65TA1 in the cleft created at the junction of the three helices of CBPKIX. (E) Close-up view on electrostatic interaction between D539 of p65TA1 and K667 of CBPKIX, and between D541 of p65TA1 and R624 of CBPKIX.
F542 of the ΦXXΦΦ motif in p65TA1 is crucial for binding to Tfb1PH and CBPKIX
To quantitatively investigate the contribution of the hydrophobic residues within the ΦXXΦΦ motif of p65TA1 for binding to CBPKIX and Tfb1PH, we prepared mutants of p65TA1 where the three hydrophobic residues were substituted with alanine (F542A, L545A and L546A) for evaluation by ITC (Figure 1C). Under the ITC conditions, no significant heat change is observed for the binding of the F542A mutant of p65TA1 with either Tfb1PH or CBPKIX and this indicates that the affinity of the p65TA1 F542A mutant has decreased by at least two orders of magnitude in both cases. The results with the F542A mutant are in agreement with our NMR structural data demonstrating that F542 of p65TA1 is a key component of the interface in both the p65TA1–Tfb1PH and the p65TA1–CBPKIX complexes. In the case of the L545A and L546A mutants of p65TA1, the effect of the alanine substitution on p65TA1 binding to either Tfb1PH or CBPKIX is less dramatic, with the two mutants displaying approximately a 1.5-fold decrease in affinity for both Tfb1PH and CBPKIX (Figure 1C). Consistent with the NMR structures, the ITC studies indicate that F542 within the ΦXXΦΦ motif is a key residue required for p65TA1 binding to both Tfb1PH and CBPKIX.
p65TA1 interaction with Tfb1PH and CBPKIX correlates with transcriptional activity
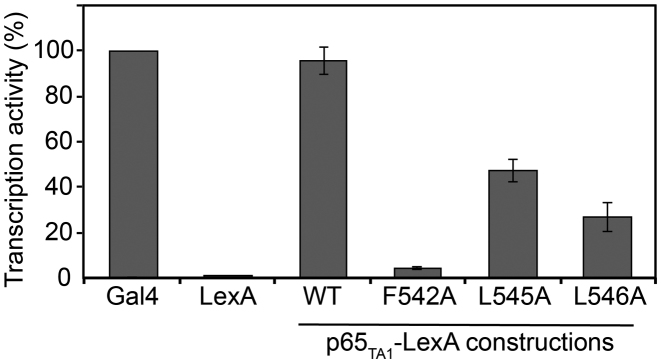
Previous studies have shown that the full activation of several NF-κB-regulated genes depends on the presence of p65TA1 and that F542 within the ΦXXΦΦ motif is essential for this activity (20). To test the relative importance of the hydrophobic residues in the ΦXXΦΦ motif on the transcriptional activation associated with p65TA1, we examined the relative activities of the F542A, L545A and L546A mutants of p65TA1 in a yeast model system. The yeast system has been previously used to characterize the activity of both p65TA1 and p65TA2 and it provides a good correlation to what has been observed in human cells (20,24). For these studies, p65TA1 and the mutants were fused to the DNA-binding domain of LexA (LexADBD) in order to measure their relative ability to activate transcription from a lacZ reporter gene. For the comparison, a LexADBD–GAL4 fusion protein served as a positive control (100% activity), whereas the LexADBD protein served as a negative control (no activity). In yeast, LexADBD–p65TA1 activates transcription at a level similar (96 ± 6%) to LexADBD–GAL4 (Figure 7), whereas the LexADBD–p65TA1 F542A mutant is almost completely devoid of activity (4.3 ± 3%). In addition, both the LexADBD–p65TA1 L545A and LexADBD–p65TA1 L546A mutants also display a reduced ability to activate transcription (47 ± 5 and 27 ± 6%, respectively), but the decrease is less dramatic than that observed with the F542A mutant. These findings are consistent with both the NMR and ITC studies, which demonstrate the important role of the three hydrophobic residues within the ΦXXΦΦ motif of p65TA1 for transcriptional activation.
Figure 7.
Identification of key residues required for p65TA1 transactivation. LexA-fusion proteins of p65TA1 and mutants were co-expressed in yeast with the reporter for the LexA operator-Lac-Z fusion plasmid pSH18–34. The percentage of activity for each fusion proteins is shown relatively to the positive control LexA-GAL4 (474–881), which was set to a maximum of activity (100%). Data represent mean and standard deviation obtained over at least six independent experiments.
DISCUSSION
Like several other acidic TADs, the TAD of p65 contains two distinct subdomains and both subdomains have been shown to be capable of independently activating transcription of select NF-κB target genes. Given the vast array of genes regulated by the p65–p50 heterodimer, it appears that the two subdomains interact with different transcriptional regulatory targets depending on the target gene being activated. In this study, we have characterized the interaction of the TA1 subdomain from the TAD of p65 (p65TA1) with two well-characterized targets of acidic TADs, the PH domain from the Tfb1/p62 (Tfb1PH/p62PH) subunit of TFIIH and the KIX domain of CBP (CBPKIX). ITC experiments demonstrate that p65TA1 binds to both p62PH/Tfb1PH and CBPKIX, and NMR structural studies show that binding to both of these transcriptional regulatory proteins stabilizes an α-helical structure in p65TA1 that is centered around the ΦXXΦΦ motif located between Phe542 and Leu546. The structure of the p65TA1–Tfb1PH complex clearly demonstrates that the three hydrophobic residues within the ΦXXΦΦ motif of p65TA1 (Phe542, Leu545 and Leu546) all play important roles at the binding interface with Tfb1PH. Consistent with what is observed in the Tfb1PH–p65TA1 complex, these same three residues also make key interactions at the interface with CBPKIX in the NMR structure of the p65TA1–CBPKIX complex. The binding interface is also complemented by electrostatic interactions involving negatively charged amino acids from the helical region of p65TA1 (D539 and D541) and positive charged amino acids from either Tfb1PH or CBPKIX. In addition, we demonstrate that the three hydrophobic residues from the ΦXXΦΦ motif are important for transcriptional activation in a yeast model system, which suggests that these residues play a key role in regulating a number of genes activated by p65 through interactions with transcription regulatory factors such as TFIIH and CBP.
The presence of two independent subdomains within the acidic TAD of p65 is similar to what has been observed in a number of other mammalian transcriptional regulatory factors including p53 (63), VP16 (64), FOX03a (65) and EKLF (66). Two key elements link these acidic TADs from different transcriptional regulators to their function: an abundance of negatively charged amino acids throughout the entire length of the sequence and the presence of a ΦXXΦΦ motif. Typically, acidic TADs are intrinsically disordered in their unbound states, but adopt a more ordered structure in the bound state. In most complexes examined to date, the bound structure consists of a mainly alpha helical conformation, although examples of extended structures have also been reported (31,32,56,60–62,67–72). Like other acidic TADs, initial NMR studies indicated that the full-length TAD of p65 was intrinsically disordered in the unbound form, but it was predicted to adopt a helical conformation when bound to target proteins with hydrophobic residues from the ΦXXΦΦ motifs being key contributors to the binding interface (24). The NMR structure of the p65TA2 subdomain bound to the TAZ1 domain of CBP clearly supports these earlier predictions (19). In complex with CBPTAZ1, p65TA2 adopts four distinct helical regions and two of these regions contain ΦXXΦΦ motifs that provide important contacts at the binding interface. Our current studies with the p65TA1–Tfb1PH and p65TA1–CBPKIX complexes are also in agreement with these earlier predictions and again highlight the importance of the ΦXXΦΦ motif in formation of the binding interface with p65TA1. However, the current NMR studies with p65TA1 indicate that the ΦXXΦΦ motif transiently adopts a short helical conformation even in the unbound state, and that a longer helical conformation is stabilized upon binding to target factors such as CBPKIX and Tfb1PH. Thus, the two complexes containing p65TA1 highlight how this domain alters its conformation so that it is able to bind to different targets in order to specifically activate transcription of p65–p50 target genes.
Several other structures of CBPKIX or p62PH/Tfb1PH have been determined previously in complex with acidic TADs from different transcriptional regulatory factors. In each complex containing CBPKIX, the acidic TADs have been shown to transition from a predominantly disordered conformation in the unbound state to a helical conformation in the bound state (61,62,69). These previous structures also indicated the presence of two distinct binding sites on the surface of CBPKIX, the so called MLL and c-Myb sites, and that TADs can bind independently to one site or in a cooperative manner to both sites (60,73). For example, the two subdomains from the TAD of FOXO3a can bind to CBPKIX either as individual subdomains to one of the sites or in a cooperative manner to both sites as the full-length TAD (68). In contrast, there is a single binding site for acidic TAD on the surface of Tfb1PH/p62PH that is formed by the β5, β6 and β7 strands of the PH domain. However, TAD binding to Tfb1PH/p62PH has been shown to occur in either a helical conformation or an extended conformation depending on the TAD (27,29,31,57,58). Our structural studies demonstrate that p65TA1 binds to both CBPKIX and Tfb1PH in a similar manner using a similar helical conformation. The main difference between p65TA1 in the two complexes appears to be at the C-terminus of the helix, where the last residue of the helix is L546 in the Tfb1PH complex compared with S550 in the CBPKIX complex. Our NMR studies together with mutational studies indicate that only one molecule of p65TA1 binds to CBPKIX at the MLL-binding site. The p65TA1 helix covers 763 Å2 on the surface of CBPKIX, but only 479 Å2 on the surface of Tfb1PH. This difference in size of the binding interfaces is consistent with the fact that p65TA1 forms a longer helix when bound to CBPKIX than when bound to Tfb1PH (17 versus 13 residues). In both complexes, the interface is primarily formed through interactions involving the three hydrophobic amino acids in the ΦXXΦΦ motif present in the α-helix. Mutation of any of the three key residues from the motif, in particular F542, leads to a decrease in binding affinity toward Tfb1PH and CBPKIX. Taken together with the fact that residues from the ΦXXΦΦ motif form a transient helix in the unbound state, this suggests that this region of p65TA1 predisposes it for binding with its target proteins.
The NF-κB-family of proteins plays an important role in controlling the transcription of over 500 human genes, and many of these genes are regulated by the p65–p50 heterodimer. In order for p65–p50 to activate these genes, the TAD of p65 forms protein–protein interactions with several different transcriptional regulatory proteins, including CBP and TFIIH (22,74). One of the keys to activation associated with p65 is the recruitment of CBP, which leads to the subsequent acetylation of select lysine residues on p65 depending on the target gene being activated (5,7). The NMR structure of the p65TA2–CBPTAZ1 complex is the only other structural information available for the TAD of p65 in complex with a target transcriptional factor (19). Our results demonstrating that p65TA1 binds to CBPKIX support that hypothesis that the individual subdomains within the TAD of p65 (p65TA1 and p65TA2) have the capacity to contact different domains of CBP at the same time, possibly in a cooperative manner. A similar mechanism has been proposed for the interaction of CBP with p53 and FOXO3a (30,68). The TAD of p53 and FOXO3a also both contain two subdomains and each subdomain has a ΦXXΦΦ motif that is important for its binding to CBP. In the case of p53, it has also been shown that its p53TAD1 and p53TAD2 have the capacity to bind to two domains of CBP when they are artificially fused together (70). The interaction between TFIIH and p65 plays a fundamental role in several processes including the regulation of transcription from the HIV promoter in the viral long terminal repeat (75,76) as well as the expression of nitric oxide synthase from the Nos2 gene in macrophages following infections by pathogenic organisms (77). The Tfb1/p62 subunit is targeted by a number of proteins containing an acidic TAD including p65, and our structural studies provide evidence of how the ΦXXΦΦ motif of p65TA1 regulates its interaction with Tfb1PH. The importance of F542 within the ΦXXΦΦ motif of p65TA1 for the formation of both complexes is consistent with the fact that it has been previously identified as essential for p65 to activate transcription (20). The structures of the two complexes explicitly illustrate the importance of this residue, as it is clear that F542 of p65TA1 is positioned in a binding cleft in both complexes where it makes crucial contacts with the transcriptional regulator. Taken together with previous structural studies involving p65TA2 (19), these studies with p65TA1 provide important insights into how the two subdomains within the TAD of p65 incorporate the ΦXXΦΦ motif in order to separately bind to the different transcriptional regulatory factors needed to help control the vast array of genes targeted by the p65–p50 heterodimer.
ACCESSION NUMBERS
1H, 13C and 15N chemical shifts of Tfb1PH–p65TA1 complex have been deposited in the BioMagResBank (BMRB) under accession number 30243 and associated 3D model structure coordinates have been deposited in the protein data bank (PDB) under the code 5URN. 1H, 13C and 15N chemical shifts of CBPKIX–p65TA1 complex have been deposited in the BMRB under accession number 26867 and associated 3D model structure coordinates have been deposited in the PDB under the code 5U4K.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Alanna Schepartz and Dr. Steven Smith for providing the constructs for expression of the KIX domain of CBP.
Footnotes
Present address: Lauriane Lecoq, Molecular Microbiology and Structural Biology, UMR 5086 CNRS, Lyon 69007, France.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Canadian Institutes for Health Research [MOP-74739 to J.G.O.]; Natural Sciences and Engineering Research Council of Canada CREATE program Postdoctoral Fellowship (to L.L.); Natural Sciences and Engineering Research Council of Canada; Canada Foundation for Innovation; Québec ministère de la recherche en science et technologie; McGill University. Funding for open access charge: Canadian Institutes for Health Research.
Conflict of interest statement. None declared.
REFERENCES
- 1. Hayden M.S., West A.P., Ghosh S.. NF-κB and the immune response. Oncogene. 2006; 25:6758–6780. [DOI] [PubMed] [Google Scholar]
- 2. Hayden M.S., Ghosh S.. Shared principles in NF-κB signaling. Cell. 2008; 132:344–362. [DOI] [PubMed] [Google Scholar]
- 3. Perkins N.D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007; 8:49–62. [DOI] [PubMed] [Google Scholar]
- 4. Gerondakis S., Grumont R., Gugasyan R., Wong L., Isomura I., Ho W., Banerjee A.. Unravelling the complexities of the NF-κB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006; 25:6781–6799. [DOI] [PubMed] [Google Scholar]
- 5. Huang B., Yang X.-D., Lamb A., Chen L.-F.. Posttranslational modifications of NF-κB: Another layer of regulation for NF-κB signaling pathway. Cell. Signal. 2010; 22:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong H., Voll R.E., Ghosh S.. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell. 1998; 1:661–671. [DOI] [PubMed] [Google Scholar]
- 7. Gerritsen M.E., Williams A.J., Neish A.S., Moore S., Shi Y., Collins T.. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. U.S.A. 1997; 94:2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D., Westerheide S.D., Hanson J.L., Baldwin A.S.. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 2000; 275:32592–32597. [DOI] [PubMed] [Google Scholar]
- 9. Vermeulen L., De Wilde G., Notebaert S., Vanden Berghe W., Haegeman G.. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem. Pharmacol. 2002; 64:963–970. [DOI] [PubMed] [Google Scholar]
- 10. Duran A., Diaz-Meco M.T., Moscat J.. Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J. 2003; 22:3910–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L.F., Williams S.A., Mu Y., Nakano H., Duerr J.M., Buckbinder L., Greene W.C.. NF-kB RelA phosphorylation regulates RelA acetylation. Mol. Cell. Biol. 2005; 25:7966–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buss H., Handschick K., Jurrmann N., Pekkonen P., Beuerlein K., Müller H., Wait R., Saklatvala J., Ojala P.M., Schmitz M.L. et al. . Cyclin-dependent kinase 6 phosphorylates NF-κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS One. 2012; 7:e51847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang Y.-J., Lee E.-W., Song J., Kim H.-R., Jun Y.-C., Hwang K.-A.. MafK positively regulates NF-κB activity by enhancing CBP-mediated p65 acetylation. Sci. Rep. 2013; 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashburner B.P., Westerheide S.D., Baldwin A.S.. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 2001; 21:7065–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen F.E., Huang D.B., Chen Y.Q., Ghosh G.. Crystal structure of the NF-kB p50/p65 heterodimer complexed to DNA. Nature. 1998; 391:410–413. [DOI] [PubMed] [Google Scholar]
- 16. Berkowitz B., Huang D.B., Chen-Park F.E., Sigler P.B., Ghosh G.. The X-ray crystal structure of the NF- B p50{middle dot}p65 heterodimer bound to the interferon—B site. J. Biol. Chem. 2002; 277:24694–24700. [DOI] [PubMed] [Google Scholar]
- 17. Escalante C.R., Shen L., Thanos D., Aggarwal A.K.. Structure of NF-κB p50/p65 Heterodimer Bound to the PRDII DNA Element from the Interferon-β Promoter. Structure. 2002; 10:383–391. [DOI] [PubMed] [Google Scholar]
- 18. Schmitz M.L., Baeuerle P.A.. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991; 10:3805–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukherjee S.P., Behar M., Birnbaum H.A., Hoffmann A., Wright P.E., Ghosh G.. Analysis of the RelA:CBP/p300 interaction reveals its involvement in NF-κB-driven transcription. PLoS Biol. 2013; 11:e1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blair W.S., Bogerd H.P., Madore S.J., Cullen B.R.. Mutational analysis of the transcription activation domain of RelA: identification of a highly synergistic minimal acidic activation module. Mol. Cell. Biol. 1994; 14:7226–7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmitz M.L., Stelzer G., Altmann H., Meisterernst M., Baeuerle P.A.. Interaction of the COOH-terminal transactivation domain of p65 NF-kappa B with TATA-binding protein, transcription factor IIB, and coactivators. J. Biol. Chem. 1995; 270:7219–7226. [DOI] [PubMed] [Google Scholar]
- 22. O'shea J.M., Perkins N.D.. Regulation of the RelA (p65) transactivation domain. Biochm. Soc. Trans. 2008; 36:603–608. [DOI] [PubMed] [Google Scholar]
- 23. Burkhart B.A., Hebbar P.B., Trotter K.W., Archer T.K.. Chromatin-dependent E1A activity modulates NF- B RelA-mediated repression of glucocorticoid receptor-dependent transcription. J. Biol. Chem. 2005; 280:6349–6358. [DOI] [PubMed] [Google Scholar]
- 24. Schmitz M.L., dos Santos Silva M.A., Altmann H., Czisch M., Holak T.A., Baeuerle P.A.. Structural and functional analysis of the NF-kappa B p65 C terminus. J. Biol. Chem. 1994; 269:25613–25620. [PubMed] [Google Scholar]
- 25. Lu H., Levine A.J.. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc. Natl. Acad. Sci. U.S.A. 1995; 92:5154–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buschmann T., Lin Y., Aithmitti N., Fuchs S.Y., Lu H., Resnick-Silverman L., Manfredi J.J., Ronai Z., Wu X.. Stabilization and activation of p53 by the coactivator protein TAFII31. J. Biol. Chem. 2001; 276:13852–13857. [DOI] [PubMed] [Google Scholar]
- 27. Di Lello P., Jenkins L.M.M., Jones T.N., Nguyen B.D., Hara T., Yamaguchi H., Dikeakos J.D., Appella E., Legault P., Omichinski J.G.. Structure of the Tfb1/p53 complex: insights into the interaction between the p62/Tfb1 subunit of TFIIH and the activation domain of p53. Mol. Cell. 2006; 22:731–740. [DOI] [PubMed] [Google Scholar]
- 28. Teufel D.P., Freund S.M., Bycroft M., Fersht A.R.. Four domains of p300 each bind tightly to a sequence spanning both transactivation subdomains of p53. Proc. Natl. Acad. Sci. 2007; 104:7009–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langlois C., Mas C., Di Lello P., Jenkins L.M.M., Legault P., Omichinski J.G.. NMR structure of the complex between the Tfb1 subunit of TFIIH and the activation domain of VP16: structural similarities between VP16 and p53. J. Am. Chem. Soc. 2008; 130:10596–10604. [DOI] [PubMed] [Google Scholar]
- 30. Lee C.W., Arai M., Martinez-Yamout M.A., Dyson H.J., Wright P.E.. Mapping the interactions of the p53 transactivation domain with the KIX domain of CBP †. Biochemistry. 2009; 48:2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okuda M., Nishimura Y.. Extended string binding mode of the phosphorylated transactivation domain of tumor suppressor p53. J. Am. Chem. Soc. 2014; 136:14143–14152. [DOI] [PubMed] [Google Scholar]
- 32. Arai M., Ferreon J.C., Wright P.E.. Quantitative analysis of multisite protein–ligand interactions by NMR: binding of intrinsically disordered p53 transactivation subdomains with the TAZ2 domain of CBP. J. Am. Chem. Soc. 2012; 134:3792–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Lello P., Nguyen B.D., Jones T.N., Potempa K., Kobor M.S., Legault P., Omichinski J.G.. NMR structure of the amino-terminal domain from the Tfb1 subunit of TFIIH and characterization of its phosphoinositide and VP16 binding sites †, ‡. Biochemistry. 2005; 44:7678–7686. [DOI] [PubMed] [Google Scholar]
- 34. Langlois C., Del Gatto A., Arseneault G., Lafrance-Vanasse J., De Simone M., Morse T., de Paola I., Lussier-Price M., Legault P., Pedone C. et al. . Structure-based design of a potent artificial transactivation domain based on p53. J. Am. Chem. Soc. 2012; 134:1715–1723. [DOI] [PubMed] [Google Scholar]
- 35. Muhandiram D.R., Kay L.E.. Gradient-enhanced triple-resonance three-dimensional NMR experiments with improved sensitivity. J. Magn. Reson. B. 1994; 103:203–216. [Google Scholar]
- 36. Kay L.E., Xu G.Y., Singer A.U., Muhandiram D.R., Formankay J.D.. A gradient-enhanced HCCH-TOCSY experiment for recording side-chain 1H and 13C correlations in H2O samples of proteins. J. Magn. Reson. B. 1993; 101:333–337. [Google Scholar]
- 37. Zhang O., Kay L.E., Olivier J.P., Forman-Kay J.D.. Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J. Biomol. NMR. 1994; 4:845–858. [DOI] [PubMed] [Google Scholar]
- 38. Zuiderweg E.R.P., McIntosh L.P., Dahlquist F.W., Fesik S.W.. Three-dimensional 13C-resolved protein NOE spectroscopy of uniformly 13C-labeled proteins for the NMR assignment and structure determination of larger molecules. J. Magn. Reson. 1990; 86:210–216. [Google Scholar]
- 39. Zwahlen C., Legault P., Vincent S.J.F., Greenblatt J., Konrat R., Kay L.E.. Methods for measurement of intermolecular NOEs by multinuclear NMR spectroscopy: application to a bacteriophage λ N-Peptide/ boxBRNA complex. J. Am. Chem. Soc. 1997; 119:6711–6721. [Google Scholar]
- 40. Delaglio F., Grzesiek S., Vuister G.W., Zhu G., Pfeifer J., Bax A.. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995; 6:277–293. [DOI] [PubMed] [Google Scholar]
- 41. Vranken W.F., Boucher W., Stevens T.J., Fogh R.H., Pajon A., Llinas M., Ulrich E.L., Markley J.L., Ionides J., Laue E.D.. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005; 59:687–696. [DOI] [PubMed] [Google Scholar]
- 42. Marsh J.A., Singh V.K., Jia Z., Forman-Kay J.D.. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: Implications for fibrillation. Protein Sci. 2006; 15:2795–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H., Neal S., Wishart D.S.. RefDB: a database of uniformly referenced protein chemical shifts. J. Biomol. NMR. 2003; 25:173–195. [DOI] [PubMed] [Google Scholar]
- 44. Shen Y., Bax A.. Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J. Biomol. NMR. 2013; 56:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen Y., Delaglio F., Cornilescu G., Bax A.. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR. 2009; 44:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Habeck M., Rieping W., Linge J.P., Nilges M.. NOE assignment with ARIA 2.0: the nuts and bolts. Methods Mol. Biol. 2004; 278:379–402. [DOI] [PubMed] [Google Scholar]
- 47. Brünger A.T., Adams P.D., Clore G.M., DeLano W.L., Gros P., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S. et al. . Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998; 54:905–921. [DOI] [PubMed] [Google Scholar]
- 48. Krieger E., Vriend G.. YASARA View—molecular graphics for all devices—from smartphones to workstations. Bioinformatics. 2014; 30:2981–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krieger E., Joo K., Lee J., Lee J., Raman S., Thompson J., Tyka M., Baker D., Karplus K.. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins. 2009; 77(Suppl. 9):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Canutescu A.A., Shelenkov A.A., Dunbrack R.L.. A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci. 2003; 12:2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bhattacharya A., Tejero R., Montelione G.T.. Evaluating protein structures determined by structural genomics consortia. Proteins. 2007; 66:778–795. [DOI] [PubMed] [Google Scholar]
- 52. Gietz R.D., Woods R.A.. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Meth. Enzymol. 2002; 350:87–96. [DOI] [PubMed] [Google Scholar]
- 53. Di Lello P., Jenkins L.M.M., Mas C., Langlois C., Malitskaya E., Fradet-Turcotte A., Archambault J., Legault P., Omichinski J.G.. p53 and TFIIE share a common binding site on the Tfb1/p62 subunit of TFIIH. Proc. Natl. Acad. Sci. U.S.A. 2008; 105:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uesugi M., Nyanguile O., Lu H., Levine A.J., Verdine G.L.. Induced alpha helix in the VP16 activation domain upon binding to a human TAF. Science. 1997; 277:1310–1313. [DOI] [PubMed] [Google Scholar]
- 55. De Guzman R.N., Goto N.K., Dyson H.J., Wright P.E.. Structural basis for cooperative transcription factor binding to the CBP coactivator. J. Mol. Biol. 2006; 355:1005–1013. [DOI] [PubMed] [Google Scholar]
- 56. Denis C.M., Chitayat S., Plevin M.J., Wang F., Thompson P., Liu S., Spencer H.L., Ikura M., LeBrun D.P., Smith S.P.. Structural basis of CBP/p300 recruitment in leukemia induction by E2A-PBX1. Blood. 2012; 120:3968–3977. [DOI] [PubMed] [Google Scholar]
- 57. Mas C., Lussier-Price M., Soni S., Morse T., Arseneault G., Di Lello P., Lafrance-Vanasse J., Bieker J.J., Omichinski J.G.. Structural and functional characterization of an atypical activation domain in erythroid Kruppel-like factor (EKLF). Proc. Natl. Acad. Sci. U.S.A. 2011; 108:10484–10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chabot P.R., Raiola L., Lussier-Price M., Morse T., Arseneault G., Archambault J., Omichinski J.G.. Structural and functional characterization of a complex between the acidic transactivation domain of EBNA2 and the Tfb1/p62 subunit of TFIIH. PLoS Pathog. 2014; 10:e1004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mielke S.P., Krishnan V.V.. Characterization of protein secondary structure from NMR chemical shifts. Prog. Nucl. Magn. Reson. Spectrosc. 2009; 54:141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goto N.K., Zor T., Martinez-Yamout M., Dyson H.J., Wright P.E.. Cooperativity in transcription factor binding to the coactivator CREB-binding protein (CBP). The mixed lineage leukemia protein (MLL) activation domain binds to an allosteric site on the KIX domain. J. Biol. Chem. 2002; 277:43168–43174. [DOI] [PubMed] [Google Scholar]
- 61. Brüschweiler S., Konrat R., Tollinger M.. Allosteric communication in the KIX domain proceeds through dynamic repacking of the hydrophobic core. ACS Chem. Biol. 2013; 8:1600–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zor T., De Guzman R.N., Dyson H.J., Wright P.E.. Solution structure of the KIX domain of CBP bound to the transactivation domain of c-Myb. J. Mol. Biol. 2004; 337:521–534. [DOI] [PubMed] [Google Scholar]
- 63. Candau R., Scolnick D.M., Darpino P., Ying C.Y., Halazonetis T.D., Berger S.L.. Two tandem and independent sub-activation domains in the amino terminus of p53 require the adaptor complex for activity. Oncogene. 1997; 15:807–816. [DOI] [PubMed] [Google Scholar]
- 64. Regier J.L., Shen F., Triezenberg S.J.. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. U.S.A. 1993; 90:883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. So C.W., Cleary M.L.. MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function. Mol. Cell. Biol. 2002; 22:6542–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen X., Bieker J.J.. Erythroid Krüppel-like factor (EKLF) contains a multifunctional transcriptional activation domain important for inter- and intramolecular interactions. EMBO J. 1996; 15:5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 67. Brzovic P.S., Heikaus C.C., Kisselev L., Vernon R., Herbig E., Pacheco D., Warfield L., Littlefield P., Baker D., Klevit R.E. et al. . The acidic transcription activator Gcn4 binds the mediator subunit Gal11/Med15 using a simple protein interface forming a fuzzy complex. Mol. Cell. 2011; 44:942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang F., Marshall C.B., Yamamoto K., Li G.Y., Gasmi-Seabrook G.M.C., Okada H., Mak T.W., Ikura M.. Structures of KIX domain of CBP in complex with two FOXO3a transactivation domains reveal promiscuity and plasticity in coactivator recruitment. Proc. Natl. Acad. Sci. U.S.A. 2012; 109:6078–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Radhakrishnan I., Pérez-Alvarado G.C., Parker D., Dyson H.J., Montminy M.R., Wright P.E.. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell. 1997; 91:741–752. [DOI] [PubMed] [Google Scholar]
- 70. Krois A.S., Ferreon J.C., Martinez-Yamout M.A., Dyson H.J., Wright P.E.. Recognition of the disordered p53 transactivation domain by the transcriptional adapter zinc finger domains of CREB-binding protein. Proc. Natl. Acad. Sci. U.S.A. 2016; 113:E1853–E1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De Guzman R.N., Martinez-Yamout M.A., Dyson H.J., Wright P.E.. Interaction of the TAZ1 domain of the CREB-binding protein with the activation domain of CITED2: regulation by competition between intrinsically unstructured ligands for non-identical binding sites. J. Biol. Chem. 2004; 279:3042–3049. [DOI] [PubMed] [Google Scholar]
- 72. Miller Jenkins L.M., Feng H., Durell S.R., Tagad H.D., Mazur S.J., Tropea J.E., Bai Y., Appella E.. Characterization of the p300 Taz2–p53 TAD2 complex and comparison with the p300 Taz2–p53 TAD1 complex. Biochemistry. 2015; 54:2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Denis C.M., Langelaan D.N., Kirlin A.C., Chitayat S., Munro K., Spencer H.L., LeBrun D.P., Smith S.P.. Functional redundancy between the transcriptional activation domains of E2A is mediated by binding to the KIX domain of CBP/p300. Nucleic Acids Res. 2014; 42:7370–7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Näär A.M., Beaurang P.A., Zhou S., Abraham S., Solomon W., Tjian R.. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999; 398:828–832. [DOI] [PubMed] [Google Scholar]
- 75. West M.J., Lowe A.D., Karn J.. Activation of human immunodeficiency virus transcription in T cells revisited: NF-kB p65 stimulates transcriptional elongation. J. Virol. 2001; 75:8524–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim Y.K., Bourgeois C.F., Pearson R., Tyagi M., West M.J., Wong J., Wu S.-Y., Chiang C.-M., Karn J.. Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. EMBO J. 2006; 25:3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Farlik M., Reutterer B., Schindler C., Greten F., Vogl C., Müller M., Decker T.. Nonconventional initiation complex assembly by STAT and NF-κB transcription factors regulates nitric oxide synthase expression. Immunity. 2010; 33:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.