Abstract
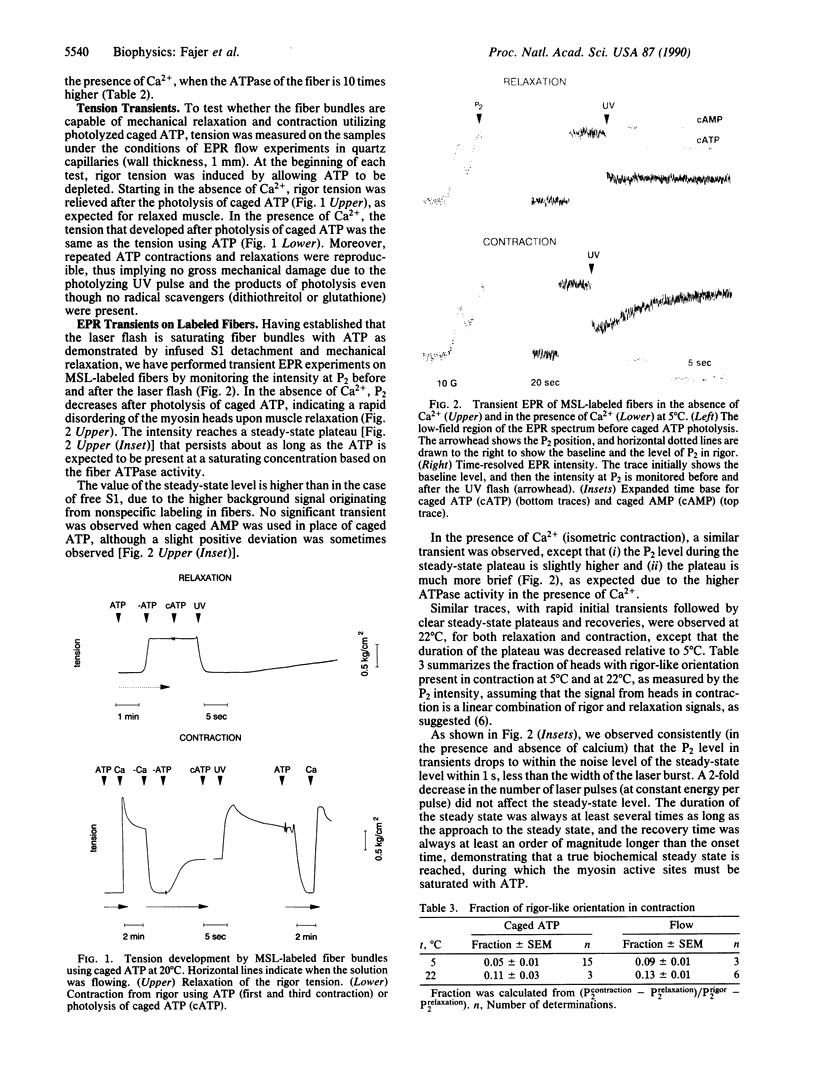
To study the orientation of spin-labeled myosin heads in the first few seconds after the production of saturating ATP, we have used a laser flash to photolyze caged ATP during EPR data acquisition. Rabbit psoas muscle fibers were labeled with maleimide spin label, modifying 60% of the myosin heads without impairing muscle fiber biochemical and physiological activity (ATPase and force). The muscle bundles were incubated for 30 min with 5 mM caged ATP prior to the UV flash. The flash, from an excimer laser, liberated 2-3 mM ATP, generating maximum force in the presence of Ca2+ and relaxing fully in the absence of Ca2+. Control experiments, using fibers decorated with labeled myosin subfragment, showed that the flash liberates sufficient ATP to saturate myosin active sites in all regions of the muscle bundles. To increase the time resolution, and to minimize the time of the contraction, we followed in time the intensity at a single spectral position (P2), which is associated with the high degree of orientational order in rigor. ATP liberation produced a rapid decrease of P2 with liberation of ATP, indicating a large decrease in orientational order in both relaxation and contraction. This transient was absent when caged AMP was used, ruling out nonspecific effects of the UV flash and subsequent photochemistry. The steady-state level of P2 during contraction was almost as low as that reached in relaxation, although the duration of the steady state was much more brief in contraction. Upon depletion of ATP in contraction, the P2 intensity reverted to the original rigor level, accompanied by development of rigor tension. The steady-state results obtained in the brief contractions induced by caged ATP are quantitatively consistent with those obtained in longer contractions by continuously perfusing fibers with ATP. In isometric contraction, most (88% +/- 4%) of the heads are in a population characterized by a high degree of axial disorder, comparable to that observed for all heads in relaxation. Since the stiffness of these fibers in contraction is 80% of the stiffness in rigor, it is likely that most of the heads in this highly disoriented population are attached to actin in contraction and that most actin-attached heads in contraction are in this disoriented population.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajtai K., French A. R., Burghardt T. P. Myosin cross-bridge orientation in rigor and in the presence of nucleotide studied by electron spin resonance. Biophys J. 1989 Sep;56(3):535–541. doi: 10.1016/S0006-3495(89)82700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V. A., Thomas D. D. Microsecond rotational motion of spin-labeled myosin heads during isometric muscle contraction. Saturation transfer electron paramagnetic resonance. Biophys J. 1989 Sep;56(3):517–523. doi: 10.1016/S0006-3495(89)82698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Wendt C. H., Barnett V. A., Thomas D. D. Muscle cross-bridges: do they rotate? Adv Exp Med Biol. 1984;170:413–427. doi: 10.1007/978-1-4684-4703-3_37. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil. 1984 Apr;5(2):131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Brunsvold N. J., Thomas D. D. Effects of AMPPNP on the orientation and rotational dynamics of spin-labeled muscle cross-bridges. Biophys J. 1988 Apr;53(4):513–524. doi: 10.1016/S0006-3495(88)83131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Matta J. J., Thomas D. D. Effect of ADP on the orientation of spin-labeled myosin heads in muscle fibers: a high-resolution study with deuterated spin labels. Biochemistry. 1990 Jun 19;29(24):5865–5871. doi: 10.1021/bi00476a031. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Active and rigor muscle stiffness [proceedings]. J Physiol. 1977 Jul;269(1):55P–57P. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Lovell S. J., Harrington W. F. Measurement of the fraction of myosin heads bound to actin in rabbit skeletal myofibrils in rigor. J Mol Biol. 1981 Jul 15;149(4):659–674. doi: 10.1016/0022-2836(81)90352-1. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Wilson M. G. Three-dimensional disorder of dipolar probes in a helical array. Application to muscle cross-bridges. Biophys J. 1982 Aug;39(2):221–227. doi: 10.1016/S0006-3495(82)84511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer G., Elliott A. Can a myosin molecule bind to two actin filaments? Nature. 1978 Jan 26;271(5643):325–329. doi: 10.1038/271325a0. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. Energetics of the actomyosin bond in the filament array of muscle fibers. Biophys J. 1988 Apr;53(4):561–573. doi: 10.1016/S0006-3495(88)83136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M., Irving M. Myosin crossbridge orientation in demembranated muscle fibres studied by birefringence and X-ray diffraction measurements. J Mol Biol. 1989 Nov 5;210(1):113–126. doi: 10.1016/0022-2836(89)90294-5. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Thomas D. D. ATP induces microsecond rotational motions of myosin heads crosslinked to actin. Biophys J. 1986 Nov;50(5):999–1002. doi: 10.1016/S0006-3495(86)83541-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Titus M. A., Ashiba G., Szent-Györgyi A. G. SH-1 modification of rabbit myosin interferes with calcium regulation. J Muscle Res Cell Motil. 1989 Feb;10(1):25–33. doi: 10.1007/BF01739854. [DOI] [PubMed] [Google Scholar]