Abstract
Recently, we identified the scrapie responsive gene 1 (SCRG1) secreted from mesenchymal stem cells (MSCs) and its receptor bone marrow stromal cell antigen 1 (BST1) as positive regulators of stem cell qualities such as self-renewal, migration abilities, and osteogenic differentiation potential. Here, we examined the effect of the paracrine activity of SCRG1 in macrophages. The mouse macrophage-like cell line Raw264.7 expressed BST1/β1 or BST1/β2 integrin as possible SCRG1 receptors. Unexpectedly, recombinant SCRG1 did not enhance cell proliferation, migration, or adhesion in these macrophages. However, further examination of the effect of SCRG1 in Raw264.7 cells did reveal a potent anti-inflammatory effect whereby SCRG1 suppressed LPS-induced CCL22 production. SCRG1 also induced the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) in these cells and, moreover, a mitogen-activated protein kinase (MAPK)/ERK kinase inhibitor U0126 significantly suppressed the effect of SCRG1 on LPS-induced chemokine CCL22 production. Taken together, these data indicate that SCRG1 signals through the MAPK pathway and suppresses the LPS signaling pathway. CCL22 is generally known to be chemotactic for monocytes, dendritic cells, natural killer cells and chronically activated T lymphocytes, suggesting that MSC-derived SCRG1 may block infiltration of these cells. A mechanism is proposed by which MSCs play their immunosuppressive role through suppressing chemokine expression in monocyte/macrophage lineage cells.
Keywords: scrapie responsive gene 1, mesenchymal stem cells, CC-chemokine ligand 22, extracellular signal-regulated kinase 1/2, macrophage
Introduction
Mesenchymal stem cells (MSCs) are adult stem cells with the ability to differentiate into mesenchymal tissue cells while retaining self-renewal and migration abilities (1). Many recent studies have revealed that MSCs possess immunomodulatory functions that they exert through cell-to-cell contacts, as well as by secreting growth factors, cytokines, and chemokines (2,3). The effect of immunosuppression with MSCs has been reported in graft-versus-host disease (4) and multiple system atrophy (5). MSCs have the ability to migrate to damaged tissue by inducing peripheral tolerance by inhibiting the release of pro-inflammatory cytokines (2). The advantages of MSC-based cell therapy have been demonstrated in acute lung injury (6), myocardial infarction (7), acute renal failure (8), cerebral ischemia (9) and Alzheimer's disease (10). At the cellular level, it has been shown that MSCs can directly inhibit both T lymphocyte and microglial cell proliferation and can negatively modulate the cytokine-secretion profile of dendritic cells and monocytes/macrophages (11–14).
In our recent study, we identified the scrapie responsive gene 1 (SCRG1) secreted from MSCs and its receptor complex bone marrow stromal cell antigen 1 (BST1)/β1 integrin, as positive regulators of stem cell qualities (15). SCRG1, which was identified by Dron et al as a protein that increased expression in the brain of scrapie infected mice, was shown to be associated with neurodegenerative changes in transmissible spongiform encephalopathy, as well as in brain injury, and is associated with autophagy (16–18). The SCRG1 gene encodes a 98-amino acid, cytokine-like peptide with an N-terminal signal peptide (19,20). Intriguingly, in stem cells SCRG1 was shown to maintain octamer-binding transcription factor 4 (Oct-4) and CD271/low-affinity nerve growth factor receptor (LNGFR) expression and thereby maintain the MSC's potential for self-renewal, migration abilities, as well as osteogenic differentiation potential, even at high stem cell passage numbers (15). Other cytokines and chemokines secreted from MSCs have been implicated in immunosuppression and repair of damaged tissues (21–24). MSC differentiation along different pathways is regulated by stimulation with various growth factors, cytokines, or chemokines, as has been demonstrated in the differentiation of bone marrow-derived MSCs (25,26).
SCRG1 secreted from MSCs is predicted to affect a variety of cell types in vivo. In this study, we hypothesized that SCRG1 secreted into the extracellular space by MSCs exhibits paracrine activity. To explore this possibility, we examined the paracrine effect of MSC-derived SCRG1 on the immune response of Raw264.7 macrophages. In particular, as a readout of macrophage function, we focused on macrophage production of CC-chemokine ligand 22 [CCL22; also known as MDC (macrophage-derived chemokine)], which is known to display chemotactic activity for monocytes, dendritic cells, natural killer cells, and chronically activated T lymphocytes (27–30).
Materials and methods
Reagents
Recombinant mouse SCRG1 (rmSCRG1), expressed in yeast, was purchased from MyBiosource, Inc. (MBS1177239, San Diego, CA, USA). The MAPK/ERK kinase inhibitor U0126 was purchased from Calbiochem (Merck Millipore, Darmstadt, Germany). Lipopolysaccharide (LPS) derived from Escherichia coli 0111:B4 was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Mouse macrophage-like Raw264.7 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in minimum essential medium Eagle's α-modification (αMEM) (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (HyClone, GE Healthcare Life Sciences, Logan, UT, USA) under the condition of 5% CO2 at 37°C.
Proliferation assay
Cell proliferation was analyzed by WST-1 assay reagent (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's instructions. Raw264.7 cells were cultured on 96-well plates (Nunc; Thermo Fisher Scientific, Waltham, MA, USA) in 100 µl complete medium containing with or without 100 ng/ml rmSCRG1. After five days, the cells were added with 10 µl WST-1 reagent and incubated for 1 h. The absorbance was measured using an MPR-A4i microplate reader (Tosoh Corp., Tokyo, Japan) at 450 nm.
Migration assay
The migration assay was performed using 8-µm pore sized Transwell cell culture inserts (BD Biosciences, Franklin Lakes, NJ, USA). Raw264.7 cells (1.0×105) were seeded on the upper well in 350 µl serum-free αMEM containing 0.1% BSA (Sigma-Aldrich). The lower well was filled in 600 µl complete medium containing with or without 100 ng/ml rmSCRG1. After incubation for 6 h, cells that had not migrated were scraped off with a cotton swab. The number of cells migrated to the lower side of the filter was stained with Diff-Quik Three-Step Stain Set (Sysmex, Kobe, Japan), and then counted using a microscope (Olympus IX70; Olympus Corp., Tokyo, Japan) under five high-power fields (x400 magnification).
Adhesion assay
Raw264.7 cells (1.0×105) were seeded onto a fibronectin-coated culture dish (BD Biosciences) and cultured in complete medium with or without 100 ng/ml rmSCRG1. After 6 h, non-adhered cells on the bottom of the dish were removed by washing twice with phosphate-buffered saline (PBS). The number of cells adhered to the culture dish were measured by the WST-1 assay described above. The absorbance of the dye in the culture directly correlates with the number of live cells.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Raw264.7 cells were either left unstimulated or stimulated with 10 ng/ml LPS, 100 ng/ml rmSCRG1, or 10 ng/ml LPS plus 100 ng/ml rmSCRG1 in the absence and presence of 1 mM U0126, for 6 h. Total RNA extraction, cDNA synthesis, RT-qPCR were performed with methods of our previous study (15). Expression of Ccl22 was normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The following primer pairs were used; Ccl22 (sense, 5′-GGCACCTATCCAGTGCCACA-3′ and antisense, 5′-TGGTGGACCAGCCTGAAACTC') and Gapdh (sense, 5′-TGTGTCCGTCGTGGATCTGA-3′ and antisense, 5′-TTGCTGTTGAAGTCGCAGGAG-3′). Relative expression levels were calculated by the 2−ΔΔCq method (31) as a fold-increase or -decrease.
Primer array
Raw264.7 cells were stimulated with 10 ng/ml LPS, 100 ng/ml rmSCRG1, or 10 ng/ml LPS plus 100 ng/ml rmSCRG1 for 6 h. Unstimulated cells were used as a control. Gene expression levels of a range of cytokines and chemokines were measured using a PrimerArray consisting of mouse cytokines and cytokine receptors (PN001, Takara Bio) and PrimerArray Analysis Tool version 2.0 (Takara Bio) according to the manufacturer's instructions. Genes whose expression levels increased more than 100-fold following LPS stimulation and less than 100-fold following SCRG1 treatment were identified.
Western blotting
Raw264.7 cells were serum-starved overnight and stimulated with 100 ng/ml rmSCRG1 for various period time. Western blotting was performed in our previously reported procedure (15). The following primary antibodies that has been purchased from Cell Signaling Technology (Danvers, MA, USA) were used; anti-p44/42 mitogen-activated protein kinase (ERK1/2), anti-phospho-ERK1/2, anti-c-Jun N-terminal kinase (JNK), anti-phospho-JNK, anti-p38, anti-phospho-p38, anti-Akt, anti-phospho-Akt, anti-focal adhesion kinase (FAK), anti-phospho-FAK. β-actin level measured were detected with an anti-β-actin antibody (Santa Cruz Biotechnology, Dallas, TX, USA) as a loading control. The densitometry of the band measured by ImageJ version 1.44 software was expressed as the ratio of phosphorylation to the total molecule.
Enzyme-linked immunosorbent assay (ELISA)
Raw264.7 cells were left unstimulated or were stimulated with 10 ng/ml LPS, 100 ng/ml rmSCRG1, or 10 ng/ml LPS plus 100 ng/ml rmSCRG1 for 48 h. The amount of secreted CCL22 in the culture medium was measured using a sandwich ELISA kit for mouse CCL22 (R&D Systems, Inc., Minneapolis, MN, USA). CCL22 levels were quantified according to the manufacturer's instructions.
Flow cytometry
A total of 1×105 Raw264.7 cells suspended in PBS containing 2 mM EDTA and 0.5% FBS were incubated with either a phycoerythrin (PE)-conjugated anti-mouse BST1/CD157 (1:10), anti-mouse β1 integrin/CD29 (1:10), or anti-mouse β2 integrin/CD18 (1:10) (all from BioLegend, Inc., San Diego, CA, USA) antibody for 1 h at 4°C. Data acquisition and analysis was performed using a flow cytometer EPICS XL (Beckman Coulter, Brea, CA, USA).
Statistical analysis
All experiments were performed in triplicate. Numerical data were presented as the mean ± standard deviation (SD), and significant differences were analyzed by Student's t-test. P<0.05 were considered statistically significant.
Results
Raw264.7 cells express BST1, and β1 and β2 integrins as an SCRG1 receptor complex
Our recent study revealed that SCRG1 secreted from MSCs forms a complex with the membrane proteins BST1 and β1 integrin, which acts as a receptor for its autocrine/paracrine activity (15). Further evidence for this novel receptor was provided by Lavagno et al, which showed BST1 also interacts with β1 and β2 integrins at the neutrophil cell surface (32). Based on these data, we investigated the expression of BST1, β1 integrin, and β2 integrin in mouse macrophage-like Raw264.7 cells by flow cytometry. As shown in Fig. 1, Raw264.7 cells co-expressed BST1, β1 integrin, and β2 integrin on the cell surface. These results indicate that Raw264.7 cells express the SCRG1 receptor complex.
Figure 1.
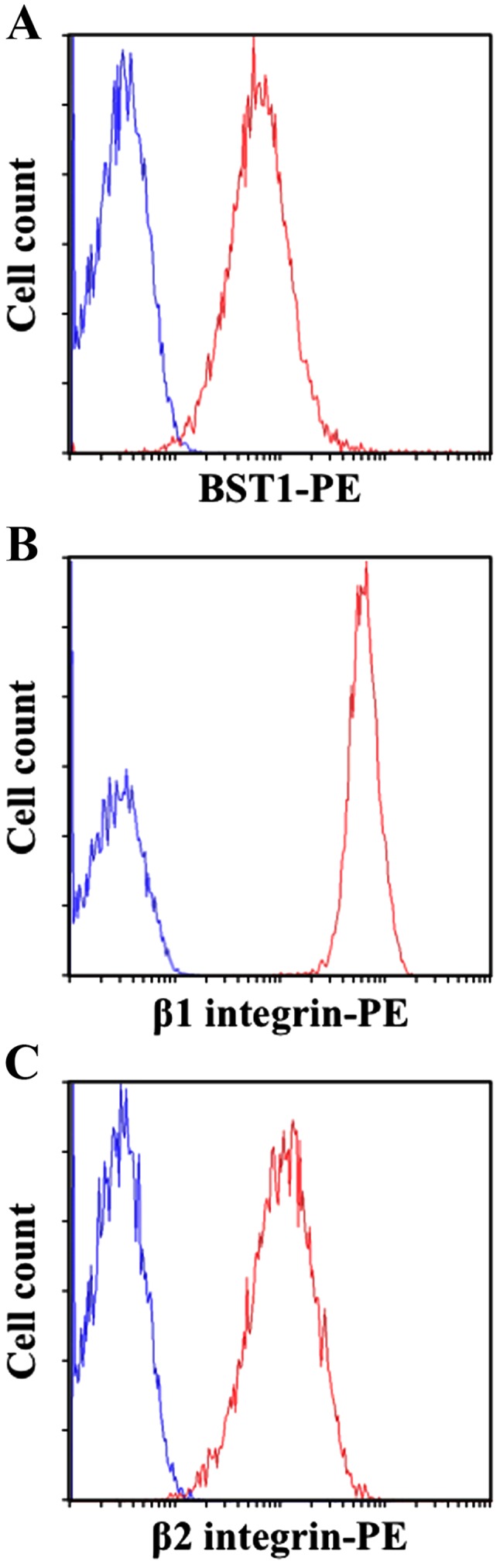
Raw264.7 cells express the bone marrow stromal cell antigen 1 (BST1), β1 integrin, and β2 integrin as a scrapie responsive gene 1 (SCRG1) receptor complex. Cell surface expression of BST1 (A), β1 integrin (B), and β2 integrin (C) was analyzed by flow cytometry with phycoerythrin (PE)-conjugated specific antibodies. Specific antibody staining (red) and isotype control IgG staining (blue) are shown.
SCRG1 does not affect the statuses of cell proliferation, migration, and adhesion of Raw264.7 cells
In MSCs, SCRG1 promotes cell migration through the β1 integrin/focal adhesion kinase (FAK) -dependent phosphoinositide 3-kinase (PI3K)/Akt pathway (15). Here, to understand the biological processes controlled by SCRG1 in macrophages, the effects of SCRG1 on cell proliferation, migration, as well as adhesion activity were investigated. As shown in Fig. 2, rmSCRG1 did not affect proliferative, migratory, or adhesive activities in Raw264.7 cells. Thus, these results indicate that the bioactivity of SCRG1 in monocyte/macrophage lineage cells is different from that in MSCs.
Figure 2.
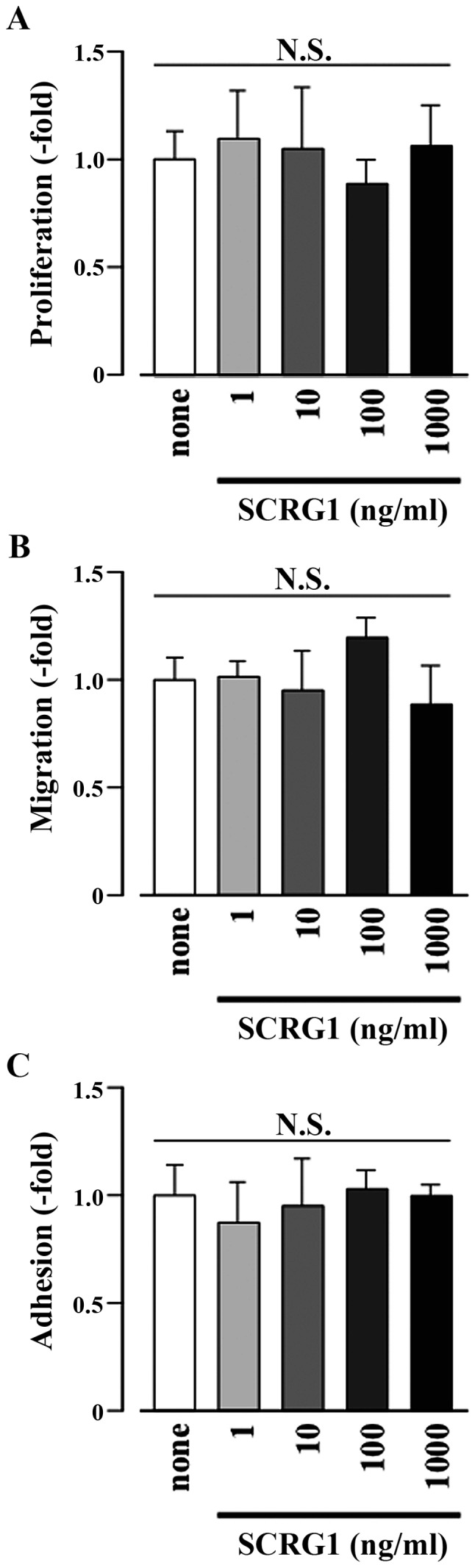
Scrapie responsive gene 1 (SCRG1) did not affect cell proliferation, migration, or adhesion of Raw264.7 cells. Cell proliferation (A), migration (B), and adhesion (C) for Raw264.7 cells were left unstimulated (none) or were stimulated with SCRG1 (1–1,000 ng/ml). The results are expressed as the fold change relative to the respective control (none). Data are presented as the means ± SD. *P<0.05 vs. unstimulated control within each cell. N.S., not significant.
SCRG1 enhances the phosphorylation of ERK1/2 in Raw264.7 cells
The MAPK pathway, in conjunction with the nuclear factor-κB (NF-κB) pathway, is closely associated with the macrophage immune response (33,34). Accordingly, the intracellular signaling pathways induced by SCRG1 in Raw264.7 cells were investigated. Treatment of cell for 30 min with rmSCRG1 significantly enhanced the phosphorylation of ERK1/2 (Fig. 3). In contrast, phosphorylation of FAK, Akt, SAPK/Jun amino-terminal kinase (JNK), or p38 MAPK by rmSCRG1 treatment in Raw264.7 cells was not observed (data not shown). These results indicate that SCRG1 specifically induces the activation of the ERK1/2 pathway.
Figure 3.
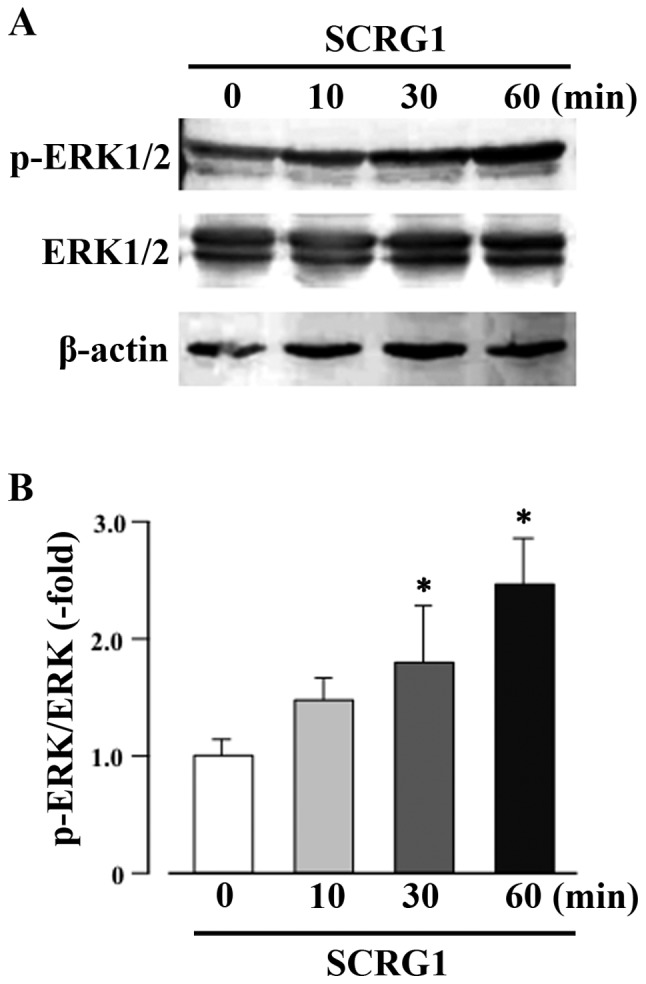
Scrapie responsive gene 1 (SCRG1) enhances the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) in Raw264.7 cells. (A) ERK1/2 phosphorylation was measured using western blotting with anti-phospho-ERK1/2 (p-ERK1/2) antibodies in Raw264.7 cells stimulated with 100 ng/ml SCRG1. Total ERK1/2 levels were measured using a primary anti-ERK1/2 (ERK1/2) antibody. As a loading control β-actin levels were measured using an anti-β-actin antibody. (B) Densitometry analysis of band intensity in western blotting was expressed as the ratio of phosphorylation to the total molecule. *P<0.05, statistically significant vs. unstimulated control.
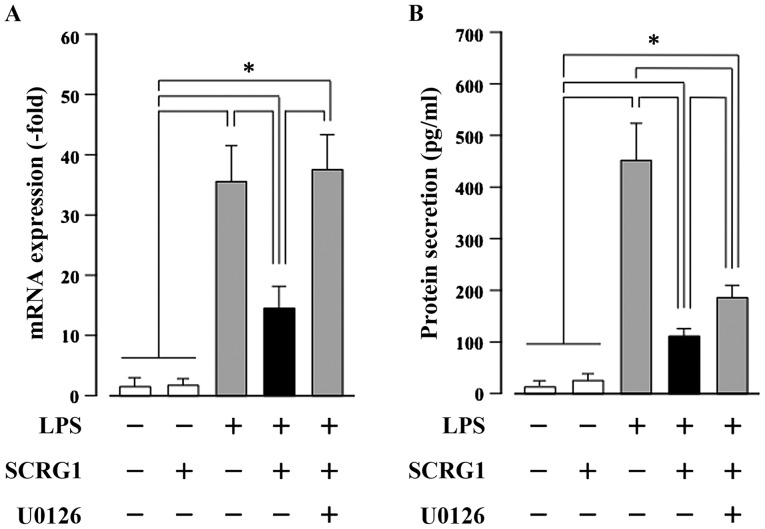
SCRG1 suppresses LPS-induced CCL22 production through the activation of ERK1/2 in Raw264.7 cells
By primer array analysis, we next investigated the effects of SCRG1 on the expression of LPS-induced chemokines and cytokines in Raw264.7 cells. As shown in Table I, rmSCRG1 suppresses the LPS-induced production of five chemokines and one cytokine in these cells. In particular, LPS-induced Ccl22 expression was reduced to less than half by rmSCRG1 treatment. Following on from this, we examined the association between suppression of CCL22 expression and ERK activation by rmSCRG1 in Raw264.7 cells. Both the LPS-induced increases in CCL22 mRNA expression, and protein secretion were significantly suppressed by rmSCRG1 treatment in Raw264.7 cells (Fig. 4). In addition, this suppressive effect of rmSCRG1 was completely abolished by treatment with the MAPK/ERK kinase inhibitor, U0126. These results indicate that LPS-induced CCL22 production in macrophages was suppressed by treatment with SCRG1 through the activation of an ERK1/2-mediated signal.
Table I.
Genes whose expression increased more than 100-fold after LPS treatment and less than 100-fold by SCRG1 treatment alone relative to unstimulated Raw264.7 cells.
| Fold change relative to unstimulated Raw 264.7 cells | ||||
|---|---|---|---|---|
| Gene symbol | Gene name | LPS | LPS+SCRG1 | SCRG1 |
| Ccl22 | Chemokine (C-C motif) ligand 22 | 4420.5 | 1520.1 | 96.335 |
| Ccl8 | Chemokine (C-C motif) ligand 8 | 2856.4 | 1770.5 | 0.84089 |
| Cxcl11 | Chemokine (C-X-C motif) ligand 11 | 1746.1 | 843.35 | 89.884 |
| Ccl12 | Chemokine (C-C motif) ligand 12 | 929.29 | 749.61 | 70.521 |
| Csf1 | Colony stimulating factor 1 | 206.50 | 125.36 | 17.876 |
| Cx3cl1 | Chemokine (C-X3-C motif) ligand 1 | 154.34 | 115.36 | 1.3755 |
LPS, lipopolysaccharide.
Figure 4.
Scrapie responsive gene 1 (SCRG1) suppresses lipopolysaccharide (LPS)-induced CC-chemokine ligand 22 (CCL22) production through the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) in Raw264.7 cells. Raw264.7 cells were stimulated with or without 10 ng/ml LPS, 100 ng/ml rmSCRG1, and 1 mM U0126 for either 6 h (A) or 48 h (B). (A) Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed with specific oligonucleotide primers. mRNA expression level of Ccl22 was normalized to glyceraldehyde-3-phosphate dehydrogenase, and the results are expressed as the fold change relative to the unstimulated control. (B) The amount of secreted CCL22 in the culture medium was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) for mouse CCL22. In (A) and (B), data are presented as the means ± SD. *P<0.05 vs. unstimulated control within each cell.
Discussion
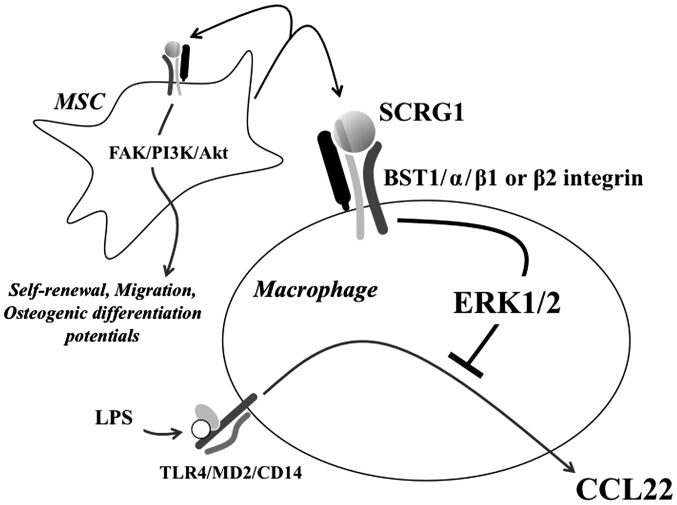
Several roles of SCRG1 suggested in this study and our previous studies are shown in Fig. 5. Recently, we identified a novel ligand-receptor combination, SCRG1/BST1, that maintains expressions of the stem cell markers Oct-4 and CD271/LNGFR in MSC, as well as self-renewal, migration, and osteogenic differentiation potential during ex vivo expansion (15). Here, we investigated the expression of the SCRG1 receptor components, BST1, β1 integrin, and β2 integrin in mouse macrophage-like Raw264.7 cells. In addition to BST1, these cells also expressed β1 and β2 integrins (Fig. 1). BST1 is an ectoenzyme with a glycosyl phosphatidylinositol anchor and a NADase/ADP-ribosyl cyclase activity that belongs to the CD38 family (35,36). It has been found on the cell surface of stromal (37) and bone marrow-derived cells (38), and it facilitates pre-B-cell growth and induces cell migration (39). Under the condition of a complex of BST1 with either β1 integrin or β2 integrin, BST1 has been shown to promote the phosphorylation of FAK through the use of an agonistic monoclonal antibody (32,40,41). Furthermore, it has also been reported that BST-1 regulates the adhesion and migration of leukocytes via phosphorylation of Akt and MAPKs (42,43). In this study, we also observed that SCRG1 enhanced the phosphorylation of ERK1/2 in Raw264.7 cells (Fig. 3).
Figure 5.
Several roles of scrapie responsive gene 1 (SCRG1) suggested in this study and our previous reports. SCRG1 is hypothesized to be secreted into the extracellular space by mesenchymal sem cells (MSCs) and to have autocrine/paracrine activity. The putative receptor for SCRG1 is a complex of bone marrow stromal cell antigen 1 (BST1) and β1 or β2 integrins on the cell surface. The SCRG1/BST1 axis positively regulates the self-renewal, migration, and osteogenic differentiation potentials of MSCs through the FAK/PI3 K/Akt signaling pathway. In macrophage, lipopolysaccharide (LPS) interacts with a heterologous receptor involving toll-like receptor 4 (TLR4), CD14, and MD2. SCRG1 suppresses LPS-induced CC-chemokine ligand 22 (CCL22) production of macrophage in a extracellular signal-regulated kinase 1/2 (ERK1/2)-dependent manner.
Notably, SCRG1 did not enhance cell proliferation, migration, or adhesion of Raw264.7 cells (Fig. 2), indicating a different biological function in these cells. In support of this, we demonstrated that SCRG1 suppresses LPS-induced CCL22 production in these mouse macrophage-like Raw264.7 cells in a MAPK/ERK-dependent manner (Table I, Fig. 4). LPS and other microbial products are recognized by the host's toll-like receptors (TLRs) family members (44). LPS interacts with a heterologous receptor involving TLR4 (45,46), CD14 (47,48) and MD2 (49–51). By LPS binds to the TLR4, two major signaling pathways via the adapter molecules TIR-domain-containing adaptor inducing IFN-β (TRIF) or myeloid differentiation factor 88 (MyD88) are activated (45,52–55). Significantly, both the Myd88 and TRIF pathways result in activation of the transcription factor, NF-κB, a central regulator of the LPS response, which induces cytokine and chemokine production as well as stress responses in many cell types, including macrophages (53,54). NF-κB is involved in regulating the expression of multiple genes involved in inflammatory and immune responses (56). Innate and adaptive immune response are mainly regulated by MAPKs, including ERK, JNK, and p38 MAPK, in addition to the NF-κB pathway. In macrophages, activation of the MEK/ERK pathway by bacterial infection regulates various inflammatory responses (57–61). The MEK/ERK pathway is one of the most studied intracellular signaling pathways in monocyte-derived macrophages activated by LPS-induced pro-inflammatory responses (62–64). In addition, findings have been previously reported that NF-κB-dependent gene expression is controlled by the activation of ERK1/2 without affecting DNA binding (65–69). Therefore, our results indicate that the SCRG1-induced ERK1/2 signaling pathway controls NF-κB-dependent chemokine CCL22 expression.
CCL22, a member of the CC chemokine families, is mainly produced by monocyte-derived macrophages, mast cells, and inflammatory dendritic cells upon stimulation with microbial products (70,71). CCL22 binds to its receptor CCR4 plays an important homeostatic role in leukocyte trafficking, activation of innate immune cells, Th2 immunopathology, as well as accumulation of regulatory T (Treg) cells in solid tumor (72–74). CCL 22 also plays a role in recruiting Treg cells into synovial fluid in inflammatory diseases like rheumatoid arthritis (75). CCL22 plays an important role in a variety of other diseases, including allergic rhinitis (76), atopic dermatitis (77), and lymphoma (78). Recent studies suggested that CCL22 can be used as a biomarker for autoimmune diseases (79). On the other hand, the role of CCL 22 in regulation of immune homeostasis is unclear (80,81).
In conclusion, here we clearly demonstrate that SCRG1, which in vivo could be derived from MSCs, suppresses LPS-induced chemokine CCL22 production in Raw264.7 macrophages. The mechanism appears to involve the MAPK ERK1/2 pathway, since SCRG1 induced the phosphorylation of ERK1/2 and a MAPK/ERK kinase inhibitor U0126 ablated the suppressive effect of SCRG1 on LPS-induced chemokine CCL22 production. These results suggest a model whereby MSCs play their immunosuppressive role by secreting SCRG1, which then suppresses microbial products-induced chemokine expression in monocyte/macrophage lineage cells in a paracrine fashion. We have additionally established fluorescently tagged immortalized MSC lines derived from different tissues of GFP- and tdTomato-transgenic mice (25,82). These cell lines can be used for in vivo imaging analyses on proliferation and differentiation of MSCs, as well as in vivo imaging studies to test cell therapies and regenerative medicine techniques, providing insight into diseases such as bone and immune disorders, fibrosis, and cancer progression or metastasis. Our findings provide new insights into the molecular mechanisms of MSCs, as well as a novel perspective for understanding the immune regulatory mechanisms of MSCs.
Acknowledgements
The present study was supported in part by the JSPS KAKENHI grant nos. JP25463053 and JP16K11654 awarded to N.C., JP26893249 and JP16K20652 awarded to E.K. and JP26670852 and JP16H05534 awarded to A.I.; and a grant from the Keiryokai Research Foundation grant no. 120 awarded to N.C., 2013.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 5.Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: Immunomodulation and neuroprotection. PLoS One. 2011;6:e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury; Proc Natl Acad Sci USA; 2007; pp. 11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh AM, Nagai A, Wakabayashi K, Narantuya D, Kobayashi S, Yamaguchi S, Kim SU. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: Contribution of fractalkine and IL-5. Neurobiol Dis. 2011;41:717–724. doi: 10.1016/j.nbd.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010;28:329–343. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 11.Lda S Meirelles, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 13.Ooi YY, Ramasamy R, Rahmat Z, Subramaiam H, Tan SW, Abdullah M, Israf DA, Vidyadaran S. Bone marrow-derived mesenchymal stem cells modulate BV2 microglia responses to lipopolysaccharide. Int Immunopharmacol. 2010;10:1532–1540. doi: 10.1016/j.intimp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aomatsu E, Takahashi N, Sawada S, Okubo N, Hasegawa T, Taira M, Miura H, Ishisaki A, Chosa N. Novel SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic differentiation potential in mesenchymal stem cells. Sci Rep. 2014;4:3652. doi: 10.1038/srep03652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandoy-Dron F, Guillo F, Benboudjema L, Deslys JP, Lasmézas C, Dormont D, Tovey MG, Dron M. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J Biol Chem. 1998;273:7691–7697. doi: 10.1074/jbc.273.13.7691. [DOI] [PubMed] [Google Scholar]
- 17.Dron M, Bailly Y, Beringue V, Haeberlé AM, Griffond B, Risold PY, Tovey MG, Laude H, Dandoy-Dron F. Scrg1 is induced in TSE and brain injuries and associated with autophagy. Eur J Neurosci. 2005;22:133–146. doi: 10.1111/j.1460-9568.2005.04172.x. [DOI] [PubMed] [Google Scholar]
- 18.Dron M, Bailly Y, Beringue V, Haeberlé AM, Griffond B, Risold PY, Tovey MG, Laude H, Dandoy-Dron F. SCRG1, a potential marker of autophagy in transmissible spongiform encephalopathies. Autophagy. 2006;2:58–60. doi: 10.4161/auto.2228. [DOI] [PubMed] [Google Scholar]
- 19.Dron M, Dandoy-Dron F, Guillo F, Benboudjema L, Hauw JJ, Lebon P, Dormont D, Tovey MG. Characterization of the human analogue of a Scrapie-responsive gene. J Biol Chem. 1998;273:18015–18018. doi: 10.1074/jbc.273.29.18015. [DOI] [PubMed] [Google Scholar]
- 20.Dron M, Tartare X, Guillo F, Haik S, Barbin G, Maury C, Tovey M, Dandoy-Dron F. Mouse scrapie responsive gene 1 (Scrg1): Genomic organization, physical linkage to sap30, genetic mapping on chromosome 8 and expression in neuronal primary cell cultures. Genomics. 2000;70:140–149. doi: 10.1006/geno.2000.6358. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 22.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 24.Lee RH, Oh JY, Choi H, Bazhanov N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem. 2011;112:3073–3078. doi: 10.1002/jcb.23250. [DOI] [PubMed] [Google Scholar]
- 25.Sawada S, Chosa N, Takizawa N, Yokota J, Igarashi Y, Tomoda K, Kondo H, Yaegashi T, Ishisaki A. Establishment of mesenchymal stem cell lines derived from the bone marrow of green fluorescent protein-transgenic mice exhibiting a diversity in intracellular transforming growth factor-β and bone morphogenetic protein signaling. Mol Med Rep. 2016;13:2023–2031. doi: 10.3892/mmr.2016.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igarashi Y, Chosa N, Sawada S, Kondo H, Yaegashi T, Ishisaki A. VEGF-C and TGF-β reciprocally regulate mesenchymal stem cell commitment to differentiation into lymphatic endothelial or osteoblastic phenotypes. Int J Mol Med. 2016;37:1005–1013. doi: 10.3892/ijmm.2016.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang Ms, McNinch J, Elias C, III, Manthey CL, Grosshans D, Meng T, Boone T, Andrew DP. Molecular cloning and functional characterization of a novel CC chemokine, stimulated T cell chemotactic protein (STCP-1) that specifically acts on activated T lymphocytes. J Biol Chem. 1997;272:25229–25237. doi: 10.1074/jbc.272.40.25229. [DOI] [PubMed] [Google Scholar]
- 29.Schaniel C, Pardali E, Sallusto F, Speletas M, Ruedl C, Shimizu T, Seidl T, Andersson J, Melchers F, Rolink AG, Sideras P. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med. 1998;188:451–463. doi: 10.1084/jem.188.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC) J Leukoc Biol. 2000;68:400–404. [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Lavagno L, Ferrero E, Ortolan E, Malavasi F, Funaro A. CD157 is part of a supramolecular complex with CD11b/CD18 on the human neutrophil cell surface. J Biol Regul Homeost Agents. 2007;21:5–11. [PubMed] [Google Scholar]
- 33.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 34.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishihara K, Hirano T. BST-1/CD157 regulates the humoral immune responses in vivo. Chem Immunol. 2000;75:235–255. doi: 10.1159/000058772. [DOI] [PubMed] [Google Scholar]
- 36.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 37.Kaisho T, Ishikawa J, Oritani K, Inazawa J, Tomizawa H, Muraoka O, Ochi T, Hirano T. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth; Proc Natl Acad Sci USA; 1994; pp. 5325–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein SC, Todd RF., III Structural and biosynthetic features of the Mo5 human myeloid differentiation antigen. Tissue Antigens. 1993;41:214–218. doi: 10.1111/j.1399-0039.1993.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 39.Funaro A, Ortolan E, Bovino P, Lo Buono N, Nacci G, Parrotta R, Ferrero E, Malavasi F. Ectoenzymes and innate immunity: The role of human CD157 in leukocyte trafficking. Front Biosci (Landmark Ed) 2009;14:929–943. doi: 10.2741/3287. [DOI] [PubMed] [Google Scholar]
- 40.Hussain AM, Lee HC, Chang CF. Functional expression of secreted mouse BST-1 in yeast. Protein Expr Purif. 1998;12:133–137. doi: 10.1006/prep.1997.0811. [DOI] [PubMed] [Google Scholar]
- 41.Okuyama Y, Ishihara K, Kimura N, Hirata Y, Sato K, Itoh M, Ok LB, Hirano T. Human BST-1 expressed on myeloid cells functions as a receptor molecule. Biochem Biophys Res Commun. 1996;228:838–845. doi: 10.1006/bbrc.1996.1741. [DOI] [PubMed] [Google Scholar]
- 42.Lo Buono N, Parrotta R, Morone S, Bovino P, Nacci G, Ortolan E, Horenstein AL, Inzhutova A, Ferrero E, Funaro A. The CD157-integrin partnership controls transendothelial migration and adhesion of human monocytes. J Biol Chem. 2011;286:18681–18691. doi: 10.1074/jbc.M111.227876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funaro A, Ortolan E, Ferranti B, Gargiulo L, Notaro R, Luzzatto L, Malavasi F. CD157 is an important mediator of neutrophil adhesion and migration. Blood. 2004;104:4269–4278. doi: 10.1182/blood-2004-06-2129. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 45.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 46.Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright SD. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 48.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.immunol.13.1.437. [DOI] [PubMed] [Google Scholar]
- 49.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: Cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 51.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. Mal and MyD88: Adapter proteins involved in signal transduction by toll-like receptors. J Endotoxin Res. 2003;9:55–59. doi: 10.1177/09680519030090010701. [DOI] [PubMed] [Google Scholar]
- 53.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/S1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 55.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 57.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 61.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 62.Rao KM. MAP kinase activation in macrophages. J Leukoc Biol. 2001;69:3–10. [PubMed] [Google Scholar]
- 63.Rao KM, Meighan T, Bowman L. Role of mitogen-activated protein kinase activation in the production of inflammatory mediators: Differences between primary rat alveolar macrophages and macrophage cell lines. J Toxicol Environ Health A. 2002;65:757–768. doi: 10.1080/00984100290071027. [DOI] [PubMed] [Google Scholar]
- 64.Bain J, Plater L, Elliott M, Shapiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003;17:1319–1321. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 66.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J Immunol. 2000;165:5606–5611. doi: 10.4049/jimmunol.165.10.5606. [DOI] [PubMed] [Google Scholar]
- 67.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–27238. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokuda M, Miyamoto R, Sakuta T, Nagaoka S, Torii M. Substance P activates p38 mitogen-activated protein kinase to promote IL-6 induction in human dental pulp fibroblasts. Connect Tissue Res. 2005;46:153–158. doi: 10.1080/03008200500182490. [DOI] [PubMed] [Google Scholar]
- 69.Zampetaki A, Mitsialis SA, Pfeilschifter J, Kourembanas S. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-kappaB: The prominent role of p42/p44 and PI3 kinase pathways. FASEB J. 2004;18:1090–1092. doi: 10.1096/fj.03-0991fje. [DOI] [PubMed] [Google Scholar]
- 70.Yamashita U, Kuroda E. Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit Rev Immunol. 2002;22:105–114. doi: 10.1615/CritRevImmunol.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- 71.Layseca-Espinosa E, Korniotis S, Montandon R, Gras C, Bouillié M, Gonzalez-Amaro R, Dy M, Zavala F. CCL22-producing CD8α- myeloid dendritic cells mediate regulatory T cell recruitment in response to G-CSF treatment. J Immunol. 2013;191:2266–2272. doi: 10.4049/jimmunol.1202307. [DOI] [PubMed] [Google Scholar]
- 72.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 74.Li YQ, Liu FF, Zhang XM, Guo XJ, Ren MJ, Fu L. Tumor secretion of CCL22 activates intratumoral treg infiltration and is independent prognostic predictor of breast cancer. PLoS One. 2013;8:e76379. doi: 10.1371/journal.pone.0076379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flytlie HA, Hvid M, Lindgreen E, Kofod-Olsen E, Petersen EL, Jørgensen A, Deleuran M, Vestergaard C, Deleuran B. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49:24–29. doi: 10.1016/j.cyto.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Yanai M, Sato K, Aoki N, Takiyama Y, Oikawa K, Kobayashi H, Kimura S, Harabuchi Y, Tateno M. The role of CCL22/macrophage-derived chemokine in allergic rhinitis. Clin Immunol. 2007;125:291–298. doi: 10.1016/j.clim.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Nakazato J, Kishida M, Kuroiwa R, Fujiwara J, Shimoda M, Shinomiya N. Serum levels of Th2 chemokines, CCL17, CCL22 and CCL27, were the important markers of severity in infantile atopic dermatitis. Pediatr Allergy Immunol. 2008;19:605–613. doi: 10.1111/j.1399-3038.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 78.Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, Jarrett RF, Te Meerman GJ, Poppema S, van den Berg A. Serum chemokine levels in hodgkin lymphoma patients: Highly increased levels of CCL17 and CCL22. Br J Haematol. 2008;140:527–536. doi: 10.1111/j.1365-2141.2007.06964.x. [DOI] [PubMed] [Google Scholar]
- 79.Jafarzadeh A, Ebrahimi HA, Bagherzadeh S, Zarkesh F, Iranmanesh F, Najafzadeh A, Khosravimashizi A, Nemati M, Sabahi A, Hajghani H, et al. Lower serum levels of Th2-related chemokine CCL22 in women patients with multiple sclerosis: A comparison between patients and healthy women. Inflammation. 2014;37:604–610. doi: 10.1007/s10753-013-9775-z. [DOI] [PubMed] [Google Scholar]
- 80.Nagata S. Apoptosis and autoimmune diseases. Ann N Y Acad Sci. 2010;1209:10–16. doi: 10.1111/j.1749-6632.2010.05749.x. [DOI] [PubMed] [Google Scholar]
- 81.Szondy Z, Garabuczi E, Joós G, Tsay GJ, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: Therapeutic implications. Front Immunol. 2014;5:354. doi: 10.3389/fimmu.2014.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furukawa S, Kuwajima Y, Chosa N, Satoh K, Ohtsuka M, Miura H, Kimura M, Inoko H, Ishisaki A, Fujimura A, Miura H. Establishment of immortalized mesenchymal stem cells derived from the submandibular glands of td to mato transgenic mice. Exp Ther Med. 2015;10:1380–1386. doi: 10.3892/etm.2015.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]