Abstract
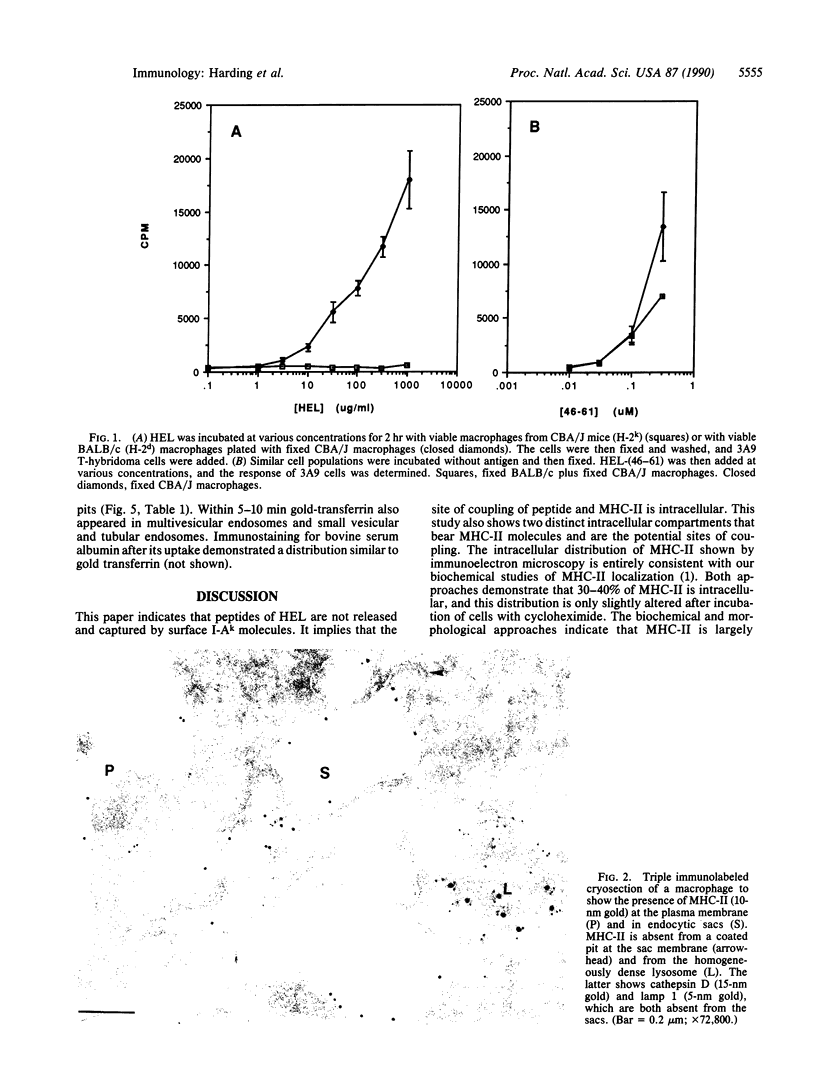
Antigen presentation requires intracellular processing of native antigens to produce immunogenic peptides that bind to major histocompatibility complex class II (MHC-II) molecules. In functional studies of antigen processing by elicited peritoneal macrophages, MHC-II-peptide complexes were formed intracellularly. Immunogenic peptides were not released to bind surface MHC-II molecules. Ultrastructural studies employing immunogold staining in ultrathin cryosections of these macrophages showed large amounts of MHC-II molecules in intracellular sac-like vacuoles in the peripheral cytoplasm; most of these were negative for the lamp 1 lysosomal/endosomal membrane protein and cathepsin D. MHC-II molecules were also present in endosomes containing cathepsin D and lamp 1 as well as previously internalized gold-transferrin. The intracellular pool of MHC-II molecules was only slightly decreased by treatment with cycloheximide for 3 hr, indicating that it consisted mainly of endocytosed, recycling molecules, as opposed to nascent ones. These ultrastructural studies support the notion that there is endocytosis of MHC-II molecules into endocytic compartments, consistent with our earlier biochemical data. Furthermore, we have defined the distinct endocytic compartments that must mediate important functions in antigen processing, including the formation of MHC-II-peptide complexes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Cardinaux F., Nagy Z. A. Competition for antigen presentation in living cells involves exchange of peptides bound by class II MHC molecules. Nature. 1989 Dec 14;342(6251):800–803. doi: 10.1038/342800a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Chen J. W., Cha Y., Yuksel K. U., Gracy R. W., August J. T. Isolation and sequencing of a cDNA clone encoding lysosomal membrane glycoprotein mouse LAMP-1. Sequence similarity to proteins bearing onco-differentiation antigens. J Biol Chem. 1988 Jun 25;263(18):8754–8758. [PubMed] [Google Scholar]
- Davis J. E., Cresswell P. Lack of detectable endocytosis of B lymphocyte MHC class II antigens using an antibody-independent technique. J Immunol. 1990 Feb 1;144(3):990–997. [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Unanue E. R. Turnover of Ia-peptide complexes is facilitated in viable antigen-presenting cells: biosynthetic turnover of Ia vs. peptide exchange. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4230–4234. doi: 10.1073/pnas.86.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J Immunol. 1989 Jan 1;142(1):12–19. [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Stollorz V., Peters P. J., Geuze H. J., Ploegh H. L. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990 Apr 6;61(1):171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Prydz K., Bomsel M., Simons K., Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989 Dec;109(6 Pt 2):3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Gieselmann V., Hasilik A. Antibody to mannose 6-phosphate specific receptor induces receptor deficiency in human fibroblasts. EMBO J. 1984 Jun;3(6):1281–1286. doi: 10.1002/j.1460-2075.1984.tb01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






