Abstract
C-X-C-motif chemokine receptor 4 (CXCR4) is a key factor for tumor growth and metastasis in several types of human cancer. We have recently reported promising first-in-man experience with CXCR4-directed endoradiotherapy (ERT) in multiple myeloma (MM).
Eight heavily pretreated MM patients underwent a total of 10 ERT cycles (7 patients with 1 cycle and a single patient with 3 cycles). ERT was administered in combination with chemotherapy and autologous stem cell support. End points were occurrence and timing of adverse events, progression-free and overall survival.
ERT was overall well tolerated without any unexpected acute adverse events or changes in vital signs. With absorbed tumor doses >30-70 Gy in intra- or extramedullary lesions, significant anti-myeloma activity was observed with 1 patient achieving complete remission and 5/8 partial remission. Directly after ERT major infectious complications were seen in one patient who died from sepsis 22 days after ERT, another patient with high tumor burden experienced lethal tumor lysis syndrome. Median progression-free survival was 54 days (range, 13-175), median overall survival was 223 days (range, 13-313). During follow-up (6 patients available), one patient died from infectious complications, 2/8 from disease progression, the remaining 3/8 patients are still alive.
CXCR4-directed ERT was well-tolerated and exerted anti-myeloma activity even at very advanced stage MM with presence of extramedullary disease. Further assessment of this novel treatment option is highly warranted.
Keywords: Multiple myeloma, PET, CXCR4, theranostics
Introduction
Multiple Myeloma (MM) is the second most common hematological malignancy. It is characterized by the proliferation of a malignant plasma cell clone in the bone marrow, causing peripheral cytopenia and secondary severe organ damage including kidney failure and bone destruction. After decades of minor changes, with most of the available drugs being inefficient 1, the introduction of “novel agents” tremendously improved response rates and survival times of MM patients 2. However, despite the availability of proteasome inhibitors (PI), immunomodulators (IMiD), antibodies or the implementation of autologous and allogeneic stem cell transplantation (SCT), patients still relapse and gain drug resistance. Of note, current treatment regimens, which combine one or two of the novel agents, induce high quality remission after initial treatment in the majority of patients. In relapsed/refractory disease, however, even most intense treatment regimens frequently fail to efficiently reduce the tumor burden and patients eventually succumb to their disease. Moreover, occurrence of extramedullary disease (EMD), as more and more seen in relapsing patients, is considered as a hallmark of ultra-high risk disease in MM. No successful treatment strategy has been established for this patient cohort so far and hardly any prospective data to guide EMD relapse salvage therapy are currently available. Recent retrospective data report low response rates of 24-54% and a median survival of not more than 4 months for patients with soft-tissue related extramedullary relapse 3 demonstrating the urgent need for novel compounds and innovative treatment concepts.
C-X-C-motif chemokine receptor 4 (CXCR4) is overexpressed on MM cells 4, 5 and has been identified as a potential drug target. Wester and co-workers successfully developed a radiolabelled CXCR4-ligand ([68Ga]Pentixafor) for PET imaging 6, 7 that we and others reported as a proof-of-concept for the visualization of CXCR4-expression in patients with hematologic as well as solid malignancies 8-15. Recently, we also reported our first-in-human experience with 177Lu- and 90Y-labelled CXCR4-directed ligand Pentixather, the therapeutic counterpart for CXCR4-targeted endoradiotherapy (ERT), in 3 patients with advanced multiple myeloma 16. In these patients, anti-tumor activity was observed, demanding validation in a larger cohort that we provide in this manuscript. Furthermore, we evaluate the safety profile and more precisely define the anti-myeloma activity of this innovative treatment approach.
Methods
Subjects
Eight patients (5 male, 3 female; aged, 61±6 y) with relapsed, refractory multiple myeloma (rrMM) were included in the study, with 6 of them presenting with ultra-high risk due to extramedullary disease (EMD). All of the included patients in this cohort were heavily pretreated including PIs (all), IMiDs (all) or monoclonal antibody (n=1; median, 4 lines of treatment; range, 2-11). All of them had undergone prior autologous stem cell transplantation. Given the lack of alternative treatment options in this advanced disease stage, experimental CXCR4-directed treatment was offered on a compassionate use basis (German Drug Act, §13,2b) in compliance with §37 of the Declaration of Helsinki. Treatment was approved by the clinical ethics committee of our institution. All subjects gave written informed consent prior to therapy. Except for patient #1 who underwent 3 repeated cycles of ERT, all other patients received a single cycle of ERT, leading to a total of 10 ERT administered. Patient characteristics are depicted in Table 1.
Table 1.
Patient characteristics
| Patient | sex | age | Myeloma type | Disease duration (m) | Pre-Treatment with PI/IMiD/Ab |
Sites of extramedullary disease | Previous auto SCT |
Conditioning Regimen (after ERT) |
Best serological response | Best metabolic response |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/1 | m | 62 | Ig G κ | 18 | +/+/+ | Soft tissue, testis | 1x | Melphalan | PR | PR |
| 1/2 | 20 | 2x | HD-BEAM | PR | PR | |||||
| 1/3 | 21 | 3x | Bortezomib, fludarabine, treosulfan | SD | PD | |||||
| 2 | m | 51 | LC λ | 59 | +/+/- | LN, pleura, soft tissue, testis, PCL | 3x | HD-BEAM | VGPR | CR |
| 3 | f | 66 | LC κ | 53 | +/+/- | LN, leptomeningeal | 4x | Melphalan, Carmustine | n/a | n/a |
| 4 | f | 53 | LC κ | 123 | +/+/- | None | 3x | Treosulfan | PR | PR |
| 5 | m | 65 | Ig A κ | 101 | +/+/- | None | 2x | Treosulfan | PR | n/a |
| 6 | m | 62 | Ig G λ | 65 | +/+/- | Soft tissue | 2x | Treosulfan | SD | n/a |
| 7 | m | 68 | LC λ | 148 | +/+/- | LN, liver | 2x | Treosulfan | SD | PR |
| 8 | f | 58 | LC κ | 16 | +/+/- | LN, pleura, soft tissue | 1x | n/a | n/a | n/a |
Response assessment was performed according to IMWG criteria and/or PET/CT imaging results.
Ab = antibody, auto SCT = autologous stem cell transplantation, CR = complete remission, ERT = endoradiotherapy, f = female, HD-BEAM = high-dose chemotherapy consisting of carmustine, etoposide, cytarabine and melphalan, LC = light chain, IMiD = immunomodulatory drugs, LN = lymph node, m = male, PCL = plasma cell leukemia, PD = progressive disease, PI = proteasome inhibitors, PR = partial response, SD = stable disease, VGPR = very good partial response
PET Imaging
CXCR4-expression was confirmed in all patients by [68Ga]Pentixafor PET/CT. In 8 cases, pre-therapeutically, [18F]FDG-PET/CT was additionally performed as standard of reference for active myeloma lesions. In the remaining 2 cases without EMD present (patient #4 and #5), computed tomography (CT) of the chest, abdomen and pelvis was available.
[68Ga]Pentixafor was prepared as previously described 17. In short, all syntheses were performed in a fully automated, GMP-compliant procedure using a GRP® module (SCINTOMICS GmbH, Fürstenfeldbruck, Germany) equipped with a disposable single-use cassette kit (ABX, Radeberg, Germany). The eluate (68Ga3+ in 0.6 M HCl) of a 68Ge/68Ga-generator (iThemba Labs, Faure, South Africa) was transferred to a cation exchange cartridge, eluted with 5 N NaCl, added to a solution of 20 µg Pentixafor (Scintomics) in HEPES-buffer and heated for 6 minutes at 125 °C. The product was immobilized on a SepPak C18 cartridge, washed with water und eluted with ethanol/water 50/50. The eluate was passed through a sterile filter (0.22 µm) into a sterile vial und diluted with phosphate buffer solution to a total volume of 15 ml. Radiochemical purity was determined by gradient high performance liquid chromatography and thin-layer chromatography. Additionally, the product was tested for ethanol content, pH, radionuclide purity, sterility, and absence of endotoxins.
[18F]Fluoride for radiolabeling was produced via a 18O(p,n)18F reaction on a 16 MeV Cyclotron (GE PETtrace 6; GE Healthcare, Milwaukee, USA) at the interdisciplinary PET centre of the University of Würzburg. [18F]FDG was synthesized in-house on a Fastlab synthesis module using [18F]FDG- Fastlab cassettes (GE Medical Systems, Uppsala, Sweden). All PET scans were performed on a dedicated PET/CT scanner (Siemens Biograph mCT 64; Siemens Medical Solutions, Erlangen, Germany). [18F]FDG- and [68Ga]Pentixafor PET/CT imaging was performed 60 minutes after injection of 43 to 165 MBq (median, 121 MBq) of [68Ga]Pentixafor and 278-300 MBq (median, 298 MBq) of [18F]FDG, respectively. Corresponding CT scans for attenuation correction were acquired using a low-dose CT protocol (20 mAs, 120 keV, a 512 × 512 matrix, 5 mm slice thickness, increment of 30 mm/s, rotation time of 0.5 s, and pitch index of 0.8) including the base of the skull to the proximal thighs. Consecutively, PET emission data were acquired in three-dimensional mode with a 200 × 200 matrix with 2-3 min emission time per bed position. After decay and scatter correction, PET data were reconstructed iteratively with attenuation correction using a dedicated software (Siemens Esoft, Siemens).
[68Ga]Pentixafor positive lesions were visually determined as focally increased tracer retention as compared to surrounding normal tissue or contralateral structures. In order to qualify for ERT, all myeloma lesions (as defined by [18F]FDG-PET/CT or CT, respectively) were required to be [68Ga]Pentixafor positive.
CXCR4-directed endoradiotherapy (ERT)
ERT was preceded in every patient by a pre-therapeutic dosimetry study with a tracer dose of [177Lu]Pentixather to exclude relevant retention in critical organs, to allow safely administrable activities and to estimate the achievable tumor doses. Based on individual dosimetry, patients were treated by intravenous injection of 177Lu- (n=6) or 90Y- (n=4) labelled Pentixather (see Table 1). ERT was performed at a median of 8 days (range, 2-22) after pre-therapy dosimetry. To prevent renal toxicity, 2 L of a solution containing arginine and lysine (25 g/L each) was co-infused in analogy to the joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy 18. Vital signs, complete blood count, and blood chemistry were documented during the infusion and within 7 days after administration. Administered doses were individually measured after each treatment conducted with [177Lu]Pentixather.
Dosimetry
Assessment of the absorbed doses to the kidneys, liver, spleen, bone marrow, and, if applicable, tumorous lesions was performed after each administration of [177Lu]Pentixather by SPECT/CT and planar scintigraphy. For liver and spleen, the mean absorbed doses were measured in large representative volumes within the organs. For kidneys, bone marrow, and tumor, the absorbed doses were deduced from the highest activity concentration measured by SPECT/CT in a contiguous 1 mL volume. All scintigraphic images were acquired with a 20% energy window at 208 keV using double head gamma cameras (Siemens Symbia; Siemens) equipped with medium energy collimators and calibrated by phantom measurements with 177Lu activity standards. Planar whole body scans (matrix 256 x 1024) were taken over several days and regions of interest were drawn for the above listed organs and corresponding background regions. The resulting count statistics were fitted by bi-exponential decay functions in order to approximate the activity kinetics in the organs. The fit functions were integrated and normalized to activity concentrations measured quantitatively by SPECT/CT. SPECT imaging was performed over a rotation of 180° with 3° angular steps (= 2 x 60 frames) at 30 s per projection, matrix size 128 x 128, voxel size 4.8 mm (0.11 cm³). Transmission was measured by low-dose CT and used to reconstruct the SPECT data iteratively by 3D-OSEM (6 subsets, 6 iterations) with corrections for scatter and attenuation.
Safety and Response Assessment
Adverse events (AE) were classified as in the Medical Dictionary for Regulatory Activities; severity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3; http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ ctcaev3.pdf).
Response was defined according to IMWG criteria 19 and/or PET/CT imaging results in analogy to the Lugano 20 and the International Harmonization Project´s 21 criteria. Post-treatment metabolic response evaluation was carried out by [18F]FDG-PET/CT in 5/10 cases (patients #1/1, #1/2, #1/3, #2, and #7) and [68Ga]Pentixafor-PET/CT in 1 case (patient #4; [68Ga]Pentixafor-PET/CT was performed to evaluate the possibility of a further ERT; however, having achieved PR and with no immediate further therapy planned, the patient rejected to undergo another [18F]FDG-PET/CT), respectively. Progression-free survival was defined as the time from ERT until disease progression (transplantation not evaluated as progression), initiation of new antitumor therapy, or death. Patients without events were censored on the last available visit date.
Results
Patient Characteristics
Between June 2014 and March 2016, 8 heavily pre-treated patients with advanced stage rrMM were enrolled with six of them having extramedullary disease relapse, thus representing an ultra-high risk cohort. In all patients intramedullary disease was additionally detectable by FDG-PET. Of interest, baseline FISH from the diagnosis sample did not show high cytogenetic risk with absence of del17p within the cohort. At inclusion, 1 patient (patient #1) suffered from oligosecretory, another (patient #3) from non-secretory disease. On average 14 EMD lesions were detectable on a patient basis (range, 2-20) involving soft tissue (n=4), lymph nodes (n=3), testes (n=2), liver (n=1) and pleura (n=1). Leptomeningeal spread was present in 1 subject.
Of note, in one patient (patient #1) 3 cycles of repeated ERT were performed. This patient experienced early relapse after having achieved partial response to the first ERT applied. Strikingly, a subsequent second cycle of ERT resulted in more sustained reduction of viable myeloma burden. However, eight weeks later, another progression was noted prompting a third cycle of CXCR4-directed ERT and allogeneic SCT but no further tumor response could be induced and progressive disease including the acquisition of additional extramedullary lesions was observed despite therapy.
Pre-therapeutic Dosimetry
The activities of [177Lu]Pentixather administered for the pre-therapeutic dosimetry study and the measured specific absorbed doses in the kidneys, liver, spleen, bone marrow, and tumor are listed in Table 2. The kidneys were the dose-limiting organ for all patients.
Table 2.
Overview of administered activities, time to SCT and achieved/estimated organ and myeloma doses
| Patient | Day | Activity | Limit | Nuclide | SCT | Liver | Spleen | Marrow | Kidneys | Tumor | PFS8 | OS9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GBq 2 | GBq 3 | No.4 | Day 5 | Gy/GBq6 | Gy/GBq6 | Gy/GBq7 | Gy/GBq7 | Gy/GBq7 | Days | Days10 | ||
| #1 | 0 | 0.24 | 177Lu | 0.38 | 0.49 | 0.35 | 1.39 | 3.3 | 29 | 204 | |||
| 6 | 15.2 | 16.5 | 177Lu | 2 | 32 | 0.37 | 0.47 | 0.23 | 0.69 | 3.5 | 41 | ||
| 73 | 4.6 | 90Y | 3 | 14 | 38 | ||||||||
| 122 | 4.5 | 90Y | 4 | 14 | |||||||||
| #2 | 0 | 0.21 | 177Lu | 0.62 | 1.70 | 2.43 | 0.63 | 4.9 | 110 | 115 | |||
| 8 | 6.3 | 7.8 | 90Y | 4 | 14 | ||||||||
| #3 | 0 | 0.20 | 177Lu | 0.79 | 1.74 | 0.71 | 1.08 | 9.5 | 22 | 22 | |||
| 8 | 23.5 | 21.3 | 177Lu | 5 | 14 | 0.56 | 1.40 | 0.41 | 0.53 | 3.0 | |||
| #4 | 0 | 0.20 | 177Lu | 0.95 | 1.00 | 1.55 | 2.53 | 4.6 | 175 | 313 | |||
| 22 | 7.8 | 9.1 | 177Lu | 4 | 20 | 0.75 | 0.81 | 0.97 | 1.53 | 4.8 | |||
| #5 | 0 | 0.21 | 177Lu | 0.85 | 0.8 | 1.08 | 2.25 | 86 | 311 | ||||
| 7 | 9.9 | 10.2 | 177Lu | 3 | 17 | 0.63 | 0.73 | 0.83 | 1.62 | ||||
| #6 | 0 | 0.20 | 177Lu | 0.54 | 0.69 | 0.62 | 2.21 | 0.70 | 67 | 241 | |||
| 14 | 7.6 | 10.4 | 177Lu | 3 | 22 | 0.45 | 0.68 | 0.58 | 1.76 | 0.57 | |||
| #7 | 0 | 0.21 | 177Lu | 0.39 | 1.03 | 0.32 | 1.36 | 5.1 | 120 | 241 | |||
| 14 | 14.6 | 16.9 | 177Lu | 3 | 21 | 0.43 | 0.83 | 0.36 | 0.96 | 4.5 | |||
| #8 | 0 | 0.21 | 177Lu | 1.27 | 1.95 | 5.4 | 3.07 | 17.6 | 13 | 13 | |||
| 2 | 2.6 | 2.1 | 90Y | 2 | n/a | ||||||||
1) Time after the pre-therapeutic dosimetry study; 2) Administered activity;
3) Activity limit calculated from the pre-therapeutic dosimetry to induce 23 Gy kidney dose;
4) Total number of stem cell transplants (SCT); 5) Time of SCT after therapy; 6) Mean absorbed dose;
7) Highest absorbed dose measured in in a contiguous 1 ml volume;
8) Progression-free survival;
9) Overall survival; 10) Censoring date of data on June, 30th, 2016
Individual activities of [177Lu]Pentixather administered for the pre-therapeutic dosimetry study and the measured specific absorbed doses in the kidneys, liver spleen, bone marrow, and tumor are listed for each patients.
If available, the specific absorbed doses measured after treatment with [177Lu]Pentixather are also shown. For the pure ß-emitter 90Y, only estimates for absorbed myeloma doses were derived from pre-therapeutic dosimetry.
Interestingly, patient #6 exhibited significantly reduced CXCR4 expression during pre-therapeutic dosimetry as compared to diagnostic [68Ga]Pentixafor-PET/CT, resulting in achievable myeloma doses per activity administered as low as 0.7 Gy/GBq of 177Lu. Of note, between both diagnostic procedures, therapy with one cycle of bortezomib, bendamustine, dexamethasone as well as dexamethasone, carmustine, etoposide and cytosine, respectively, had been performed without any significant influence on serological myeloma parameters.
Adverse Events
The radionuclide (90Y or 177Lu) to be administered for ERT was chosen depending on tumor burden, biological half-life of the radiopharmaceutical and the associated time interval to SCT. According to these considerations, 90Y was administered in 4/10 treatment cycles, 177Lu in the remaining 6. Activities ranging from 2.6 to 6.3 GBq for 90Y and 7.6 to 23.5 GBq for 177Lu were infused which were calculated to limit the absorbed doses to the kidneys to 23 Gy. The radionuclide administered, the tolerable activity for 23 Gy kidney absorbed dose, and the actually infused activity are listed in Table 2 for each patient. Importantly, during application of endoradiotherapy, no immediate toxic or allergic reactions or changes in vital signs occurred. Furthermore, no acute adverse renal, hepatic or cardiac events were observed. As expected, ERT resulted in myeloablation in all patients after a median of 11 days (range, 7-23 d).
One patient with very high tumor burden and high tumor proliferation (patient #8) experienced tumor lysis syndrome two days after ERT with hyperuricemia, hyperkalemia and subsequent acute kidney injury. Despite intense supportive care this patient died 13 days after ERT. Patient #3 developed infectious complications during aplasia and died from sepsis 22 days after ERT (8 days after SCT).
As part of conditioning prior to SCT (after ERT), patients were treated with high-dose chemotherapy including treosulfan (n=4), high-dose BEAM (n=2), a combination of melphalan and carmustine (n=1), a combination of bortezomib, fludarabine and treosulfan (n=1) and melphalan (n=1).
Autologous (n=8) and allogeneic (n=1) SCT was performed after a median of 21 days (177Lu, range, 14-32 d) and 14 days (90Y), respectively when the residual dose in the medullary cavities was reliably below 750 mGy and resulted in successful engraftment in all cases. Median times from stem cell infusion to engraftment (as defined as the first 3 days with a neutrophil count > 0.5x109/L) ranged from 7 to 30 days (median, 17 days), respectively.
Of note, the patient undergoing 3 cycles of ERT successfully engrafted after 30, 16 and 13 days, respectively. With successful transplant engraftment after each cycle, no cumulative bone marrow or other organ toxicity could be observed.
Dosimetry during ERT
The specific absorbed doses measured after treatment with [177Lu]Pentixather are shown in Table 2. As compared to the corresponding data in the pre-therapeutic dosimetry, the specific absorbed doses were reduced during ERT to on average 86%, 88%, 78%, and 82% in liver, spleen, bone marrow, and tumors, respectively. The specific kidney absorbed doses were 64% ± 13% of the pre-therapeutic values which can be explained by the nephro-protective medication during ERT.
Anti-Myeloma Activity
Response to ERT was assessed after 8/10 treatments (in patients #1/1, #1/2, #1/3, #2, #4, #5, #6, #7). Patients #3 and #8 died within the first three weeks after ERT and were therefore not available for analysis; thus 6/8 patients could be assessed.
CXCR4-directed ERT demonstrated anti-myeloma activity after ERT and SCT. According to IMWG criteria, best serological responses included a very good partial response (VGPR) after 1/10 treatments (patient #2), partial response (PR) after 4/10 (patients #1/1, #1/2, #4, #5), and stable disease after 3/10 (patients #1/3, #6 and #7) ERT (and subsequent SCT).
Regarding imaging-based response assessment (available after 6/10 treatments), the patient with VGPR (patient #2) presented with a complete metabolic response after ERT. PR was recorded after 4/10 treatments (patient #1/1, #1/2, #4, and #7; Figure 1) and PD was observed after the third ERT application in patient #1 (patient #1/3). The overall response rate was 83% (5/6 patients; 6/8 treatments [75%]). Until censoring of data on June 30th, 2016, 1 patient (patient #1) died from infectious complications (d+204), 2/8 from disease progression (patients #2 and #4; d+115 and d+313, respectively), the remaining 3/8 patients (patients #5, #6, #7; d+241, d+241 and d+311, respectively) are still alive. For the entire cohort, median PFS was 54 days (range, 13-175), and median OS was 223 days (range, 13-313), respectively. Excluding the 2 patients who died from sepsis and tumor lysis syndrome, median PFS and OS were 77 days (range, 29-175) and 241 days (range, 115-313), respectively.
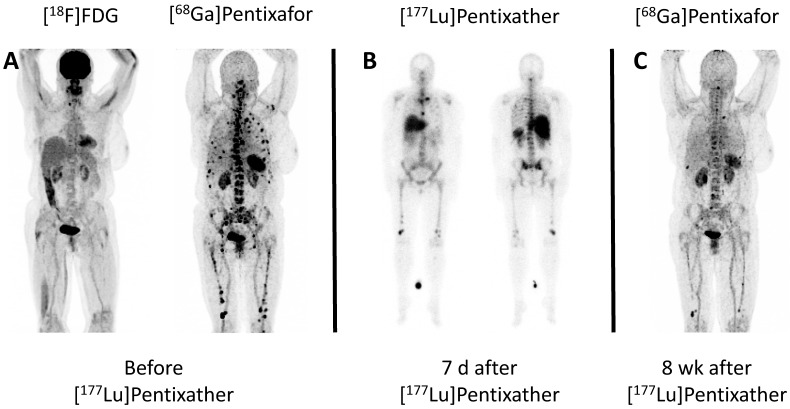
Figure 1.
Example of partial response to CXCR4-directed treatment with [177Lu]Pentixather. A: Maximum Intensity Projections (MIP) of [68Ga]Pentixafor- and [18F]FDG-PET/CT of patient #4 prior to Pentixather therapy indicating high CXCR4-expression in multiple intramedullary [18F]FDG-negative myeloma lesions. B: Scintigraphic images of patient #4 7 days after administration of 7.8 GBq [177Lu]Pentixather confirming the long-lasting binding to the CXCR4-target after treatment. The patient is seen from ventral (left) and dorsal (right). C: MIP of [68Ga]Pentixafor-PET/CT 8 weeks after [177Lu]Pentixather therapy displaying partial response with disappearance of most of the myeloma manifestations. In concordance, serological response was assessed as stable disease.
Treatment efficiency correlated with absorbed tumor doses: A single patient (patient #6) achieved maximum myeloma doses of not more than 4 Gy (due to unexplained downregulation of CXCR4 prior to ERT) and experienced disease progression shortly after ERT. In all other patients, doses ≥30 Gy (up to 71 Gy) could be calculated. Interestingly, due to intense tracer retention in patient #8, doses as high as 17.6 Gy/GBq administered activity were obtained (Figure 2).
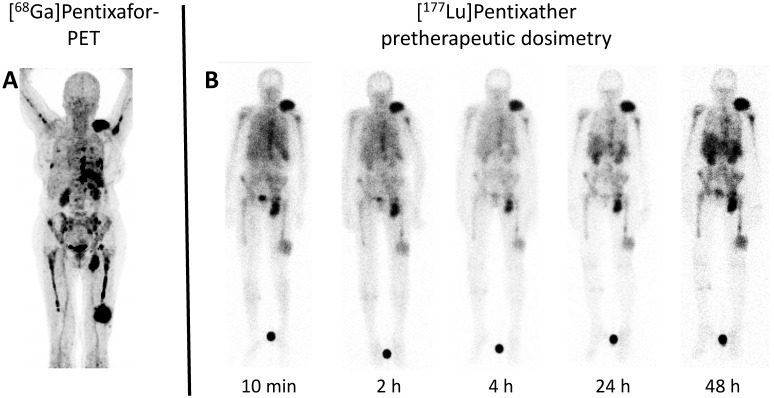
Figure 2.
Example of pre-therapeutic dosimetry for individual treatment tailoring. A: Maximum Intensity Projection (MIP) of [68Ga]Pentixafor-PET/CT of patient #8 prior to Pentixather therapy indicating high CXCR4-expression in multiple intra- and extramedullary lesions. B: Scintigraphic images (seen from ventral) of the patient #8 during different time points of pre-Pentixather dosimetry confirming intense and long-lasting binding to the CXCR4-target. Pre-therapeutic dosimetry is performed to calculate estimated kidney doses (as the organ of risk) and to derive an estimate of achievable myeloma doses. Maximum myeloma doses of up to 17.6 Gy/GBq could be achieved.
Discussion
This paper summarizes the currently available experience with CXCR4-targeted radionuclide therapy using 177Lu- and 90Y-labeled Pentixather in patients with multiple myeloma. This novel treatment option emerges as a feasible method to induce anti-tumor response in highly refractory, even EMD relapsed MM patients. Furthermore, we are first to demonstrate feasibility of repeated CXCR4-targeted ERT, without accumulation of toxicity.
It has to be emphasized that no successful salvage therapy after extramedullary rrMM relapse has been established so far and that published data are limited to a handful of retrospectively investigated patients. In these patients of highest risk, despite most intense treatments applied, response rates are being described as consistently poor (ranging from 24-54%) and overall survival times are disappointingly short (4-7 months) 3, 22, 23. Similarly in our cohort, time to disease relapse was relatively short in all studied patients with median progression-free and overall survival times of 54 and 223 days, respectively. It is to speculate if more durable responses would have been achieved by additional consolidation or maintenance therapy after tumor debulking CXCR4 directed ERT or at earlier disease stage.
However, intensified stem cell boost containing therapy combined with overall well- tolerated ERT induced significant tumor response (>=PR) in the majority of patients. Of note, treatment efficiency correlated with achievable absorbed tumor doses: The single patient with the lowest achieved target dose of approximately only 4 Gy experienced disease progression after ERT, whereas myeloma doses ≥30 Gy (up to 71 Gy) resulted in partial or complete (metabolic) responses with PFS as long as 175 days. Interestingly, we were able to demonstrate an influence of concomitant conventional drug regiments like chemotherapy and/or proteasome inhibitors on the expression of the CXCR4 cell surface receptor expression: Visually, in patient #6, intermittent therapy with one cycle of bortezomib, bendamustine, dexamethasone as well as dexamethasone, carmustine, etoposide and cytosine, respectively, significantly reduced CXCR4 expression during pre-therapeutic dosimetry as compared to diagnostic [68Ga]Pentixafor-PET/CT -a finding which could already be observed in small cell lung cancer 12- partly explaining the low tumor doses achieved in this patient (≤4 Gy). One might speculate that surface expression of CXCR4 is a dynamic process that is influenced by therapeutic interventions in a time and dose dependent manner. Whereas in vitro experiments on the influence of chemotherapy on receptor expression are already ongoing in our laboratory, future studies to further investigate therapy-induced down- and -preferably- up-regulation of CXCR4 are warranted.
In parallel with the previously published data 16, application of endoradiotherapy was safe and generally well tolerated. As expected, ERT resulted in myeloablation in all patients and was therefore combined with additional high-dose chemotherapy and subsequent autologous stem cell rescue. One patient developed fatal tumor lysis syndrome but in the remaining patients, apart from expected hematotoxicity, no acute adverse renal, hepatic or cardiac events specifically associated with Pentixather were observed. Classical complications of autologous SCT like mucositis were not recorded. No toxic or allergic reactions occurred. The higher amount of substance and nephro-protection by peri-therapeutic hydration and amino acid infusion reduced the radiation doses to the kidneys to 64% of the values estimated in pre-therapeutic dosimetry performed without nephro-protective medication.
Of note, only one patient within the heavily pre-treated, largely extramedullary high-risk cohort experienced major infectious complications. Acute tumor lysis syndrome occurred in the subject with extensive myeloma burden and the highest radiation dose per activity administered. Therefore, a close collaboration between hematologists, nuclear medicine physicians and medical physicists is mandatory to optimize patient management. Also, the radionuclide used should be chosen carefully: Whereas [177Lu] offers the advantage of post-therapeutic imaging due to its γ-radiation, the time interval from ERT to SCT itself can last up to 4 weeks (due to its -compared to 90Y- longer half-life of 6.7 days), thereby increasing the risk of infectious complications. In contrast, apart from its higher β-energy, [90Y] reliably allows SCT after 14 days, but lacks the diagnostic option. Pre-therapy dosimetry can aid in decision-making in those cases.
Alternatively, ERT using α-emitters like Actinium-225 (225Ac) which are characterized by a high linear energy transfer, a higher fraction of nuclear double strand breaks per track length and consequently a higher biological effectiveness might further improve anti-myeloma activity, especially in patients not satisfactorily responding to therapy with β-emitters. The concept of re-treating non-β-responders with α-emitting ERT is currently investigated for prostate cancer patients and might also be transferable to MM in future studies.
We acknowledge the limitations of our retrospective study, the number of included patients, the use of different radionuclides as well as variable concomitant and subsequent treatment protocols. Nevertheless, our data clearly demonstrate that CXCR4-directed, ERT-intensified stem cell boost containing therapy is feasible, repeatable and able to induce significant tumor response (>=PR) even in highly refractory, EMD relapsed MM patients. Further investigation including application at earlier diseases stages as well as in other malignancies is needed; a pilot trial investigating ERT in relapsed/refractory lymphoma prior to allogeneic SCT is ongoing at our center. Furthermore, the value of chemokine receptor CXCR4-directed theranostics of advanced lymphoproliferative cancers by radiopeptide-based imaging and therapy will be assessed in a multicenter prospective phase I/II study (COLPRIT trial, Eudra-CT 2015-001817-28).
Acknowledgments
We thank Gabriele Bohley, Cornelia Schubert, Monika Siemer, Simone Seifert, Michael Schulze-Glück (members of the nuclear medicine team), Inge Grelle and the whole staff of the ward M63 for their support and assistance.
This publication was funded in part by the Wilhelm-Sander-Stiftung (grant no. 2013.906.1) and the Deutsche Forschungsgemeinschaft (DFG).
Authorship Contributions
Initials: Constantin Lapa (CL), Ken Herrmann (KH), Heribert Hänscheid (HH), Katharina Lückerath (KL), Margret Schottelius (MS), Malte Kircher (MK), Rudolf A Werner (RAW), Martin Schreder (MaS), Andreas Schirbel (AS), Samuel Samnick (SSa), Saskia Kropf (SKr), Stefan Knop (SK), Andreas K. Buck (AKB), Hermann Einsele (HE), Hans-Juergen Wester (HJW), Klaus Martin Kortüm (KMK).
Conception and design: CL, KH, HH, HJW, AKB, HE, KMK.
Development of methodology: CL, KH, HH, KL, As, SSa, MS, SKr.
Acquisition of data: CL, KH, HH, MK, RAW, MaS.
Analysis and interpretation of data: CL, KH, HH, KL, AS, SSa, AKB, HE, HJW, KMK.
Writing, review and/or revision of the manuscript: all authors.
Administrative, technical, or material support: MS; Mas; AS, SSa, SKr, SK, HJW.
Supervision: SSa, AKB, HE, HJW.
References
- 1.Kortuem KM, Zidich K, Schuster SR, Khan ML, Jimenez-Zepeda VH, Mikhael JR. et al. Activity of 129 single-agent drugs in 228 phase I and II clinical trials in multiple myeloma. Clinical lymphoma, myeloma & leukemia. 2014;14:284–90. doi: 10.1016/j.clml.2013.12.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S. et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L. et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99:360–4. doi: 10.3324/haematol.2013.094409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 5.Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. OncoTargets and therapy. 2013;6:1347–61. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demmer O, Gourni E, Schumacher U, Kessler H, Wester HJ. PET imaging of CXCR4 receptors in cancer by a new optimized ligand. ChemMedChem. 2011;6:1789–91. doi: 10.1002/cmdc.201100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourni E, Demmer O, Schottelius M, D'Alessandria C, Schulz S, Dijkgraaf I. et al. PET of CXCR4 expression by a (68)Ga-labeled highly specific targeted contrast agent. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011;52:1803–10. doi: 10.2967/jnumed.111.098798. [DOI] [PubMed] [Google Scholar]
- 8.Lapa C, Luckerath K, Kleinlein I, Monoranu CM, Linsenmann T, Kessler AF. et al. (68)Ga-Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression in Glioblastoma. Theranostics. 2016;6:428–34. doi: 10.7150/thno.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapa C, Luckerath K, Rudelius M, Schmid JS, Schoene A, Schirbel A. et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression in small cell lung cancer - initial experience. Oncotarget. 2016;7:9288–95. doi: 10.18632/oncotarget.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vag T, Gerngross C, Herhaus P, Eiber M, Philipp-Abbrederis K, Graner FP. et al. First Experience with Chemokine Receptor CXCR4-Targeted PET Imaging of Patients with Solid Cancers. J Nucl Med. 2016;57:741–6. doi: 10.2967/jnumed.115.161034. [DOI] [PubMed] [Google Scholar]
- 11.Abbrederis K, Hermann K, Knop S, Schottelius M, Eiber M, Lueckerath K, In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wester HJ, Keller U, Schottelius M, Beer A, Philipp-Abbrederis K, Hoffmann F. et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5:618–30. doi: 10.7150/thno.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herhaus P, Habringer S, Philipp-Abbrederis K, Vag T, Gerngross C, Schottelius M. et al. Targeted positron emission tomography imaging of CXCR4 expression in patients with acute myeloid leukemia. Haematologica. 2016;101:932–40. doi: 10.3324/haematol.2016.142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bluemel C, Hahner S, Heinze B, Fassnacht M, Kroiss M, Bley TA. et al. Investigating the Chemokine Receptor 4 as Potential Theranostic Target in Adrenocortical Cancer Patients. Clin Nucl Med. 2017;42:e29–e34. doi: 10.1097/RLU.0000000000001435. [DOI] [PubMed] [Google Scholar]
- 15.Lapa C, Schreder M, Schirbel A, Samnick S, Kortum KM, Herrmann K. et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - Comparison to [18F]FDG and laboratory values. Theranostics. 2017;7:205–12. doi: 10.7150/thno.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hanscheid H. et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J Nucl Med. 2016;57:248–51. doi: 10.2967/jnumed.115.167361. [DOI] [PubMed] [Google Scholar]
- 17.Martin R, Juttler S, Muller M, Wester HJ. Cationic eluate pretreatment for automated synthesis of [(6)(8)Ga]CPCR4.2. Nuclear medicine and biology. 2014;41:84–9. doi: 10.1016/j.nucmedbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O'Dorisio MS. et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. European journal of nuclear medicine and molecular imaging. 2013;40:800–16. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K. et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:3059–68. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A. et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 22.Rasche L, Strifler S, Duell J, Rosenwald A, Buck A, Maeder U. et al. The lymphoma-like polychemotherapy regimen "Dexa-BEAM" in advanced and extramedullary multiple myeloma. Annals of hematology. 2014;93:1207–14. doi: 10.1007/s00277-014-2023-2. [DOI] [PubMed] [Google Scholar]
- 23.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127:971–6. doi: 10.1182/blood-2015-07-635383. [DOI] [PubMed] [Google Scholar]