Abstract
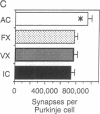
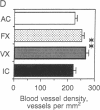
The role of the cerebellar cortex in motor learning was investigated by comparing the paramedian lobule of adult rats given difficult acrobatic training to that of rats that had been given extensive physical exercise or had been inactive. The paramedian lobule is activated during limb movements used in both acrobatic training and physical exercise. Acrobatic animals had greater numbers of synapses per Purkinje cell than animals from the exercise or inactive groups. No significant difference in synapse number or size between the exercised and inactive groups was found. This indicates that motor learning required of the acrobatic animals, and not repetitive use of synapses during physical exercise, generates new synapses in cerebellar cortex. In contrast, exercise animals had a greater density of blood vessels in the molecular layer than did either the acrobatic or inactive animals, suggesting that increased synaptic activity elicited compensatory angiogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker R. L., Cragg B. G. Estimation of the number of synapses in a volume of nervous tissue from counts in thin sections by electron microscopy. J Neurocytol. 1974 Dec;3(6):725–735. doi: 10.1007/BF01097194. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. E., Polinsky M., Greenough W. T. Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiol Aging. 1989 Jul-Aug;10(4):353–358. doi: 10.1016/0197-4580(89)90048-1. [DOI] [PubMed] [Google Scholar]
- Black J. E., Sirevaag A. M., Greenough W. T. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987 Dec 29;83(3):351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Braendgaard H., Gundersen H. J. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods. 1986 Oct;18(1-2):39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Chang F. L., Greenough W. T. Lateralized effects of monocular training on dendritic branching in adult split-brain rats. Brain Res. 1982 Jan 28;232(2):283–292. doi: 10.1016/0006-8993(82)90274-8. [DOI] [PubMed] [Google Scholar]
- Chen S., Hillman D. E. Plasticity of the parallel fiber-Purkinje cell synapse by spine takeover and new synapse formation in the adult rat. Brain Res. 1982 May 27;240(2):205–220. doi: 10.1016/0006-8993(82)90217-7. [DOI] [PubMed] [Google Scholar]
- Collins R. C., Santori E. M., Der T., Toga A. W., Lothman E. W. Functional metabolic mapping during forelimb movement in rat. I. Stimulation of motor cortex. J Neurosci. 1986 Feb;6(2):448–462. doi: 10.1523/JNEUROSCI.06-02-00448.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M., Beaulieu C. An empirical assessment of stereological formulae applied to the counting of synaptic disks in the cerebral cortex. J Comp Neurol. 1985 Jan 8;231(2):175–179. doi: 10.1002/cne.902310205. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. An instruction-selection theory of learning in the cerebellar cortex. Brain Res. 1977 May 27;127(2):327–352. doi: 10.1016/0006-8993(77)90550-9. [DOI] [PubMed] [Google Scholar]
- Floeter M. K., Greenough W. T. Cerebellar plasticity: modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979 Oct 12;206(4415):227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Fujita M. Simulation of adaptive modification of the vestibulo-ocular reflex with an adaptive filter model of the cerebellum. Biol Cybern. 1982;45(3):207–214. doi: 10.1007/BF00336193. [DOI] [PubMed] [Google Scholar]
- Gilbert P. F., Thach W. T. Purkinje cell activity during motor learning. Brain Res. 1977 Jun 10;128(2):309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Glick R., Bondareff W. Loss of synapses in the cerebellar cortex of the senescent rat. J Gerontol. 1979 Nov;34(6):818–822. doi: 10.1093/geronj/34.6.818. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Juraska J. M., Volkmar F. R. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979 Jul;26(3):287–297. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Larson J. R., Withers G. S. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985 Sep;44(2):301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., McDonald J. W., Parnisari R. M., Camel J. E. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986 Aug 13;380(1):136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Hillman D. E., Chen S. Vulnerability of cerebellar development in malnutrition-II. Intrinsic determination of total synaptic area on purkinje cell spines. Neuroscience. 1981;6(7):1263–1275. doi: 10.1016/0306-4522(81)90186-x. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper R. M., Harvey R. J. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988 Aug 8;274(2):168–177. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- Pysh J. J., Weiss G. M. Exercise during development induces an increase in Purkinje cell dendritic tree size. Science. 1979 Oct 12;206(4415):230–232. doi: 10.1126/science.482938. [DOI] [PubMed] [Google Scholar]
- Ruela C., Matos-Lima L., Sobrinho-Simões M. A., Paula-Barbosa M. M. Comparative morphometric study of cerebellar neurons. II. Purkinje cells. Acta Anat (Basel) 1980;106(2):270–275. doi: 10.1159/000145190. [DOI] [PubMed] [Google Scholar]
- Santori E. M., Der T., Collins R. C. Functional metabolic mapping during forelimb movement in rat. II. Stimulation of forelimb muscles. J Neurosci. 1986 Feb;6(2):463–474. doi: 10.1523/JNEUROSCI.06-02-00463.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambes G. M., Gibson J. M., Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15(2):94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Turner A. M., Greenough W. T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985 Mar 11;329(1-2):195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Volkmar F. R., Greenough W. T. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972 Jun 30;176(4042):1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Wesa J. M., Chang F. L., Greenough W. T., West R. W. Synaptic contact curvature: effects of differential rearing on rat occipital cortex. Brain Res. 1982 Jun;256(2):253–257. doi: 10.1016/0165-3806(82)90049-9. [DOI] [PubMed] [Google Scholar]
- Yeo C. H., Hardiman M. J., Glickstein M. Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behav Brain Res. 1984 Sep;13(3):261–266. doi: 10.1016/0166-4328(84)90168-2. [DOI] [PubMed] [Google Scholar]