Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and one of the most lethal human cancers. Inflammation is a critical component in PDAC initiation and progression. Inflammation also contributes to the aggressiveness of PDAC indirectly via induction of epithelial-mesenchymal transition (EMT), altogether leading to enhanced resistance to chemotherapy and poor survival rates. This review gives an overview of the key pro-inflammatory signaling pathways involved in PDAC pathogenesis and discusses the role of inflammation in induction of EMT and development of chemoresistance in patients with PDAC.
Keywords: Inflammation, PDAC, EMT, chemoresistance
Introduction
The year 2015 witnessed a surge in the estimated new cases of pancreatic cancer to 48 960 and the associated 40 560 deaths in the United States, making it the third leading cause of cancer deaths.1 Moreover, 1 in 67 American is at a risk of pancreatic cancer. Pancreatic ductal adenocarcinoma (PDAC), a predominant histologic subtype making 90% of all pancreatic cancers, exhibits local invasion and distant metastasis during early disease stages that directly correlate with an extremely poor prognosis and an overall survival rate of only 5%.2 At the time of diagnosis, 80% of patients are considered inoperable, and surgery is the only hope for the remaining 20%. Pancreatic ductal adenocarcinoma postsurgical 5-year survival rates are significantly low spanning from 15% to 20% with most of the patients dying due to local recurrence or metastasis.3 Nonsurgical approaches have been attempted in advanced-stage PDAC via targeting tumor growth using adjuvant chemotherapies or chemoradiotherapy (CRT) in combination with gemcitabine, 5-fluorouracil (5-FU), cisplatin, erlotinib, or interferon alfa-2b. This approach demonstrated improved prognosis but the curative effects are limited.4–8 Poor prognosis of PDAC is attributed to anatomic and biological reasons. Pancreatic ductal adenocarcinoma–associated inflammation9 and subsequent epithelial-mesenchymal transition (EMT)10 are key factors in the development of chemoresistance in patients with PDAC, resulting in failure of therapy.11
Inflammation and PDAC: The Underlying Mechanisms
Interleukin 6-STAT3 signaling pathway
Under inflammatory conditions, the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) induce secretion of interleukin 6 (IL-6) in myeloid cells, a process known as trans-signaling where IL-6 forms a complex with soluble IL-6 receptors, which mediates the effects of secreted IL-6. Interleukin 6 induces phosphorylation of signal transducer and activator of transcription 3 (STAT3) and promotes synthesis of the neutrophil attractant CXCL1 in pancreatic acinar cells.12 In addition to IL-6, multiple growth factors and pro-inflammatory cytokines are involved in mediating STAT3 phosphorylation.13 As opposed to normal pancreatic microenvironment, in PDAC, tyrosine phosphorylation triggers STAT3 activation and nuclear translocation leading to the transcription of numerous target genes involved in inflammation as well as stem cell renewal.14–16 STAT3 plays a vital role in the development of acinar-to-ductal metaplasia (ADM) lesions; in some instances, these ADM lesions may develop into pancreatic cancer.17 The role of STAT3 as an inflammatory mediator of the development of pancreatic precursor lesion formation was observed in vitro, and in vivo studies confirmed its role in the development of preneoplastic lesions.18–21 Moreover, several studies using pancreatic cell lines and murine animal models highlighted the critical role of STAT3 in driving cancer progression at different stages.18 Corcoran et al18 demonstrated that STAT3 is vital both for the formation of precursor lesions (ie, ADM, pancreatic intraepithelial neoplasia [PanIN]) and progression to PDAC. Another study showed that STAT3 contributes to PDAC initiation by enhancing the development of prepancreatic cancer lesions, cell proliferation, and inflammatory responses associated with metaplasia.19 Fukuda et al19 validated that STAT3 overexpressed in the epithelial cells after cerulein-induced inflammation in a KrasG12D mouse model assists in the initialization of tumor development and progression. However, STAT3 inhibition attenuates precursor lesion formation, cell proliferation and enhances apoptosis.19 In addition, the loss of STAT3 in the epithelial tissue reduces inflammatory cell infiltration and expression of inflammatory cytokines, indicating that STAT3 does not only influence the proliferative and dedifferentiated state of epithelial cells but also regulate inflammatory processes associated with metaplasia.19 In an extension of this study, Lesina et al20 identified the myeloid origin cells as a source of pro-inflammatory cytokine IL-6 that activates STAT3 in the pancreas and nourishes the formation and progression of PanIN lesions.20 The recognition of this mechanism endorses the role of the inflammatory microenvironment in the development of PDAC in mouse models and stands true for human PDAC based on the analysis of human PDAC specimen and patient data. Increased levels of mitochondrial pSTAT3 enhance the pool of available adenosine triphosphate and increase cellular proliferation.22
NF-κB signaling pathway
Nuclear factor κB is a key transcription factor that regulates inflammation and thus plays a critical role in the development of pancreatitis and pancreatic carcinogenesis.23 Under normal physiological conditions in pancreas, the IκB family of inhibitory proteins (IκB-α, IκB-β, IκB-γ, IκB-ε, Bcl-3, p105/NF-κB1, and p100/NF-κB2) keeps the NF-κB signaling pathway in an inactive state by sequestering the regulatory subunits of NF-κB in the cytoplasm.24–27 However, under the influence of microbial or viral infections or pro-inflammatory cytokines, the IκB kinase (IKK) complex is activated and phosphorylates the IκB proteins28 leading to its ubiquitination and subsequent degradation by the 26S proteasomal system.29 This allows the regulatory subunits of NF-κB to translocate to the nucleus and regulate the transcription of various genes responsible for survival and inflammation.30,31 The activation of NF-κB pathway is one of the early events in pancreatitis where it promotes the pro-inflammatory response through the upregulation of inflammatory genes in addition to boosting antiapoptotic genes32–34 assisting pancreatic cancer cells in evading apoptosis.35,36 Nuclear factor κB delivers its antiapoptotic effects on pancreatic cancer cells by upregulation of the antiapoptotic gene B-cell lymphoma extra large (Bcl-xL) and the cell cycle gene cyclin D1.37 Another report demonstrates that low expression of the NF-κB subunit p65 in pancreatic cancer cells leads to downregulation of the antiapoptotic gene B-cell lymphoma 2 (Bcl-2), cyclin D1, vascular endothelial growth factor (VEGF) in addition to activation of caspase-3 leading to growth attenuation in the pancreatic cancer cell line BxPC-3.38 Nuclear factor κB seems to act downstream of the epidermal growth factor receptor (EGFR) because EGFR pathway inhibition in the pancreatic cancer cell line MDA Panc-28 results in lesser NF-κB binding activity and downregulation of the antiapoptotic genes Bcl-xL and Bfl-1.39
Recently, it was reported that persistent activation of NF-κB in pancreatic acinar cells leads to the development of chronic pancreatitis characterized by severe pancreatic damage, immune cell infiltration, and fibrosis.40 Another study showed that the deletion of IKK, IKK2, in all pancreatic epithelial cells averts the development of PanIN lesions in PdxCre/+, LSL-KrasG12D/+ mice.41 IκB protein is a substrate of β-TrCP that encodes a member of the F-box protein family and plays an important role in regulating cell cycle checkpoints.42 High levels of β-TrCP1 and constitutive activation of NF-κB are hallmarks of chemoresistant PDAC cell lines compared with chemosensitive PDAC cell lines. Overexpression of β-TrCP1 in chemosensitive PDAC cell lines results in enhanced NF-κB activity and reduced sensitivity to chemotherapy drugs, whereas small interfering RNA–dependent knockdown of β-TrCP1 in chemoresistant PDAC cell lines attenuates NF-κB activation and chemoresistance.43 Nuclear factor κB seems to enhance the development of chronic pancreatitis, pancreatic precursor lesions, and their transformation to invasive PDAC at least in part through mediating the interplay between oncogenic Kras signaling and inflammatory responses.40,44
Pancreatic ductal adenocarcinoma is believed to be mainly originated from the pancreatic duct cells. Nevertheless, under the activation mutation of KRasG12D, during pancreatitis, acinar cells can go through ADM and form duct cells and eventually PanIN and PDAC.45,46 Hence, PDAC can also originate from acinar cells by means of ADM.45,46 Mitogen-activated protein kinase (MAPK), Wnt, Notch, and PI3K/Akt signaling are involved in this acinar transdifferentiation process. Moreover, during this transdifferentiation to ADM, acinar cells lose their grape-like phenotype and alter the transcriptome from acinar-like (carboxypeptidase, amylase, elastase, and Mist expression) to duct-like (expressing cytokeratin-19, 20, and carbonic anhydrase II).45–47 Also, in vivo studies have demonstrated that both acute and chronic pancreatitis can lead to ADM.48,49 These findings complement that chronic pancreatitis may be one of the etiologic factors of pancreatic cancer.45,50
In addition, mutant Kras mouse model system supports the idea that ADM might be a prerequisite for PanIN and PDAC development.47,51 Furthermore, inflammation is critical in mediating tumorigenesis was demonstrated in an in vivo study where the acceleration to PDAC lesions was seen with the chronic administration of cerulein to mutant Kras mice.52 Fascinatingly, in mice, direct targeting of acinar cells with KrasG12D is sufficient for spontaneous transformation of acinar cells to PanIN lesions even in the absence of injury or inflammation.53 However, the development of PanIN lesions in vivo may involve the emergence of a progenitor population that is either an indirect or direct precursor to cells that will contribute to a PanIN.54 This progenitor population expresses Pdx1, which is normally low or absent in ductal cells.54 It is possible that a resident progenitor population exists among ductal cells or centroacinar cells, which undergoes neoplastic transformation without ADM. The existence of such a population is suggested by the finding that a subset of adult mouse centroacinar cells/terminal duct cells harbor high aldehyde dehydrogenase isoform 1 (ALDH1) enzymatic activity, which is important in retinoic acid metabolism and has been associated with stem and progenitor cells in a variety of tissue types.54,55
Inhibition of the MAPK and NF-κB survival pathways with U0126 and caffeic acid phenethyl ester (CAPE), respectively, potently blocks pancreatic tumor growth without inducing apoptotic death. Interestingly, apoptosis was induced by U0126 and CAPE after inhibition of autophagy in a caspase-independent manner in Panc-1 cells and in a caspase-dependent manner in MiaPaCa-2 cells.56
Transforming growth factor β signaling pathway
Transforming growth factor β (TGF-β), a secreted anti-inflammatory cytokine that regulates apoptosis, cell growth, and differentiation, has been associated with advanced tumor stages57–59 where TGF-β plays an antitumorigenic role via restricting cell growth and enhancing apoptosis. On ligand binding, TGF-β receptors type I (TGF-βR1) and type II (TGF-βR2) undergo heterodimerization. The TGF-βR2 phosphorylates TGF-βR1 kinase domain triggering phosphorylation and activation of various isoforms of SMAD proteins.60 Phosphorylated SMAD shuttles to the nucleus and stimulates transcription of target genes responsible for tumor suppression.61
Similar to its overall tumor suppressive roles under homeostatic conditions, TGF-β signaling inhibits cell growth in early stages of pancreatic cancer and in a number of pancreatic cancer cell lines such as Colo-357.62 However, during late stages of pancreatic cancer, TGF-β signaling is dysregulated on multiple levels. Defects in TGF-β receptors and mutations in SMADs have been observed in numerous pancreatic cancer cell lines.63 These defects result in the emergence of an opposite role of TGF-β signaling where it promotes tumorigenesis through enhancing cancer cell growth, survival, invasion, and metastasis leading to reduced survival of patients with pancreatic cancer.61,64–66 The defective response of TGF-β signaling following TGF-β stimulation has been confirmed in several pancreatic cancer cell lines including Panc-1, MiaPaCa, and BxPC3 by 3[H] thymidine incorporation, and TGF-β–sensitive reporter assays. Along the same line, treatment of Panc-1 and IMIM-PC1 cells with recombinant TGF-β enhances their invasiveness, an effect that is completely blocked in the presence of TGF-β–neutralizing antibody. Transforming growth factor β–induced invasiveness could be attributed at least in part to the enhanced expression of matrix metalloproteinase 2 (MMP2) and the urokinase plasminogen activator (uPA) system in Panc-1 and IMIM-PC1 cell lines.67 Although SMAD2 and SMAD3 do not seem to be part of the dysregulated TGF-β system in pancreatic cancer, SMAD4 seems to be directly involved in the malfunctional response of TGF-β. Introducing SMAD4 into the SMAD4 homozygous-deficient pancreatic cell line, BxPC3 restores responsiveness to TGF-β.68 Similarly, inhibition of NF-κB pathway impairs invasiveness of BxPC3 and Capan-1 cells only on restoration of SMAD4 expression, indicating the downstream role of SMAD4 in NF-κB signaling.69 Kindlin-2, a target protein that is upregulated by TGF-β1 in PDAC cells, is another mediator of TGF-β1–induced tumorigenic effects, where it enhances PDAC cell growth, migration, and invasion and promotes overall PDAC progression via downregulation of HOXB9 and E-cadherin.70 In addition, SMAD371 and SMAD472 together contribute to TGF-β1–induced invasiveness in PDAC cells by inducing expression of EMT-associated transcription factors and subsequent phenotypic changes.
Various tumor-stroma interactions have been reported of having the capability to foster pancreatic cancer cell invasion and metastasis. Growth factors that have been derived from cancer cells, mainly TGF-βs, along with fibroblast growth factors (FGFs), platelet-derived growth factor BB (PDGF-BB), and insulin-like growth factor 1 (IGF-1).73 These growth factors gets encompassed within the stromal areas and thus acts as a site of storage for these growth factors.73 The invading cancer cells release MMPs that cause the release of these growth factors.73–75 The stroma itself is a very complex structure consisting of various cell types including mesenchymal cells (cancer-associated fibroblasts [CAF]), endothelial cells, extracellular matrix (ECM) proteins (mainly, type I collagen), nerve cells, endothelial cells and pericytes, bone marrow–derived stem cells, and immune cells.76 Transforming growth factor β receptors are expressed by all these cell types, and the TGF-β pathway can thus influence tumor microenvironment by affecting fibrosis, angiogenesis, and immune cell infiltration.77 Both the generation of cancer from a nontumoral environment and the maintenance of a favorable tumoral microenvironment are governed by the TGF-β pathway activation.76 The activated TGF-β pathway enhances production and lowers the degradation of ECM components, mainly type I collagen, as well as mesenchymal cell proliferation.78–80 Furthermore, TGF-β promotes reactive oxygen species production via several mechanisms (such as activation of nicotinamide adenine dinucleotide phosphate oxidases family members), leading to targeting downstream signaling pathways such as Src, EGFR, SMADs, and MAPK family, thus promoting profibrotic gene expression (eg, TGF-β1, angiotensinogen, PAI-1, and connective tissue growth factor).81 Overproduction of TGF-β not only drives the fibrotic process/chronic phases of inflammatory diseases but also precedes tumor formation and thus creates a favorable microenvironment for cancer cells’ growth.76,78,82
In addition, TGF-β activates surrounding CAFs and stellate cells. These activated CAFs and stellate cells are responsible for the secretion of several factors (such as PDGF, FGF, MMP, EGF, type I collagen, and IGF-1) that enhance tumor proliferation, growth, invasion, metastasis, and above all chemoresistance.83 Furthermore, they take part in the creation of hypoxic microenvironment, thus applying a selection pressure leading to an invasive cancer cell phenotype.83 In conclusion, the stroma, depending on collagen I structure, can behave as a barrier or a promoter to metastatic dissemination of cancer.76,84
IL-1α and IL-1β signaling pathways
Numerous pro-inflammatory molecules have been recognized as key players in PDAC invasion and metastasis. Interleukin 1α (IL-1α) is a major inflammatory cytokine that promotes adhesion, proliferation, and migration of the pancreatic cancer cell lines SW1990, BxPC3, and Capan-2 by upregulating the expression of the urokinase receptor and integrin subunits α6 and β1. These effects are linked with the activation of RAS-ERK (extracellular signal–regulated kinase) signaling pathway.85 Inhibition of α6 and β1 integrins and uPA leads to downstream inhibition of ERK signaling and subsequent impairment of proliferative, migratory, and adhesive responses of pancreatic cells.85 Xu et al86 showed that IL-1α synthesized by pancreatic cancer cells induces expression of hepatocyte growth factor (HGF) in fibroblasts. Coculture experiments demonstrated a paracrine effect of IL-1α–dependent fibroblast-driven HGF on neighboring cells where fibroblast-secreted HGF promotes invasive and proliferative behavior of pancreatic cancer cells and human umbilical vein endothelial cells.86 In another study, forced expression of IL-1α in the pancreatic cancer cell line MiaPaCa-2 results in activation of NF-κB signaling pathway leading to an increase in the invasive phenotype of pancreatic cancer cells. Along the same line, blocking NF-κB pathway by the expression of a dominant-negative IκB protein impairs the metastatic behavior of pancreatic cancer cells. Similar responses were observed when IL-1α was silenced in the metastatic pancreatic cancer cell line L3.6pl supporting the notion that IL-1α–induced NF-κB expression promotes the invasive and metastatic behavior of pancreatic cancer cells.87
The pro-inflammatory cytokine interleukin 1β (IL-1β) is another member of the IL-1 family that influences metastasis and tumor growth in various types of cancers.88 Interleukin 1β along with IL-1α induces the expression of pro-inflammatory genes including inducible nitric oxide synthase, cyclooxygenase 2 (COX-2), and IL-6. Pancreatic cancer cell lines treated with recombinant IL-1β show a strong invasive behavior with no influence on ECM adhesion.89
CXC chemokine signaling
Recent studies also suggest the dysregulation of CXC chemokines in late-stage PDAC. Expression of CXCL5, a ligand for CXCR2, is enhanced in human PDAC and has been linked to increased tumor size, advanced tumor stage, and poor outcome. Genetic mutations that dysregulate chemokine sign-aling, such as TP53 mutation, have been attributed to promoting invasion and metastasis in PDAC.9 Another example is SMAD4 mutations that are found in 50% of PDAC cases and known to dysregulate chemokine signaling.90 In a mutant Kras mouse model–based study,91 TGF-βR2 knockout leads to aggressive PDAC that histologically resembles human disease. Mutant Kras mice are also characterized by enhanced secretion of CXCR2-specific chemokines, including CXCL1 and CXCL5, which are regulated by TGF-β and NF-κB signaling. Interestingly, stromal fibroblasts express markedly higher levels of CXCR2 than epithelial cells, and acute inhibition of CXCR2 improves survival and reduces microvessel density, further validating the involvement of CXCR2 ligands in driving PDAC progression.9,90
Current reports suggest that metastasis may occur in PDAC even before primary tumor formation, a behavior associated with early epidermal mesenchymal transformation.92 This is accelerated in the presence of pancreatic inflammation where the most invasive areas of tumor are located at the foci of inflammation. This phenomenon is quenched by dexamethasone indicating the integral role played by tumor-associated inflammation. Hence, identification of inflammatory signaling pathways involved in PDAC metastasis is critical for developing combinatorial antimetastatic therapies in the near future.
The Role of EMT in PDAC Development and Drug Resistance
Epithelial-mesenchymal transition is a process by which epithelial cells undergo numerous genotypic and phenotypic changes to attain mesenchymal phenotype. The mesenchymal phenotype is characterized by enhanced migratory capacity, invasiveness, resistance to apoptosis, and production of ECM.93,94 Newly transformed mesenchymal cells typically show poor cell adhesion parallel to loss of E-cadherin. This phenomenon also features the gain of mesenchymal markers, including vimentin, N-cadherin, and fibronectin.93,94 Epithelial-mesenchymal transition plays a crucial role during development and in adult tissue repair following injury.95 Epithelial-mesenchymal transition initiated by genetic and epigenetic changes in the tumor microenvironment represents a pivotal event during cancer progression and metastasis.93–97
Transition to mesenchymal phenotype is regulated at the cellular level by certain key zinc finger transcription factors, such as Snail, Slug, Zeb-1, and Twist, which perturb the regulation of genes driving epithelial phenotype.93,94,98 Tumor-budding cells in the tumor microenvironment of aggressive PDAC express EMT markers at both messenger RNA (mRNA) and protein levels. These budding cells display classical EMT phenotypic changes and are surrounded by a heterogeneous population of stromal cells that express high levels of the E-cadherin repressors ZEB1, ZEB2, and SNAIL1.99
There is a close association between chemoresistance and the gain of the EMT phenotype in various carcinoma cells including PDAC.100 Pancreatic ductal adenocarcinoma cell lines BxPC3, L3.6pl, CFPAC-1, and SU86.86 with enhanced E-cadherin expression and reduced expression of the mesenchymal marker Zeb-1 display sensitivity to the chemotherapeutic agents 5-FU, gemcitabine, and cisplatin, whereas other cell lines Hs766T, Panc-1, MiaPaCa-2, AsPC-1, and MPanc96 express low E-cadherin, high Zeb-1 levels and display EMT as well as exhibit resistance to the aforementioned chemotherapeutic drugs.101 Zeb-1 downregulation in PDAC cells with EMT phenotype enhances the expression of epithelial markers and retrieve drug sensitivity, indicating the involvement of Zeb-1 and other EMT regulators in enhancing the resistance of PDAC cells to chemotherapy.102 This notion was further validated in an in vivo mouse model. In this report, although EMT suppression enhanced cancer cell proliferation, it also increased expression of nucleoside transporters leading to enhanced sensitivity to gemcitabine treatment and prolonged survival in mice.103 Furthermore, this study highlights the need of a combination of EMT inhibitors to efficiently blunt chemotherapeutic resistance during treatment of pancreatic cancer.103
A small population of permanent proliferating cells and a large population of differentiated cells (with limited proliferation potential) exist in the carcinoma tissue.102 Within the permanently proliferating cells, cancer stem cells (CSCs) are believed to be culpable for the initiation, chemoresistance, metastases, and tumor recurrence.102,104 Cancer stem cells are self-renewing cells that bear the potential to differentiate into other cell types, as well as initiate tumors in the immunodeficient mice.105,106 It is known from the recent studies that CSC and EMT-type cells not only show similarities such as higher metastatic potential and chemoresistance but also have the molecular pathways such as Notch and Wnt in common, indicating the direct correlation among CSC property and EMT program.107 Moreover, constant Notch-1 overexpression is known to induce self-renewal potential, expression of CSC markers CD44 and epithelial surface antigen, as well as EMT properties via upregulation of Zeb-1 in the PDAC cell line AsPC-1.108 Similarly, forced expression of forkhead box protein M1 (FoxM1) induced EMT state by enhancing expression of vimentin, Zeb-1, and Snail2, as well as promoted the gain of the CSC phenotype in PDAC cells.109 Furthermore, reduced expression of stem cell–related transcription factors Sox2 and Oct4, reversal of the EMT phenotype, decreased sphere formations, and the in vivo tumorigenicity in PDAC cells were seen after silencing of Snail with small hairpin RNA introduction.110
One of the most critical property of CSCs is to gain the EMT-induced stemness phenotype that leads them to resistance to several chemotherapeutic agents.111 Pancreatic ductal adenocarcinoma cells with the CSC phenotype under the influence of hypoxia gain EMT and enhanced migration ability.112 In addition, it was reported that only the CSC-like cells acquire high migratory potential and thus may be responsible for invasion and metastasis.102,112
Also, human pancreatic cancers have a cell subset known as side population.111 These side population cells are highly resistant to gemcitabine, a very routine chemotherapeutic agent in used in the therapy of pancreatic cancer.113 In addition, these cells exhibit enhanced gene expression profiles associated with multidrug resistance (ABCG2 and ABCA9), EMT (SNAI2, LEF1), and regulation of apoptosis (ETS1, FASLG,).113 Also, it is reported that in pancreatic CSCs, microRNAs (miRNAs) such as miR99a, miR100, miR-125b, miR-192, and miR-429 are differentially expressed. These miRNA clusters are related to the stem cell–associated mRNAs in pancreatic CSCs.114 These findings indicate that stem cell–like properties imparted during EMT could attribute to chemoresistance in pancreatic cancer.111
Tumor-Infiltrating Inflammatory Cells and EMT: Crosstalk in Cancer Pathogenesis and Progression
An important question that needs further investigation is “How do tumor-infiltrating inflammatory cells and EMT impact one another toward cancer progression?” Many mechanisms have been described in literature, including autocrine/paracrine extracellular signals as well as genetic and epigenetic modifications.
Epithelial-mesenchymal transition–inducing signals are released through a process where a reactive stroma is formed after the recruitment of variety of inflammatory cells, such as myofibroblasts, fibroblasts, macrophages, granulocytes, myeloid cell–derived suppressor cells, lymphocytes, and mesenchymal stem cells, under the influence of certain factors synthesized by islands of cancer cells in advanced primary carcinomas.115 Using human PDAC primary tumors and Kras(G12D)/Snail mice, it was shown that SNAIL overexpression is associated with enhanced infiltration of mast cells via stem cell factor.116 Enhanced recruitment of Gr-1+ and F4/80+ cells was also reported in Kras(G12D)/Snail mice compared with control Kras (G12D) mice.116 Interaction between inflammatory and EMT pathways toward cancer progression is observed in multiple types of cancers and not restricted to PDAC. Coculture of tumor-associated macrophages (TAMs) and ovarian cancer cells demonstrated that TAMs promote the invasive phenotype of cancer cells in tumor necrosis factor α (TNF-α) and NF-κB–dependent manner.117,118 In PDAC, macrophage infiltration is seen at a significantly higher numbers than in normal pancreatic tissue, and their infiltration does not match with chronic pancreatitis-like features in the neighboring tissue.119,120 The TAM M2 subtype has been associated with a poor prognosis.121 It was shown in an in vivo mouse model that when human tumor cells were co-engrafted with high numbers of human monocytes, enhanced tumor growth was seen.122 But, when they co-engrafted tumor cells with a low ratio of human monocytes, they noticed inhibition of tumor growth.122 Continuous and regular contact of monocytes with tumor cells downregulates the production of cytotoxic molecules (such as reactive oxygen intermediates, TNF-α, and IL-12) and upregulates the levels of immunosuppressive cytokine IL-10.122,123 This indicates that there could be a threshold ratio of the tumor cells to the number of monocytes and a set-limit of the immune mediators/molecules they make, which when exceeded antitumor effects are not seen and more protumor phenotype is displayed. Furthermore, it was reported in an in vitro study that TNF-α made by TAMs increased with macrophage motility as well as pancreatic tumor cell numbers and ultimately transforming the phenotype of tumor cells to EMT phenotype.124 These findings support the notion that the increment in the number of TAMs and their products such as TNF-α in PDAC could overpower a definite threshold and transform from an antitumor to a protumor response.119 However, further studies are required to better understand the importance and impact of the number and type of TAMs that play a critical role in PDAC.
Cancer-associated fibroblasts represent another major cell type present in chronic inflammatory microenvironment and express growth factors such as FGF and HGF in addition to matrix-degrading enzymes, which are known inducers of EMT.125–127 Pancreatic ductal adenocarcinoma cells and CAFs reciprocally enhance each other’s proliferation and differentiation. Cell culture supernatants from PDAC cells trigger the production of ECM proteins and proliferation of pancreatic stellate cells (PSCs).128,129 Similarly, coculture of PDAC cells with CAF cell culture supernatant enhances the proliferation and migration of PDAC cells, as well as the rate of growth of PDAC cells when PSCs are coinjected into nude mice.129–131 It was demonstrated that coculture of PSCs with PDAC cells leads to downregulation of epithelial markers, E-cadherin, cytokeratin 19, and β-catenin, and upregulation of mesenchymal markers, vimentin and Snail, subsequently leading to enhanced cancer cell migration.132 Furthermore, in an in vivo study, male human CAFs were orthotopically coinjected along with female PDAC cells as a xenograft into the pancreas of female mice. It was observed that CAFs followed the pancreatic cancer cells to the metastatic sites, indicating that CAFs could play a potential role in the colonization of metastatic PDAC cells.133
Furthermore, it has been reported that CAFs protect pancreatic cancer cells from CRT.130 In an in vitro study, it was shown that when pancreatic cancer cells were cultured in the presence of culture supernatant (conditioned medium) from PSCs, the components in the PSC-conditioned media blocked the apoptosis of the gemcitabine-treated (100 µmol/L) or radiation therapy (100 Gy)–treated pancreatic cancer cells.130 Moreover, pancreatic cancer cell survival during radiation was enhanced in the presence of PSCs in both the monocultures or direct coculture-based conditions131,134 However, in another study, contact between CAFs and the PDAC cells was necessary for PDAC cells to gain radioprotective, but when β1-integrin signaling was blocked using blocking antibodies, this radioprotective effect of CAFs was significantly attenuated.131 Furthermore, in an in vivo xenograft model system, it was shown that CAFs provide radioprotection to the implanted tumor cells when coinjected with pancreatic cancer cells, indicating critical role of CAFs in pancreatic cancer.128,131,134
Inflammation and EMT: A Vicious Cycle in PDAC Progression
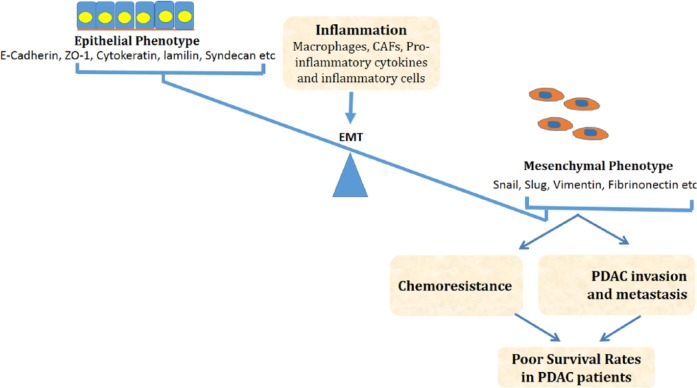
Inflammation, EMT and cancer are closely interconnected (Figure 1).78,135–137 In this section, we will discuss the molecular mechanisms involved in the regulation of inflammation and EMT in cancer pathogenesis and progression with a focus on the interplay between NF-κB, TGF-β, TNF-α, and STAT3 signaling pathways.
Figure 1.
Proposed mechanisms of induction of inflammation-mediated EMT and its subsequent effects on PDAC chemoresistance and progression, which eventually end up in poor survival rates in patients with PDAC. In this figure, we show that protumor inflammation can shift the balance and transform the epithelial cells toward mesenchymal phenotype. These newly gained mesenchymal traits promote tumor invasion and resistance to chemotherapy leading to bad prognosis. CAFs indicates cancer-associated fibroblasts; EMT, epithelial-mesenchymal transition; PDAC, pancreatic ductal adenocarcinoma.
Nuclear factor κB is not only a direct and powerful inducer of EMT but also promotes mobilization of innate immunity and inflammation, thus representing a molecular bridge between inflammation, EMT, and cancer.78,138–147 Akt-mediated activation of NF-κB leads to enhanced SNAIL expression and induction of EMT.143,148 Subsequently, upregulated SNAIL inhibits expression of the metastasis suppressor gene products Raf kinase inhibitor protein (RKIP) and phosphatase and tensin homology (PTEN) leading to blocking of NF-κB/MAPK and PI3K/AKT pathways, respectively.149–151 Nuclear factor κB has been shown to regulate a number of miRNAs. Nuclear factor κB upregulates expression of miR-9,152 a miRNA whose overexpression in breast cancer cells directly targets CDH1 (the E-cadherin–encoding messenger RNA) leading to enhanced cell motility and invasiveness.153 Nuclear factor κB also directly binds to miR-448 promoter and downregulates miR-448 transcription leading to EMT induction. miR-448 suppression induces EMT via targeting special AT-rich sequence-binding protein-1 (SATB1) mRNA, enhancing EGFR-mediated TWIST1 expression and NF-κB activation. Moreover, patients who were subject to combinatorial chemotherapy exhibited lower miR-448 levels and higher SATB1 and TWIST1 levels. Thus, a feedback loop between miR-448 and NF-κB seems to play a critical role in the regulation of chemotherapy-induced EMT.154 Nuclear factor κB activation in myeloid cells has also been associated with EMT and tumor progression in inflammation-associated cancer models.155
Transforming growth factor β is another major regulator of EMT through canonical SMAD-dependent156 and noncanonical SMAD-independent pathways. Transforming growth factor β also modulates the expression of other EMT regulators, such as SLUG157 and SNAIL,158,159 through SMAD and MAPK activation in both normal and malignant mammary epithelial cells (MECs).160–163 In addition, TGF-β-TGF-βR-SMAD2 signaling axis controls maintenance of epigenetic silencing of crucial EMT genes in breast cancer progression.164 Along with canonical SMAD-dependent pathways, several reports demonstrate that TGF-β can also regulate MECs behavior and induce EMT independently of SMADs. Noncanonical SMAD-independent effectors include phos-phatidylinositol-4,5-bisphosphate 3-kinase (PI3K), MAPKs, guanine triphosphate–binding proteins, and NF-κB.165–171 In addition, TGF-β targets include Na and K-ATPase,172 IGFBP3,173 ZAG174 SKIP, TGF-βR1,175 Dab2, ROCK and LIMK, PIAS1, as well as multiple nuclear transcription factors, including members of SNAIL, SIP1, TWIST, and 6 family of homeobox (Six1).176,177 Transforming growth factor β regulation of EMT does take place at the miRNA level as well in both normal and cancerous cells. In normal MECs, TGF-β stimulation enhances miR-155 expression through a SMAD4-dependent pathway. Transforming growth factor β also mediates miR-21 and miR-29a expression leading to EMT induction.178,179 miR-200 is another miRNA that falls under the umbrella of TGF-β–regulated small RNAs. Transforming growth factor β downregulates miR-200 expression, thus enhancing expression of E-cadherin repressors ZEB1 and ZEB2, which in turn results in E-cadherin downregulation and EMT induction.180,181 Moreover, TGF-β signaling induces hypermethylation of E-cadherin promoter leading to differentiation of Ras-transformed MECs that have undergone a serum-induced stable EMT.182 Overall, a long list of targets have been identified downstream of TGF-β in the regulation of EMT. Nevertheless, the relative importance of these downstream targets and the crosstalk among them in TGF-β–mediated EMT is not yet fully understood. However, TGF-β signaling in EMT has been shown to be regulated by a number of miRNAs, such as miR-30 and/or miR-200 family members, in cells derived from anaplastic thyroid carcinoma cells.183
Similar to NF-κB and TGF-β, TNF-α is a potent stimulator of EMT. Transforming growth factor α induces SNAIL1 promoter activity and stabilizes SNAIL1 protein.124,184,185 Transforming growth factor α–induced EMT is partly mediated by TGF-β1 activation.185,186 Transforming growth factor α and TGF-β act in a synergistic manner expediting EMT via a p38 MAPK-dependent pathway.187 Transforming growth factor α also promotes CD44 expression and moesin phosphorylation via TGF-β and protein kinase C activation along with actin remodeling. This leads to the dissociation of cell-cell contacts and increase in cellular motility.188 In addition to TGF-β–mediated EMT induction, TNF-α induces EMT via NF-κB activation or IKK2 constitutive upregulation and activation.189,190 As previously discussed, the downstream targets of TNF-α, TGF-β, and NF-κB are also interconnected.160,191 Transforming growth factor β–mediated NF-κB activation induces EMT and metastasis by upregulation of an autocrine cascade of Cox-2/prostaglandin E2 (PGE2) receptor 2 (EP2) signaling.170,192–196 Altogether, these findings elucidate the regulation of EMT induction via a triad system of NF-κB, TGF-β, and TNF-α pro-inflammatory signaling pathways.
Another pro-inflammatory mechanism that primarily contributes to EMT induction is STAT3-mediated expression of TWIST.146 However, STAT3 has been reported to be a negative regulator of adenoma-carcinoma transition in colon cancer197 in contrast to the general dogma where pro-inflammatory signals induce EMT and promote tumor progression.
Current Treatment Options and Therapeutic Approach
For patients diagnosed with PDAC, at the moment, only surgical resection is the hope.198,199 But, about 80% of the patients with PDAC at the time of diagnosis already have a locally advanced or metastatic disease, thus rendering surgical intervention ineffective.199,200 For the past 2 decades, the standard therapeutic strategy for these patients has been a combinatorial strategy of chemotherapy along with the nucleoside analogue gemcitabine.199 Despite this, only a meager 5-week increase in median survival of these patients has been observed using gemcitabine.201 Moreover, therapeutic strategy to combine either thymidylate synthetase inhibitor (capecitabine) or platin-based agents (cisplatin and oxaliplatin) along with gemcitabine has been unsuccessful in enhancing the therapeutic efficacy.202–204 A limited increase in the median survival (6.24 vs 5.91 months) for the patients with unresectable PDAC was seen in a phase 3 study with the combinatorial treatment of erlotinib, an EGFR inhibitor along with gemcitabine in comparison with gemcitabine alone.205 Recent advances show that the use of FOLFIRINOX (irinotecan, oxaliplatin, leucovorin, and FU) has shown a significant increase in the median survival of patients by more than 4 months in comparison with gemcitabine alone (11.1 vs 6.8 months).206
Precision medicine in oncology bas been critical in understanding diverse molecular mechanisms of PDAC oncogenesis.207 Nevertheless, transforming this knowledge toward the development of targeted therapy has been a daunting task due to the complex biology of PDAC.207 Axitinib, an oral inhibitor of VEGF receptors (VEGFR), was investigated in a randomized, placebo-controlled phase 2 study enrolling 103 patients with unresectable or metastatic PDAC as supplement to gemcitabine. Median overall survival for gemcitabine with axitinib was 6.9 months, whereas for gemcitabine alone was 5.6 months.208,209 Although the study was extended with a phase 3 trial including 632 patients,210 an interim analysis suggested that the study was a failure and hence was terminated.208
Germline mutations in the BRCA1 or BRCA2 genes render PDAC tumors highly sensitive to poly (ADP-ribose) polymerase (PARP) inhibitors.211 To this effect, several PARP inhibitors, such as olaparib, are being tested in clinical trials. In a recent multicenter phase 2 study, olaparib (400 mg twice per day) was given to the enrolled 298 patients, including a subgroup of patients with pancreatic cancer with a germline BRCA1/2 mutation.212 The overall response rate for patients with PDAC (treated previously with gemcitabine) was 21.7 (5 of 23).212 In another study, treatment of Marimastat 25 mg, an oral MMP inhibitor did not change the survival rate for patients in a randomized study enrolling 414 patients with unresectable pancreatic cancer in comparison with patients receiving gemcitabine alone.213 In a mouse model–based study, a combination of gemcitabine along with saridegib, an inhibitor of the Hh pathway, depleted desmoplastic stroma, enhanced delivery of gemcitabine to tumor cells, and thus displayed a significant improvement in the survival of tumor-bearing mice.214 Conversely, a randomized, double-blind, placebo-controlled phase 2 trial with gemcitabine plus saridegib resulted in worse median survival in comparison with gemcitabine plus placebo arm; this study was discontinued.208
In patients with solid tumors, targeting ERBB family members (eg, EGFR) and VEGF) and VEGFR using monoclonal antibodies has been most effective.208 But some of these antibodies have not been successful in the trials with patients with advanced PDAC. Monoclonal antibodies targeting PD-1, PD-L1, and CTLA-4 (so-called checkpoint blockade, reviewed by Postow et al215) have been shown in recent clinical trials to promote endogenous antitumor immune activity.216–218 Various phase 1 and 2 trials are going on to study the effect of antibodies against PD-1, PD-L1, and CTLA-4 in the solid tumors including advanced or metastatic pancreatic adenocarcinoma.208 Furthermore, new studies have been started to test monoclonal antibodies against tissue factor (CD142), Notch, human growth factor receptor, and tumor endothelial marker 1 (TEM1, endosialin) in patients with PDAC.208
In addition, vaccines and immunotherapies are being used to target PDAC. Algenpantucel-L is a vaccine derived of 2 irradiated allogeneic pancreatic cancer cell lines (HAPa-1 and HAPa-2) transfected to express murine α-1,3-galactosyltransferase has reached phase 3. It was successfully tested in a phase 2 trial (multicenter, open label) with 70 resected (R0-1) patients with PDAC along with the combination of gemcitabine chemotherapy and chemoradiation.208 In this study, the median overall survival was 86% and disease-free survival was 62% for the first year during a followup of 21 months.208 The GVAX, a granulocyte-macrophage colony-stimulating factor–secreting allogenic pancreatic tumor cell vaccine was investigated recently in 90 patients with metastatic PDAC along with low-dose cyclophosphamide (Cy/GVAX) to block regulatory T cells, and with or without CRS-207, a live-attenuated Listeria monocytogenes expressing mesothelin. This was performed in a prime/boost vaccination manner, ie, Cy/GVAX followed by CRS-207 (arm A) in comparison with Cy/GVAX alone (arm B),219 where overall survival of 6.1 months in arm A and 3.9 months in arm B (P = .02) was seen. Higher levels of mesothelin-specific CD8+ T-cell responses were linked to the longer overall survival.208
For adoptive immunotherapy, ex vivo genetic engineered T cells collected from patients are used to generate chimeric antigen receptors (CAR), efficient in detecting mesothelin expressed on PDAC cells.220,221 The CAR-T cell infused back into the patient immediately detects tumor cells and thus avoids antigen processing and HLA expression. In preclinical studies, CAR-T cells displayed strong antitumor activity.222 Also, CAR-T cell therapy is now a discipline of active research in PDAC and there are ongoing studies in this field. (ClinicalTrials.gov identifiers: NCT01897415 and NCT01583686).207
In context to this article, although targeting signaling pathways downstream from KRAS has been unsuccessful so far,207 but there is a renewed fascination for targeting the outcome of an activated Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway in PDAC due to the association of PDAC and cachexia.20,223 Furthermore, addition of ruxolitinib, the JAK inhibitor to capecitabine for refractory metastatic patients with PDAC in a phase 2 trial exhibited overall benefit for a patient subgroup with increased levels of C-reactive protein207,224 and thus has created the rationale for phase 3 trials for the evaluation of ruxolitinib in patients with metastatic PDAC (ClinicalTrials.gov identifiers: NCT02117479 and NCT02119663). Analysis of data from global genomic studies also disclosed alterations in the gene expression patterns of the Wnt/Notch and TGF-β signaling pathways in all PDACs.225 To this effect, at present, there are ongoing clinical trials to study the potency of specific inhibitors of these pathways (ClinicalTrials.gov identifiers: Wnt inhibitors: NCT02050178, NCT01764477; mAb against Notch:NCT01647828; Oral anti TGF-β receptor type 1: NCT01373164).207
Conclusions
Low survival rates of patients with PDAC have been primarily attributed to the resistance to chemotherapy. Inflammation does not only contribute to PDAC initiation but also promote cell survival, inhibit apoptosis, and induce EMT eventually leading to chemoresistance and enhanced invasiveness and metastasis of PDAC. Hence, simultaneous targeting of inflammation and EMT is crucial to overcome chemoresistance and improve survival in the battle against PDAC.
Footnotes
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 502 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
MWK conceived and designed the experiments. MWK and FGK analyzed the data. MWK wrote the first draft of the manuscript. FGK and MWK contributed to the writing of the manuscript, agree with manuscript results and conclusions, jointly developed the structure and arguments for the paper, made critical revisions, and approved the final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics
As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the National cancer database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 3.Ahrendt SA, Pitt HA. Surgical management of pancreatic cancer. Oncology (Williston Park) 2002;16:725–734. discussion 734, 736–728, 740, 743. [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 7.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 8.Picozzi VJ, Abrams RA, Decker PA, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2011;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele CW, Jamieson NB, Evans TR, et al. Exploiting inflammation for therapeutic gain in pancreatic cancer. Br J Cancer. 2013;108:997–1003. doi: 10.1038/bjc.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krantz SB, Shields MA, Dangi-Garimella S, Munshi HG, Bentrem DJ. Contribution of epithelial-to-mesenchymal transition and cancer stem cells to pancreatic cancer progression. J Surg Res. 2012;173:105–112. doi: 10.1016/j.jss.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitkara D, Mittal A, Mahato RI. miRNAs in pancreatic cancer: therapeutic potential, delivery challenges and strategies. Adv Drug Deliv Rev. 2015;81:34–52. doi: 10.1016/j.addr.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Neuhofer P, Song L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019–1031. doi: 10.1172/JCI64931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science (New York, NY) 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 14.Shuai K, Stark GR, Kerr IM, Darnell JE., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science (New York, NY) 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 15.Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 16.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 17.Miyatsuka T, Kaneto H, Shiraiwa T, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corcoran RB, Contino G, Deshpande V, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda A, Wang SC, Morris JP, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zhang Y, Feurino LW, et al. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008;99:733–737. doi: 10.1111/j.1349-7006.2008.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang R, Tang D, Lotze MT, Zeh HJ., 3rd AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6-STAT3pathway. Autophagy. 2012;8:989–991. doi: 10.4161/auto.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 24.Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 27.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 28.Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci U S A. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Hagler J, Palombella VJ, et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 30.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 31.Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 33.Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. doi: 10.1016/s0016-5085(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 34.Karin M. The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am. 1998;4:S92–S99. [PubMed] [Google Scholar]
- 35.Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:735–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 36.Greten FR, Weber CK, Greten TF, et al. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–2063. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong R, Sun B, Jiang H, et al. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010;291:90–98. doi: 10.1016/j.canlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Sclabas GM, Fujioka S, Schmidt C, Fan Z, Evans DB, Chiao PJ. Restoring apoptosis in pancreatic cancer cells by targeting the nuclear factor-kappaB signaling pathway with the anti-epidermal growth factor antibody IMC-C225. J Gastrointest Surg. 2003;7:37–43. doi: 10.1016/s1091-255x(02)00088-4. discussion 43. [DOI] [PubMed] [Google Scholar]
- 40.Huang H, Liu Y, Daniluk J, et al. Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144:202–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maniati E, Bossard M, Cook N, et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Pparγ expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121:4685–4699. doi: 10.1172/JCI45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busino L, Donzelli M, Chiesa M, et al. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Maitra A, Wang H. The emerging roles of F-box proteins in pancreatic tumorigenesis. Semin Cancer Biol. 2015;36:88–94. doi: 10.1016/j.semcancer.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. 1082.e1–1082.e6. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong CH, Li YJ, Chen YC. Therapeutic potential of targeting acinar cell reprogramming in pancreatic cancer. World J Gastroenterol. 2016;22:7046–7057. doi: 10.3748/wjg.v22.i31.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Means AL, Meszoely IM, Suzuki K, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development (Cambridge, England) 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 48.Collins MA, Yan W, Sebolt-Leopold JS, Pasca di Magliano M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology. 2014;146:822–834.e827. doi: 10.1053/j.gastro.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 51.Song SY, Gannon M, Washington MK, et al. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 52.Stanger BZ, Stiles B, Lauwers GY, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papademetrio DL, Lompardia SL, Simunovich T, et al. Inhibition of survival pathways MAPK and NF-kB triggers apoptosis in pancreatic ductal adenocarcinoma cells via suppression of autophagy. Targeted oncology. 2015;11:183–195. doi: 10.1007/s11523-015-0388-3. [DOI] [PubMed] [Google Scholar]
- 57.Patterson GI, Padgett RW. TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- 58.Lu Z, Friess H, Graber HU, et al. Presence of two signaling TGF-beta receptors in human pancreatic cancer correlates with advanced tumor stage. Dig Dis Sci. 1997;42:2054–2063. doi: 10.1023/a:1018814416903. [DOI] [PubMed] [Google Scholar]
- 59.Daroqui MC, Vazquez P, Bal de Kier Joffe E, Bakin AV, Puricelli LI. TGF-β autocrine pathway and MAPK signaling promote cell invasiveness and in vivo mammary adenocarcinoma tumor progression. Oncol Rep. 2012;28:567–575. doi: 10.3892/or.2012.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 61.Friess H, Yamanaka Y, Buchler M, et al. Enhanced expression of the type II transforming growth factor beta receptor in human pancreatic cancer cells without alteration of type III receptor expression. Cancer Res. 1993;53:2704–2707. [PubMed] [Google Scholar]
- 62.Kleeff J, Korc M. Up-regulation of transforming growth factor (TGF)-beta receptors by TGF-beta1 in COLO-357 cells. J Biol Chem. 1998;273:7495–7500. doi: 10.1074/jbc.273.13.7495. [DOI] [PubMed] [Google Scholar]
- 63.Villanueva A, Garcia C, Paules AB, et al. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–1978. doi: 10.1038/sj.onc.1202118. [DOI] [PubMed] [Google Scholar]
- 64.Friess H, Yamanaka Y, Buchler M, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 65.Kleeff J, Ishiwata T, Maruyama H, et al. The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene. 1999;18:5363–5372. doi: 10.1038/sj.onc.1202909. [DOI] [PubMed] [Google Scholar]
- 66.Kleeff J, Maruyama H, Friess H, Buchler MW, Falb D, Korc M. Smad6 suppresses TGF-beta-induced growth inhibition in COLO-357 pancreatic cancer cells and is overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 1999;255:268–273. doi: 10.1006/bbrc.1999.0171. [DOI] [PubMed] [Google Scholar]
- 67.Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer. 2001;93:204–211. doi: 10.1002/ijc.1330. [DOI] [PubMed] [Google Scholar]
- 68.Simeone DM, Pham T, Logsdon CD. Disruption of TGFbeta signaling pathways in human pancreatic cancer cells. Ann Surg. 2000;232:73–80. doi: 10.1097/00000658-200007000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chow JY, Ban M, Wu HL, et al. TGF-beta downregulates PTEN via activation of NF-kappaB in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2010;298:G275–G282. doi: 10.1152/ajpgi.00344.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhan J, Song J, Wang P, et al. Kindlin-2 induced by TGF-beta signaling promotes pancreatic ductal adenocarcinoma progression through downregulation of transcriptional factor HOXB9. Cancer Lett. 2015;361:75–85. doi: 10.1016/j.canlet.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 71.Yamazaki K, Masugi Y, Effendi K, et al. Upregulated SMAD3 promotes epithelial-mesenchymal transition and predicts poor prognosis in pancreatic ductal adenocarcinoma. Lab Invest. 2014;94:683–691. doi: 10.1038/labinvest.2014.53. [DOI] [PubMed] [Google Scholar]
- 72.Chen YW, Hsiao PJ, Weng CC, et al. SMAD4 loss triggers the phenotypic changes of pancreatic ductal adenocarcinoma cells. BMC Cancer. 2014;14:181. doi: 10.1186/1471-2407-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleeff J, Ishiwata T, Kumbasar A, et al. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J Clin Invest. 1998;102:1662–1673. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding K, Lopez-Burks M, Sanchez-Duran JA, Korc M, Lander AD. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J Cell Biol. 2005;171:729–738. doi: 10.1083/jcb.200508010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neuzillet C, Tijeras-Raballand A, Cohen R, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Therapeut. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Neuzillet C, de Gramont A, Tijeras-Raballand A, et al. Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget. 2014;5:78–94. doi: 10.18632/oncotarget.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pohlers D, Brenmoehl J, Loffler I, et al. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: opportunities for new therapeutic intervention. Adv Wound Care (New Rochelle) 2013;2:122–141. doi: 10.1089/wound.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 83.Duner S, Lopatko Lindman J, Ansari D, Gundewar C, Andersson R. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. 2010;10:673–681. doi: 10.1159/000320711. [DOI] [PubMed] [Google Scholar]
- 84.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sawai H, Okada Y, Funahashi H, et al. Interleukin-1alpha enhances the aggressive behavior of pancreatic cancer cells by regulating the alpha6beta1-integrin and urokinase plasminogen activator receptor expression. BMC Cell Biol. 2006;7:8. doi: 10.1186/1471-2121-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu W, Li Y, Wang X, et al. PPARgamma polymorphisms and cancer risk: a meta-analysis involving 32,138 subjects. Oncol Rep. 2010;24:579–585. [PubMed] [Google Scholar]
- 87.Melisi D, Niu J, Chang Z, et al. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624–633. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Apte RN, Krelin Y, Song X, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006;42:751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 89.Greco E, Basso D, Fogar P, et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1beta not by transforming growth factor-beta1. Int J Biol Markers. 2005;20:235–241. doi: 10.1177/172460080502000406. [DOI] [PubMed] [Google Scholar]
- 90.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 91.Ijichi H, Chytil A, Gorska AE, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsch T, Kleeff J, Friess H. Molecular pathogenesis of pancreatic cancer: advances and challenges. Curr Mol Med. 2007;7:504–521. doi: 10.2174/156652407781387082. [DOI] [PubMed] [Google Scholar]
- 97.Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galvan JA, Zlobec I, Wartenberg M, et al. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br J Cancer. 2015;112:1944–1950. doi: 10.1038/bjc.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Z, Li Y, Ahmad A, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 101.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Satoh K, Hamada S, Shimosegawa T. Involvement of epithelial to mesenchymal transition in the development of pancreatic ductal adenocarcinoma. J Gastroenterol. 2015;50:140–146. doi: 10.1007/s00535-014-0997-0. [DOI] [PubMed] [Google Scholar]
- 103.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 105.Clevers H. The cancer stem cell: premises, promises and challenges. Nature Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 106.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2013;338:94–100. doi: 10.1016/j.canlet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bao B, Wang Z, Ali S, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Bao B, Wang Z, Ali S, et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–2306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Zhou W, Lv R, Qi W, et al. Snail contributes to the maintenance of stem cell-like phenotype cells in human pancreatic cancer. PLoS ONE. 2014;9:e87409. doi: 10.1371/journal.pone.0087409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vaz AP, Ponnusamy MP, Seshacharyulu P, Batra SK. A concise review on the current understanding of pancreatic cancer stem cells. J Cancer Stem Cell Res. 2014;2:e1004. doi: 10.14343/jcscr.2014.2e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salnikov AV, Liu L, Platen M, et al. Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLoS ONE. 2012;7:e46391. doi: 10.1371/journal.pone.0046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van den Broeck A, Gremeaux L, Topal B, Vankelecom H. Human pancreatic adenocarcinoma contains a side population resistant to gemcitabine. BMC Cancer. 2012;12:354. doi: 10.1186/1471-2407-12-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jung DE, Wen J, Oh T, Song SY. Differentially expressed microRNAs in pancreatic cancer stem cells. Pancreas. 2011;40:1180–1187. doi: 10.1097/MPA.0b013e318221b33e. [DOI] [PubMed] [Google Scholar]
- 115.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science (New York, NY) 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 116.Knab LM, Ebine K, Chow CR, et al. Snail cooperates with Kras G12D in vivo to increase stem cell factor and enhance mast cell infiltration. Mol Cancer Res. 2014;12:1440–1448. doi: 10.1158/1541-7786.MCR-14-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 118.Hagemann T, Wilson J, Kulbe H, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 119.Wachsmann MB, Pop LM, Vitetta ES. Pancreatic ductal adenocarcinoma: a review of immunologic aspects. J Investig Med. 2012;60:643–663. doi: 10.231/JIM.0b013e31824a4d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 122.Mytar B, Baj-Krzyworzeka M, Majka M, Stankiewicz D, Zembala M. Human monocytes both enhance and inhibit the growth of human pancreatic cancer in SCID mice. Anticancer Res. 2008;28:187–192. [PubMed] [Google Scholar]
- 123.Mytar B, Woloszyn M, Szatanek R, et al. Tumor cell-induced deactivation of human monocytes. J Leukoc Biol. 2003;74:1094–1101. doi: 10.1189/jlb.0403140. [DOI] [PubMed] [Google Scholar]
- 124.Baran B, Bechyne I, Siedlar M, et al. Blood monocytes stimulate migration of human pancreatic carcinoma cells in vitro: the role of tumour necrosis factor-alpha. Eur J Cell Biol. 2009;88:743–752. doi: 10.1016/j.ejcb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 125.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 127.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 128.Nielsen MF, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol. 2016;22:2678–2700. doi: 10.3748/wjg.v22.i9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 130.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 133.Xu Z, Vonlaufen A, Phillips PA, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Assar O, Demiciorglu F, Lunardi S, et al. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother Oncol. 2014;111:243–251. doi: 10.1016/j.radonc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 135.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sansone P, Bromberg J. Environment, inflammation, and cancer. Curr Opin Genet Dev. 2011;21:80–85. doi: 10.1016/j.gde.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 138.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 139.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 140.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science (New York, NY) 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 141.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 142.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Julien S, Puig I, Caretti E, et al. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 144.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 146.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rehman AO, Wang CY. CXCL12/SDF-1 alpha activates NF-kappaB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 2009;1:105–118. doi: 10.4248/IJOS.09059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Strippoli R, Benedicto I, Perez Lozano ML, Cerezo A, Lopez-Cabrera M, del Pozo MA. Epithelial-to-mesenchymal transition of peritoneal mesothelial cells is regulated by an ERK/NF-kappaB/Snail1 pathway. Dis Model Mech. 2008;1:264–274. doi: 10.1242/dmm.001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-κB/Snail/RKIP/PTEN circuit. Genes Cancer. 2010;1:409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pantuck AJ, An J, Liu H, Rettig MB. NF-kappaB-dependent plasticity of the epithelial to mesenchymal transition induced by Von Hippel-Lindau inactivation in renal cell carcinomas. Cancer Res. 2010;70:752–761. doi: 10.1158/0008-5472.CAN-09-2211. [DOI] [PubMed] [Google Scholar]
- 151.Baritaki S, Chapman A, Yeung K, Spandidos DA, Palladino M, Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene. 2009;28:3573–3585. doi: 10.1038/onc.2009.214. [DOI] [PubMed] [Google Scholar]
- 152.Bazzoni F, Rossato M, Fabbri M, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microR-NA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li QQ, Chen ZQ, Cao XX, et al. Involvement of NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ. 2011;18:16–25. doi: 10.1038/cdd.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gavert N, Vivanti A, Hazin J, Brabletz T, Ben-Ze’ev A. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res. 2011;9:14–24. doi: 10.1158/1541-7786.MCR-10-0406. [DOI] [PubMed] [Google Scholar]
- 156.de Graauw M, van Miltenburg MH, Schmidt MK, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci U S A. 2010;107:6340–6345. doi: 10.1073/pnas.0913360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 158.Franco DL, Mainez J, Vega S, et al. Snail1 suppresses TGF-beta-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J Cell Sci. 2010;123:3467–3477. doi: 10.1242/jcs.068692. [DOI] [PubMed] [Google Scholar]
- 159.Hoot KE, Lighthall J, Han G, et al. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–2732. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 161.Bardeesy N, Cheng KH, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dooley S, Hamzavi J, Ciuclan L, et al. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 164.Papageorgis P, Lambert AW, Ozturk S, et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 2010;70:968–978. doi: 10.1158/0008-5472.CAN-09-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 166.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 167.Zavadil J, Bitzer M, Liang D, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arsura M, Panta GR, Bilyeu JD, et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22:412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- 169.Park JI, Lee MG, Cho K, et al. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene. 2003;22:4314–4332. doi: 10.1038/sj.onc.1206478. [DOI] [PubMed] [Google Scholar]
- 170.Neil JR, Schiemann WP. Altered TAB1:I kappaB kinase interaction promotes transforming growth factor beta-mediated nuclear factor-kappaB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol. 2010;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rajasekaran SA, Huynh TP, Wolle DG, et al. Na, K-ATPase subunits as markers for epithelial-mesenchymal transition in cancer and fibrosis. Mol Cancer Ther. 2010;9:1515–1524. doi: 10.1158/1535-7163.MCT-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Natsuizaka M, Ohashi S, Wong GS, et al. Insulin-like growth factor-binding protein-3 promotes transforming growth factor-{beta}1-mediated epithelial-to-mesenchymal transition and motility in transformed human esophageal cells. Carcinogenesis. 2010;31:1344–1353. doi: 10.1093/carcin/bgq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kong B, Michalski CW, Hong X, et al. AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-beta-mediated ERK signaling. Oncogene. 2010;29:5146–5158. doi: 10.1038/onc.2010.258. [DOI] [PubMed] [Google Scholar]
- 175.Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Six1 increases TGF-beta type I receptor and converts TGF-beta signaling from suppressive to supportive for tumor growth. Cancer Res. 2010;70:10371–10380. doi: 10.1158/0008-5472.CAN-10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 177.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 178.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 179.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 180.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 181.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou C, Liu J, Tang Y, Liang X. Inflammation linking EMT and cancer stem cells. Oral Oncol. 2012;48:1068–1075. doi: 10.1016/j.oraloncology.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 183.Braun J, Hoang-Vu C, Dralle H, Huttelmaier S. Downregulation of microR-NAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 184.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England) 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 185.Yan C, Grimm WA, Garner WL, et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Takahashi E, Nagano O, Ishimoto T, et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem. 2010;285:4060–4073. doi: 10.1074/jbc.M109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luo JL, Tan W, Ricono JM, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 190.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 191.Zhang Q, Helfand BT, Jang TL, et al. Nuclear factor-kappaB-mediated transforming growth factor-beta-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2009;15:3557–3567. doi: 10.1158/1078-0432.CCR-08-1656. [DOI] [PubMed] [Google Scholar]
- 192.Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.You HJ, How T, Blobe GC. The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis. 2009;30:1281–1287. doi: 10.1093/carcin/bgp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Neil JR, Tian M, Schiemann WP. X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-{beta}-mediated nuclear factor-{kappa}B activation during breast cancer progression. J Biol Chem. 2009;284:21209–21217. doi: 10.1074/jbc.M109.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 197.Musteanu M, Blaas L, Mair M, et al. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology. 2010;138:1003–1011. e1–e5. doi: 10.1053/j.gastro.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 198.Bachmann J, Michalski CW, Martignoni ME, Buchler MW, Friess H. Pancreatic resection for pancreatic cancer. HPB (Oxford) 2006;8:346–351. doi: 10.1080/13651820600803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma. In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum, India: Transworld Research Network; 2012. [PubMed] [Google Scholar]
- 200.Passadouro M, Faneca H. Managing pancreatic adenocarcinoma: a special focus in microRNA gene therapy. Int J Mol Sci. 2016;17:E718. doi: 10.3390/ijms17050718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 202.Bernhard J, Dietrich D, Scheithauer W, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial—SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol. 2008;26:3695–3701. doi: 10.1200/JCO.2007.15.6240. [DOI] [PubMed] [Google Scholar]
- 203.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 204.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 205.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 206.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 207.Narayanan V, Weekes CD. Molecular therapeutics in pancreas cancer. World J Gastrointest Oncol. 2016;8:366–379. doi: 10.4251/wjgo.v8.i4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol. 2015;21:9297–9316. doi: 10.3748/wjg.v21.i31.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet (London, England) 2008;371:2101–2108. doi: 10.1016/S0140-6736(08)60661-3. [DOI] [PubMed] [Google Scholar]
- 210.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 211.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 212.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447–3455. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 214.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 217.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 218.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Beatty GL. Engineered chimeric antigen receptor-expressing T cells for the treatment of pancreatic ductal adenocarcinoma. Oncoimmunology. 2014;3:e28327. doi: 10.4161/onci.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao Y, Moon E, Carpenito C, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gilabert M, Calvo E, Airoldi A, et al. Pancreatic cancer-induced cachexia is Jak2-dependent in mice. J Cell Physiol. 2014;229:1437–1443. doi: 10.1002/jcp.24580. [DOI] [PubMed] [Google Scholar]
- 224.Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol. 2015;33:4039–4047. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, NY) 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]