Abstract
Purpose
As an autoimmune inflammatory disorder, active thyroid-associated ophthalmopathy (TAO) is managed optimally by immunosuppression. In this study, we aimed to evaluate octreotide scintigraphy and the level of serum extraocular muscle antibodies in TAO activity.
Patients and methods
This prospective study comprised 304 patients with active TAO (the clinical activity score; CAS≥3), 73 with inactive TAO (CAS<3), 128 with Graves' disease (GD) without ophthalmopathy, and 100 healthy subjects. Moderate-to-severe active TAO patients (CAS≥5) received intravenous injection of methylprednisolone; mild active patients (3≤CAS≤4) received periocular injection of triamcinolone acetonide. 99Tcm-octreotide scintigraphy and serum levels of calsequestrin, uveal auto-antigen with coiled-coil domains and ankyrin repeats (UACA) and G2s antibodies were detected before and after treatment.
Results
99Tcm-octreotide scintigraphy was positive in active TAO patients (97%) with elevated uptake ratio (UR) (P<0.05), and showed a significant correlation with CAS (r=0.816, P<0.01). After treatment both UR and CAS decreased significantly (P<0.05). The receiving operator characteristic curve (ROC) showed that the best UR threshold for discriminating active and inactive TAO was 1.34 (sensitivity, 100% specificity, 89.4%). The level of serum calsequestrin antibody was higher in active TAO (P<0.05), showed a significant correlation with CAS (r=0.738, P<0.05), and also decreased after treatment (P<0.05). The best serum calsequestrin antibody threshold of the ROC curve was 138 ng/l (sensitivity, 88.4% specificity, 89.2%). The UACA antibody was elevated in both TAO and GD patients (P<0.05), with no significant difference (P>0.05). As to G2s, no significant difference was found between all groups (P>0.05). Moreover, six GD patients (4.69%) with elevated calsequestrin developed active TAO 12 weeks later.
Conclusion
99Tcm-octreotide scintigraphy played a critical role in the evaluation of the clinical activity and therapeutic efficacy of TAO. Autoimmunity against calsequestrin in the pathogenesis of the eye muscle components may provide further objective evidence of myopathy in active TAO. Furthermore, calsequestrin antibody may predict myopathy in active TAO.
Introduction
Thyroid-associated ophthalmopathy (TAO) is the most common extrathyroidal manifestation of autoimmune thyroid disease occurring in ~50% of patients with Graves' disease (GD)1 and 25% with Hashimoto's thyroiditis.2 Although the pathogenesis of TAO is not clearly understood, most researchers now agree that it is an autoimmune disease with immunological cross-reactivity between the thyroid and the orbital preadipocytes, myoblasts, and fibroblasts.3 Generally, the natural history of TAO follows Rundle's curve with early inflammatory/congestive phase (the active phase) lasting 1–2 years, followed by stabilization (the plateau phase), and eventually, remission (the inactive phase).1 The treatment for TAO varies according to inflammatory activity and severity. Inflammatory suppression therapy with corticosteroids is always suggested to treat moderate-to-severe active TAO to decrease orbital inflammation and congestion, and surgery is indicated for the stable, inactive TAO.4, 5 Clinical activity score (CAS), based on characteristic ocular signs and symptoms, has been widely used for TAO evaluation and as criteria and guideline for therapeutic management.6, 7, 8 Clinically, most of the diagnosed TAO patients have mild TAO (76.9%),9 and 15–25% of these patients will progress to more severe disease as reflected in a change in CAS.10 So, it is necessary to find more sensitive and objective criteria to access TAO activity.
Nowadays, somatostatin receptor scintigraphy has been used to predict efficacy of immunosuppressive therapy for TAO. Somatostatin, a neuroendocrine peptide hormone, has receptors on many human cells, including fibroblasts, myoblasts, and lymphocytes, which are involved in the pathology of active TAO. Orbital increase of somatostatin analogs has been found to be correlated with CAS and clinical disease severity.11 As a result of its clinical associations, octreotide scintigraphy in TAO may guide treatment by identifying and grading disease activity.12 Previous studies have shown a sensitivity of ~90%, and a specificity of 100%, when octreoscan was used for evaluation of treatment efficacy.13, 14
As an autoimmune-related orbital inflammation, TAO comprises two main subtypes (1) congestive ophthalmopathy, which is characterized by swelling of the eyelid and periorbital connective tissues, and (2) ocular myopathy, which reflects an autoimmune attack against the extraocular muscle.15 The thyroid stimulating hormone-receptor (TSH-R) is the most popular candidate antigen for the development of TAO, but TSH-R cannot explain the development of eye muscle swelling and dysfunction.16 Several studies have implicated the role of autoimmunity against eye muscle antigens, including the calcium-binding protein calsequestrin, G2s, and the uveal auto-antigen with coiled-coil domains and ankyrin repeats (UACA). Calsequestrin was expressed 4.8 times higher in eye muscle compared to other skeletal muscle.17 As reported, anti-calsequestrin antibodies were detected in 40% of patients with clinically active TAO but only in 5% of normal subjects.18 G2s, the terminal 141 amino-acid fragment of the winged-helix transcription factor FOXP1, were found both in the extraocular muscles and thyroid, but not in orbital connective or adipose tissue.19 Anti-G2s antibodies were also reported to be associated with active ophthalmopathy in the patients with Graves's hyperthyroidism, being detected in 54% of patients with active TAO compared to 33% of those with inactive disease.20 UACA is an auto-antigen associated with panuveitis, and is expressed in eye muscle as well as human thyroid follicular cells. Previous studies have shown that the level of anti-UACA antibodies in TAO was significantly higher than that in GD patients without ophthalmopathy (29 vs 11%). Also, 75% of severe ocular myopathy showed high UACA titers.21
The aim of this study was to evaluate 99Tcm-octreotide scintigraphy, and serum levels of antibodies against extraocular muscles antigens, such as calsequestrin, G2s, and UACA antibodies, in the assessment of TAO activity and response to medical treatment.
Materials and methods
Clinical subjects
In this prospective study, we analyzed a series of 377 patients with TAO, 128 with GD without ophthalmopathy, and 100 healthy subjects attending First Hospital of Shanxi Medical University from August 2011 to October 2014. Local Ethical Committee approval was received for the studies, and informed consent of each participating subject was obtained. All subjects were euthyroid at the time of the study, as shown by normal values of serum free thyroxine (FT4), free triiodothyronine (FT3), and thyrotropin (TSH). According to CAS,8 TAO patients were classified as active TAO (CAS≥3, n=304) and inactive TAO (CAS<3, n=73). All patients had no corticosteroids contraindication. The pregnant and lactating women were excluded from the study. Ocular examination was performed by the same ophthalmologist, who evaluated the inflammatory/congestive symptoms and signs of active TAO. The general data of all subjects were summarized in Supplementary Table 1.
Treatment protocols
Corticosteroids were used for management of active TAO. Patients with moderate-to-severe active TAO (CAS≥5) received intravenous methylprednisolone 500 mg (SOLU-MEDROL, 0.5 g, Pfizer Manufacturing, Puurs, Belgium NV) weekly for 6 weeks followed by 250 mg weekly for another 6 weeks for a cumulative dose of 4.5 g.22 The response rate of this intravenous corticosteroid pulse therapy was about 85%. The eyes suffering from mild active TAO (3≤CAS≤4) received periocular injection of triamcinolone acetonide 20 mg (TRANSTON, 1 ml: 40 mg; Kunming Jida Pharmaceutical Co., Ltd., China) 3 weeks intervals, 5 times total; the response rate was about 90%. The activity of TAO before and after treatment was defined by CAS.
99Tcm-octreotide scintigraphy
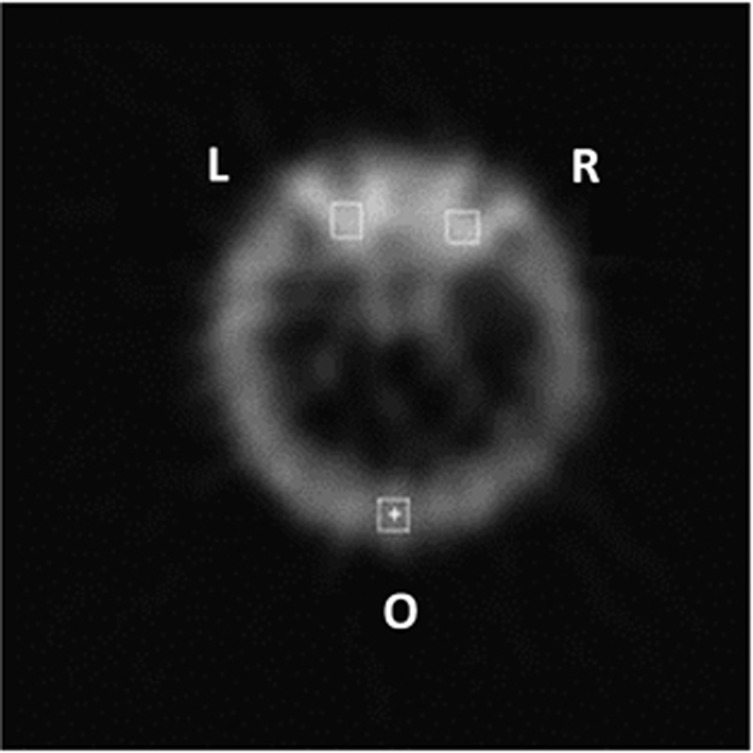
The orbital scintigraphy was carried out in all subjects using 99Tcm-labeled somatostatin analog octreotide, which was prepared as described before.23 Minimum 4 h fasting and good hydration was maintained, then 740 MBq 99Tcm-octreotide was administered intravenously through a forearm vein. 1.0–1.5 h planar and tomographic (single-photon emission computed tomography, SPECT; Adac, USA) imaging of the skull was performed. The transaxial slices with both eyes having optimum visualization were selected for semiquantitative analysis. The regions of interest (ROI) of right orbit (R), left orbit (L), and occipital (O) were selected as Figure 1. The uptake ratios (UR) were calculated as (R+L)/2O, and expressed as mean pixel count ratios.23 99Tcm-octreotide scintigraphy was performed in all active TAO patients before and after therapy. Blood glucose level was measured for each patient before radiopharmaceutical injection.
Figure 1.
The rectangular ROIs on one of the transverse images of 99Tcm-octreotide scintigraphy in a patient with active TAO. Mean pixel counts were measured in right (R) and left (L) orbital ROIs, and in the occiptal (O) ROI. The UR were calculated as (R+L)/2O.
Serum extraocular muscle antibody measurement
The standard enzyme-linked immunosorbent assay (ELISA) was used to measure serum extraocular muscle antibodies, including anti-calsequestrin, anti-UACA, and anti-G2s IgG (Anti-Calsequestrin IgG ELISA kit, Anti-UACA IgG ELISA kit, Anti-G2s IgG ELISA kit; Shanghai Future Industrial Co., Ltd, Shanghai, China). In preliminary studies, the assays were optimized with respect to serum dilution (1 : 50).
Statistical analysis
Statistical analysis was carried out using SPSS 16.0. All data were expressed as mean±SD. One-way ANOVA analysis was used for statistical analysis of the differences between groups. Spearman's correlation test was used to compare the CAS and the UR ratio or antibodies levels. The efficiency of 99Tcm-octreotide scintigraphy or serum extraocular muscle autoantibodies to assess TAO activity and predict the response to corticosteroid treatment was evaluated by receiving operator characteristic curve analysis. The level of statistical significance was determined as P<0.05.
Results
99Tcm-octreotide scintigraphy and correlation analysis with CAS
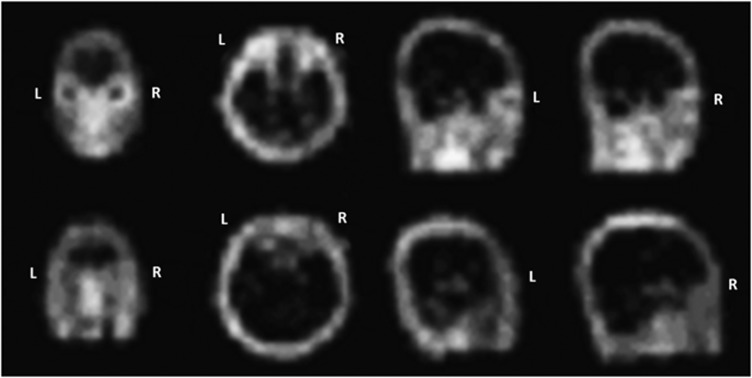
In 97% of patients with active TAO (CAS≥3), 99Tcm-octreotide accumulated intensively in the orbital area (Figure 2), while patients with inactive TAO, GD, and normal controls showed no obvious 99Tcm-octreotide accumulation.
Figure 2.
99Tcm-octreotide scintigraph images from an active TAO patient and a normal control subject. The active patient (upper images) showed intensive accumulatioin of 99Tcm-octreotide in the orbital area, while the normal control (lower images) showed no obvious signal.
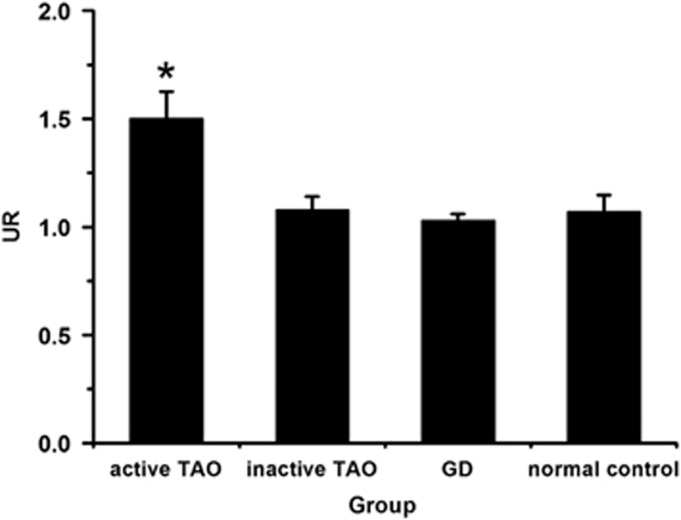
The URs were calculated as (R+L)/2O. On the 2-h 99Tcm-octreotide scintigraphy, the UR in active TAO patients was significantly higher than that in patients with inactive TAO, GD, and normal controls (P<0.05) (Figure 3). Moreover, there was a significant correlation between UR and CAS in TAO patients (r=0.816, P<0.01).
Figure 3.
The UR of 99Tcm-octreotide scintigraph images in different patient groups. The 2-h UR in active TAO was significantly higher than that in inactive TAO, GD patients, and normal controls (*P<0.05).
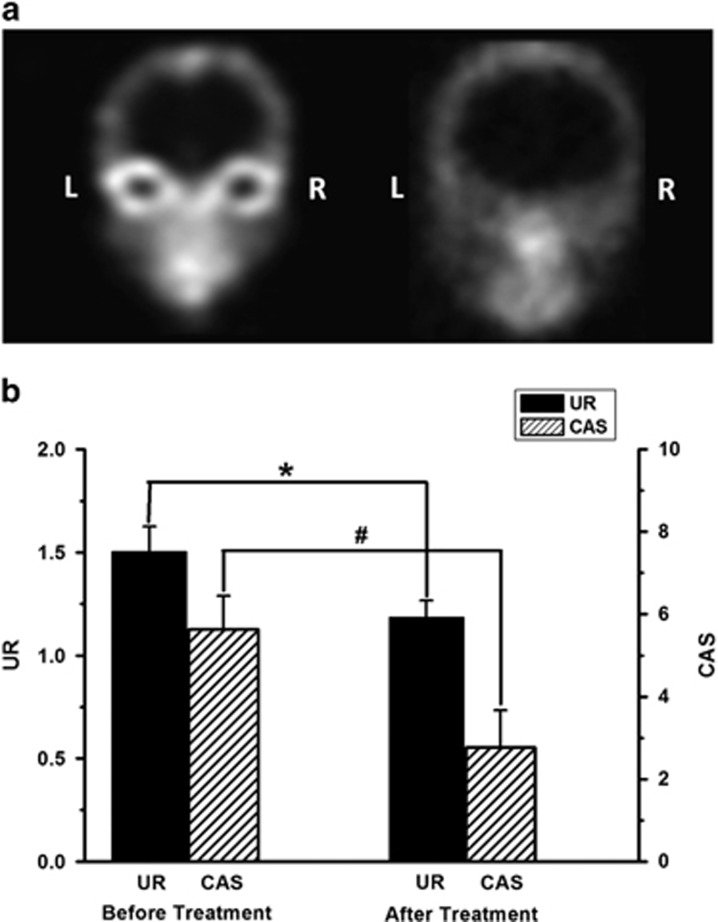
After treatment, with the stabilization of ophthalmopathy, CAS and UR were decreased accordingly in active TAO patients (P<0.05) (Figure 4).
Figure 4.
A 99Tcm-octreotide scintigraph images from an active TAO patient before and after corticosteroid therapy. (a) 99Tcm-octreotide scintigraph image before (left image) and after treatment (right image). The accumulation of 99Tcm-octreotide in the orbital areas was significantly decreased after therapy. (b) For active TAO patients, the 2-h UR (*) and CAS (#) were both decreased after treatment (*P<0.05, #P<0.05).
Serum extraocular muscle antibody levels and correlation analysis with CAS
Before and after treatment, we tested sera from patients with TAO, GD, and normal controls for autoantibodies against calsequestrin, UACA, and G2s by ELISA.
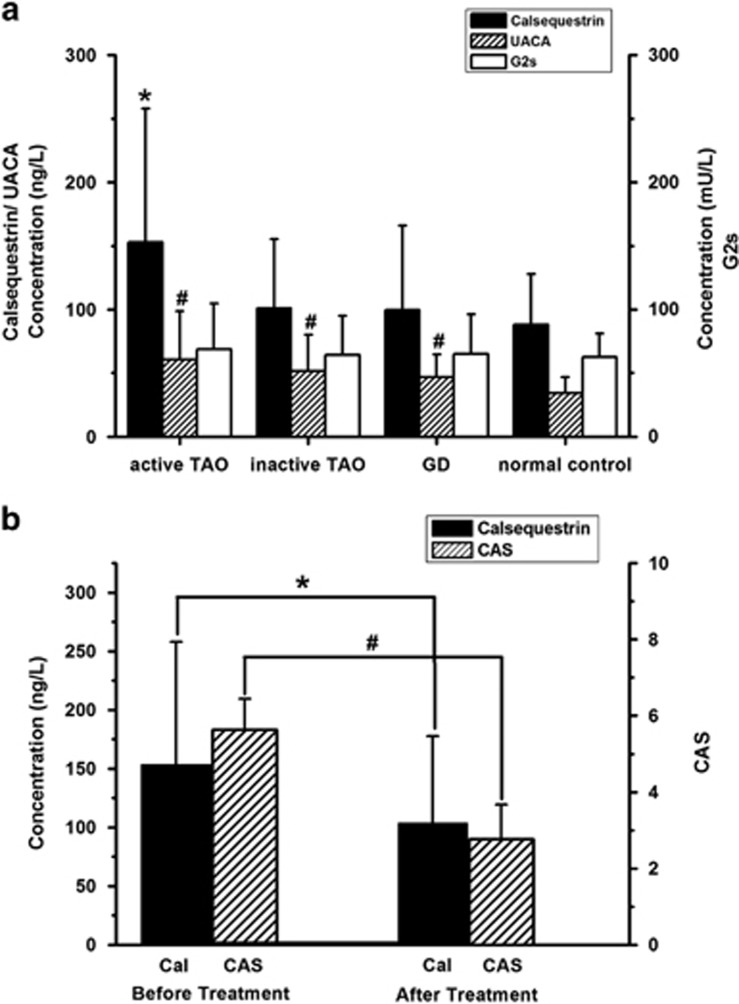
The calsequestrin antibody levels were significantly higher in patients with active ophthalmopathy than in patients with inactive TAO, GD, and normal controls (P<0.05) (Figure 5a) and also showed strong correlation with CAS (r=0.738, P<0.05). We also evaluated the efficiency of calsequestrin antibody levels for assessment of TAO activity and response to the corticosteroid therapy. After treatment, the level of calsequestrin antibody was also decreased significantly (P<0.05) (Figure 5b).
Figure 5.
The level of serum extraocular muscle antibodies and efficacy in evaluation of the treatment of active TAO. (a) The concentration of calsequestrin antibody in active TAO patients was significantly higher than that in inactive TAO, GD patients, and normal controls (*P<0.05). Compared with normal controls, the level of UACA antibodies in patients with TAO and GD were higher (#P<0.05). Serum G2s antibody levels showed no difference between the groups (P>0.05). (b) After treatment, both serum calsequestrin antibody and CAS in active TAO patients decreased significantly (*P<0.05, #P<0.05).
Compared with normal controls, serum UACA antibody levels were higher in TAO and GD patients (P<0.05), although there was no significant difference between active and inactive TAO (P>0.05) (Figure 5a) and, no correlation with CAS (r=0.213, P>0.05).
As to serum G2s antibody levels, no significant difference was observed between the TAO group, GD group, and normal controls (P>0.05) (Figure 5a).
Correlation of 99Tcm-octreotide scintigraphy and calsequestrin antibody levels with active TAO
The efficiency of the orbit 99Tcm-octreotide 2 h UR for assessing disease activity and for predicting a response to corticosteroid therapy was evaluated by receiving operator characteristic curve analysis. The area under the curve was 99.6%. The optimal threshold for discriminating active and inactive TAO was 1.34 (sensitivity, 100% specificity, 89.4%), therefore, there was a positive predictive value of 90.7%, and a negative predictive value of 100%.
Another parameter for evaluation of TAO activity and the response to the therapy was the serum calsequestrin antibody level. The area under the curve was 87.9%. The threshold was 138 ng/l (sensitivity, 88.4% specificity, 89.2%), therefore, there was a positive predictive value of 83.5%, and the negative predictive value of 81.9%.
More interestingly, six GD patients with higher concentration of calsequestrin antibody (4.69%) showed active ophthalmopathy 12 weeks later.
Discussion
The TAO is an autoimmune disorder of the extraocular muscles and orbital connective tissues closely associated with autoimmune thyroid disease.15 The natural history of TAO undergoes two main phases, the active inflammatory phase and the inactive chronic fibrotic phase.1 It is vital to identify the phase of ophalthmopathy to achieve an effective response to corticosteroid therapy. As a measure of periocular soft tissue inflammation, CAS was devised to identify active TAO; however, we should be aware of the deficiencies of the CAS scale. Each clinical feature of CAS is weighted equally and scored in binary fashion. CAS cannot predict the risk of developing significant complications. Thus, we should recognize that some patients with severe disease complications can present with low-CAS scores, and other patients with high CAS may have mild long-standing congestive changes. Even so, because the CAS scale is relatively easy to score, we still used it as a preliminary diagnostic criterion to identify TAO activity.24
Based on the CAS, the average disease duration for active TAO in this study was 20.7 months, which almost reached the reported maximum duration for the active inflammatory/congestive phase of 24 months.25 Most of the patients who came to our clinic, and were identified as active TAO, had long-standing pre-existing congestive changes. In future studies, we will focus on the TAO patients with disease duration of less than 6 months.
The expression of somatostatin on fibroblasts, myoblasts, and lymphocytes may contribute to the pathology of active TAO, and a number of studies have revealed that the octreotide analog 111In-octreoscan/99Tcm-octreoscan is able to efficiently assess the TAO orbital soft tissue inflammation/congestion.23, 26, 27 In this study, 99Tcm-octreotide scintigraphy in patients with TAO had a strong correlation with the CAS (r=0.816, P<0.01), and the 99Tcm-octreotide UR was higher in the active TAO patients than in the inactive and normal control subjects (P<0.05). More importantly, the positive orbital octreoscan that was performed in clinically active eye disease in which corticosteroid treatment might be of therapeutic benefit had a positive predictive value of 90.7% and a negative predictive value of 100%. The 99Tcm-octreoscan showed improvement when the ophthalmopathy stabilized after 12 weeks of treatment. Our findings were similar to results of previous studies.28 Thus, 99Tcm-octreotide scintigraphy might provide a useful criterion in prediction of immunosuppressive treatment in active TAO patients.
In TAO, the eye muscles are targets of the autoimmune reaction, and more than 90% of GD patients with or without clinical signs of ophthalmopathy have extraocular muscle enlargement by orbital imaging techniques.29 The extraocular muscles and thyroid share antigens with clinical relevance in TAO, such as calsequestrin, G2s,19 and UACA21 antigens. In this study, we observed a close relationship between positive reactivity of serum calsequestrin antibodies (87.9%) and active TAO, which corroborates previous report.30 Moreover, the level of calsequestrin antibody was strongly correlated with CAS in active TAO (r=0.738, P<0.05) and also could be used to assess the efficacy of corticosteroids therapy (P<0.05), however, calsequestrin antibody levels were not as efficient as 99Tcm-octreotide scintigraphy in evaluation of therapy response. Since calsequestrin was expressed 4.8 times higher in eye muscles compared to other skeletal muscle,17 calsequestrin antibodies may be correlated with the onset of ocular myopathy30 rather than congestive ophthalmopathy. More interestingly, six GD patients (4.96% of GD patients) without ophthalmopathy, but with elevated levels of calsequestrin antibodies, developed signs of active TAO after 12 weeks. A recent cohort study on the natural history of Graves' ophthalmopathy showed that about 4.9% of patients without ophthalmolpathy or mild TAO progressed to moderate-to-severe TAO.9 Perhaps, serum calsequenstrin antibody can be used as a sensitive predictor for active ocular myopathy of TAO. In future studies, we will collect more data to support this finding.
As reported previously, the prevalence of anti-UACA antibodies was significantly higher in GD patients than in normal subjects (15 vs 0%), and higher in TAO patients compared with GD patients without ophthalmopathy (29 vs 11%).21 In this study, we found that UACA antibodies to be elevated in all active TAO, inactive TAO and GD patients compared with normal controls (P<0.05). There was no significant difference between TAO and GD patients (P>0.05). TSH may upregulate the expression of UACA in skeletal muscles, including extraocular muscles and thyroid tissue,21 and TAO activity is not correlated with TSH. The thyroid function of all subjects in this study was controlled at normal level, so it was reasonable to detect no difference in the levels of serum UACA antibody between TAO and GD groups.
As to G2s, we found no significant difference between patients with TAO, GD, and normal controls. Actually, the function of anti-G2s antibody in TAO or GD is still disputed.31, 32
In conclusion, we have demonstrated that 99Tcm-octreotide scintigraphy played a critical role in evaluation of therapeutic efficacy in active TAO. In addition, autoimmunity against calsequestrin could provide another objective evidence for the pathogenesis of the eye muscle components in active TAO. It is possible that serum calsequestrin antibody can predict the activity of ocular myopathy in TAO.
Acknowledgments
This research was supported by the Natural Science Foundation of Shanxi Province, Grant No. 2013011059-2. We thank Xianfeng Li, MD, PhD, from the First Hospital of Shanxi Medical University for help with 99Tcm-octreotide scintigraphy.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
The authors declare no conflict of interest.
Supplementary Material
References
- Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev 2000; 21(2): 168–199. [DOI] [PubMed] [Google Scholar]
- Tjiang H, Lahooti H, McCorquodale T, Parmar KR, Wall JR. Eye and eyelid abnormalities are common in patients with Hashimoto's thyroiditis. Thyroid 2010; 20(3): 287–290. [DOI] [PubMed] [Google Scholar]
- Naik VM, Naik MN, Goldberg RA, Smith TJ, Douglas RS. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol 2010; 55(3): 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 2008; 158(3): 273–285. [DOI] [PubMed] [Google Scholar]
- Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid 2008; 18(3): 333–346. [DOI] [PubMed] [Google Scholar]
- Werner SC. Classification of the eye changes of Graves' disease. Am J Ophthalmol 1969; 68(4): 646–648. [DOI] [PubMed] [Google Scholar]
- Werner SC. Modification of the classification of the eye changes of Graves' disease. Am J Ophthalmol 1977; 83(5): 725–727. [DOI] [PubMed] [Google Scholar]
- Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol 1989; 73(8): 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L et al. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab 2013; 98(4): 1443–1449. [DOI] [PubMed] [Google Scholar]
- Terwee CB, Prummel MF, Gerding MN, Kahaly GJ, Dekker FW, Wiersinga WM. Measuring disease activity to predict therapeutic outcome in Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2005; 62(2): 145–155. [DOI] [PubMed] [Google Scholar]
- Burggasser G, Hurtl I, Hauff W, Lukas J, Greifeneder M, Heydari B et al. Orbital scintigraphy with the somatostatin receptor tracer 99mTc-P829 in patients with Graves' disease. J Nucl Med 2003; 44(10): 1547–1555. [PubMed] [Google Scholar]
- Krassas GE, Dumas A, Pontikides N, Kaltsas T. Somatostatin receptor scintigraphy and octreotide treatment in patients with thyroid eye disease. Clin Endocrinol (Oxf) 1995; 42(6): 571–580. [DOI] [PubMed] [Google Scholar]
- Kahaly G, Diaz M, Hahn K, Beyer J, Bockisch A. Indium-111-pentetreotide scintigraphy in Graves' ophthalmopathy. J Nucl Med 1995; 36(4): 550–554. [PubMed] [Google Scholar]
- Rabinowitz MP, Carrasco JR. Update on advanced imaging options for thyroid-associated orbitopathy. Saudi J Ophthalmol 2012; 26(4): 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar M, Archibald C, De BA, Li AW, Yamada M, Chang CH et al. Eye muscle antibodies and subtype of thyroid-associated ophthalmopathy. Thyroid 2002; 12(3): 187–191. [DOI] [PubMed] [Google Scholar]
- Tani J, Wall JR. Autoimmunity against eye-muscle antigens may explain thyroid-associated ophthalmopathy. CMAJ 2006; 175(3): 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR et al. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc Natl Acad Sci USA 2001; 98(21): 12062–12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunji K, Kubota S, Stolarski C, Wengrowicz S, Kennerdell JS, Wall JR. A 63 kDa skeletal muscle protein associated with eye muscle inflammation in Graves' disease is identified as the calcium binding protein calsequestrin. Autoimmunity 1999; 29(1): 1–9. [DOI] [PubMed] [Google Scholar]
- Gunji K, De Bellis A, Li AW, Yamada M, Kubota S, Ackrell B et al. Cloning and characterization of the novel thyroid and eye muscle shared protein G2s: autoantibodies against G2s are closely associated with ophthalmopathy in patients with Graves' hyperthyroidism. J Clin Endocrinol Metab 2000; 85(4): 1641–1647. [DOI] [PubMed] [Google Scholar]
- Gunji K, De Bellis A, Kubota S, Swanson J, Wengrowicz S, Cochran B et al. Serum antibodies against the flavoprotein subunit of succinate dehydrogenase are sensitive markers of eye muscle autoimmunity in patients with Graves' hyperthyroidism. J Clin Endocrinol Metab 1999; 84(4): 1255–1262. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Taniguchi S, Yamada K, Nishio N, Okamura T, Yoshida A et al. Detection of the novel autoantibody (anti-UACA antibody) in patients with Graves' disease. Biochem Biophys Res Commun 2004; 321(2): 432–440. [DOI] [PubMed] [Google Scholar]
- Zang S, Ponto KA, Kahaly GJ. Clinical review: intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 2011; 96(2): 320–332. [DOI] [PubMed] [Google Scholar]
- Duan L, Li XF, Lu KY, Zhang CG, Hu G, Liu JZ et al. Clinical application of orbital scintigraphy with 99Tc(m)-octreotide in patients with thyroid associated ophthalmopathy. Zhonghua Yan Ke Za Zhi 2006; 42(12): 1068–1072. [PubMed] [Google Scholar]
- Dolman PJ. Evaluating Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab 2012; 26(3): 229–248. [DOI] [PubMed] [Google Scholar]
- Verity DH, Rose GE. Acute thyroid eye disease (TED): principles of medical and surgical management. Eye 2013; 27(3): 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi L, Rambaldi PF, Bizzarro A, Panza N, Di Martino S, De Bellis A et al. Indium-111 octreotide in Graves' disease and in the evaluation of active exophthalmos. Q J Nucl Med 1995; 39(2): 105–110. [PubMed] [Google Scholar]
- Kainz H, Bale R, Donnemiller E, Gabriel M, Kovacs P, Decristoforo C et al. Image fusion analysis of 99 m Tc-HYNIC-octreotide scintigraphy and CT/MRI in patients with thyroid-associated orbitopathy: the importance of the lacrimal gland. Eur J Nucl Med Mol Imaging 2003; 30(8): 1155–1159. [DOI] [PubMed] [Google Scholar]
- Sun H, Jiang XF, Wang S, Chen HY, Sun J, Li PY et al. (99m)Tc-HYNIC-TOC scintigraphy in evaluation of active Graves' ophthalmopathy (GO). Endocrine 2007; 31(3): 305–310. [DOI] [PubMed] [Google Scholar]
- Villadolid MC, Yokoyama N, Izumi M, Nishikawa T, Kimura H, Ashizawa K et al. Untreated Graves' disease patients without clinical ophthalmopathy demonstrate a high frequency of extraocular muscle (EOM) enlargement by magnetic resonance. J Clin Endocrinol Metab 1995; 80(9): 2830–2833. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Musselman R, Beard N, El-Kaissi S, Tani J, Adams CL et al. Antibodies targeting the calcium binding skeletal muscle protein calsequestrin are specific markers of ophthalmopathy and sensitive indicators of ocular myopathy in patients with Graves' disease. Clin Exp Immunol 2006; 145(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B, Gopinath B, Tani J, Wescombe L, Wall JR. Peripheral blood T lymphocyte sensitisation against calsequestrin and flavoprotein in patients with Graves' ophthalmopathy. Autoimmunity 2008; 41(5): 372–376. [DOI] [PubMed] [Google Scholar]
- De Bellis A, Conzo G, Cennamo G, Pane E, Bellastella G, Colella C et al. Time course of Graves' ophthalmopathy after total thyroidectomy alone or followed by radioiodine therapy: a 2-year longitudinal study. Endocrine 2012; 41(2): 320–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.