Abstract
An open question in evolutionary biology is how does the selection–drift balance determine the fates of biological interactions. We searched for signatures of selection and drift in genomes of five endosymbiotic bacterial groups known to evolve under strong genetic drift. Although most genes in endosymbiotic bacteria showed evidence of relaxed purifying selection, many genes in these bacteria exhibited stronger selective constraints than their orthologs in free-living bacterial relatives. Remarkably, most of these highly constrained genes had no role in the host–symbiont interactions but were involved in either buffering the deleterious consequences of drift or other host-unrelated functions, suggesting that they have either acquired new roles or their role became more central in endosymbiotic bacteria. Experimental evolution of Escherichia coli under strong genetic drift revealed remarkable similarities in the mutational spectrum, genome reduction patterns and gene losses to endosymbiotic bacteria of insects. Interestingly, the transcriptome of the experimentally evolved lines showed a generalized deregulation of the genome that affected genes encoding proteins involved in mutational buffering, regulation and amino acid biosynthesis, patterns identical to those found in endosymbiotic bacteria. Our results indicate that drift has shaped endosymbiotic associations through a change in the functional landscape of bacterial genes and that the host had only a small role in such a shift.
Introduction
The interactions between biological entities and their role in evolution has enthralled scientists for decades, but the causes and consequences of such interactions remain poorly characterized. Starring these biological interactions is the symbiosis of bacteria with plants and animals, considered an important engine of eukaryote ecological diversification (McFall-Ngai et al., 2013; Archibald, 2014).
The mutualistic symbiosis between bacteria and insects is one of the most widespread associations in nature. The obligate symbiosis between the pea aphid (Acyrthosiphon pisum) and its bacterial endosymbiont Buchnera aphidicola (Shigenobu et al., 2000) provides a good example of such mutualistic associations. These bacteria are restricted to highly specialized host cells, are maternally inherited (Koga et al., 2012) and exhibit specific molecular trafficking with the host (Nakabachi et al., 2014; Price et al., 2014). The genomes of the aphid and the bacterium have been co-transmitted for millions of generations and each seems to influence its partner: the bacterium provides nutrients to the host (Shigenobu et al., 2000; Tamas et al., 2002; van Ham et al., 2003) and allows it to thrive on an otherwise unbalanced diet (Law and Lewis, 1983), while the host houses and transmits the bacterium under benign environmental conditions (Hansen and Moran, 2011; Macdonald et al., 2012). This association, however stable, is constrained by the small effective population sizes and asexual reproduction of endosymbiotic bacteria (Moran, 1996; Mira et al., 2001; Nilsson et al., 2005) and the inherent nucleotide deletion bias of their genomes (Mira et al., 2001; Kuo and Ochman, 2009), leading to their genome size reduction (McCutcheon and Moran, 2012). The population bottlenecks during bacterial transmission to the host offspring makes natural selection less efficient, which combined with the lack of recombination and repair genes has led to an increase in the mutational load of endosymbiotic genomes (Moran, 1996; Fares et al., 2002b; van Ham et al., 2003; Wernegreen, 2011). Nonetheless, signatures of purifying selection (Toft and Fares, 2008) and positive selection (Fares et al., 2002a) have been identified in endosymbiotic genes not directly linked to the purpose of providing the host with nutrients.
Patterns of selection in endosymbiotic bacteria may result from different levels of selection, including selection imposed by the host and that emerging in a symbiotic context but being independent from the host. Teasing apart these levels of selection remains a major challenge. In this study, we investigate whether the evolutionary landscape of endosymbiotic genes has changed as a result of genetic drift or selection imposed by the diet requirements of the host. To address this question, here we investigate the selective patterns of five major endosymbiotic bacterial groups and characterize the genome and transcriptome changes of the bacterium Escherichia coli K12 evolving experimentally under population dynamics that emulate those of maternally inherited endosymbiotic bacteria.
Materials and methods
Endosymbiotic and free-living bacterial genomes
Endosymbiotic bacterial genomes and those of their free-living relatives were downloaded from the SymbioGenomesDB database (Reyes-Prieto et al., 2015). We used endosymbiotic genomes of: aphids (B. aphidicola strain JF98, from A. pisum; B. aphidicola strain Sg, from Schizaphis graminum), carpenter ants (Candidatus Blochmannia floridanus and Ca. Blochmannia pennsylvanicus strain BPEN), tsetse flies (Wigglesworthia glossinidia, from Glossina brevipalpis; and W. morsitans from Glossina morsitans morsitans), sharpshooters (Candidatus Baumannia cicadellinicola strains HC and BGSS) and cockroaches (Blattabacterium strain Bge, from Blatella germanica; and strain BPLAN, from Periplaneta americana). Pairs of endosymbiotic genomes used in this study were similar in size. B. aphidicola strain Ak from Acyrthosiphon kondoi was used to ascertain the lack of saturation of synonymous sites. We used E. coli strain K12 substrain MG1655 and Salmonella enterica serovar Typhi (S. typhi) as free-living relatives of gamma-proteobacteria endosymbionts. Flavobacterium branchiophilum and F. psychrophilum were used as free-living relatives of Bacteroidetes endosymbionts.
Analysis of selective constraints
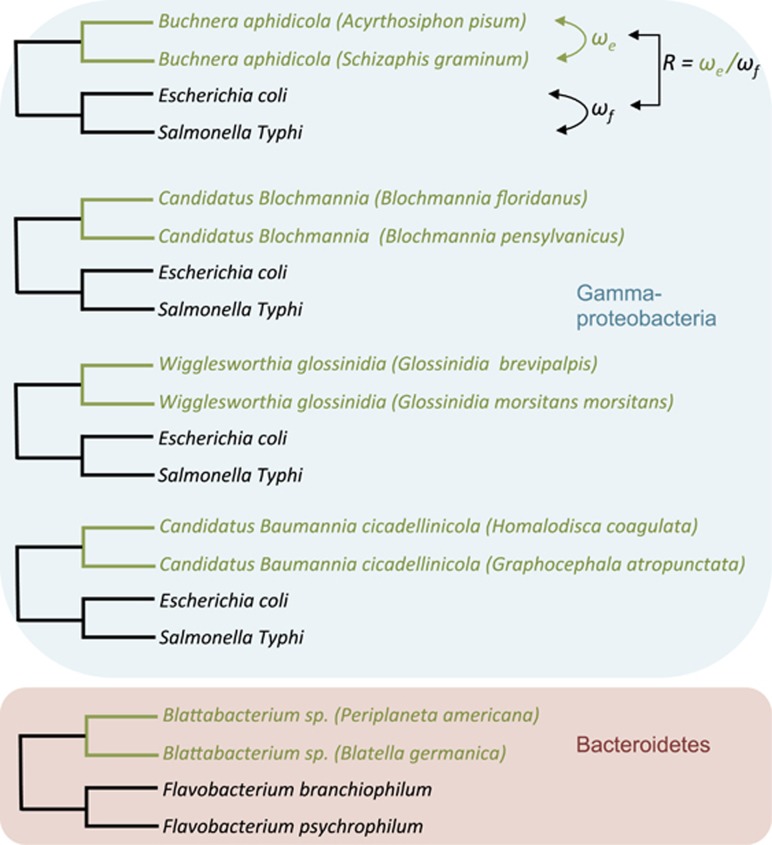
For each pair of bacterial genomes, we built pairwise sequence alignments for orthologous genes. This resulted in seven groups of alignments corresponding to five pairs of endosymbiotic bacteria (namely Buchnera, Ca. Blochmannia, Wigglesworthia, Ca. Baumannia and Blattabacterium) and two pairs of free-living bacterial genomes (E. coli/S. typhi and Flavobacterium). We used MAFFT version 7 (Katoh and Standley, 2013) to align amino acid sequences and then used these alignments to guide the alignment of nucleotide sequences. In total, we obtained reliable multiple sequence alignments for 483, 462, 514, 448 and 348 protein-coding genes in Buchnera, Ca. Blochmannia, Wigglesworthia, Ca. Baumannia and Blattabacterium sp., respectively. We estimated the strength of selection by calculating the non-synonymous-to-synonymous divergence ratio (ω=dN/dS) using yn00 implemented in PAML version 4.7 (Yang, 2007) (Figure 1). The parameter ω is an indicator of selective pressure, with values of ω=1, ω<1 and ω>1, indicating neutral evolution, purifying selection and positive selection, respectively. The closer ω is to 0, the stronger is purifying selection in purging deleterious nonsynonymous mutations. Conversely, the closer ω is to 1, the weaker is selection in eliminating deleterious mutations. To compare the selective pressures acting in endosymbiotic bacteria vs those acting in free-living bacteria, we used two strains within each of the endosymbiotic bacterial groups and obtained two ω estimates for each of the genes: one ω was estimated by comparing the gene sequences of the endosymbiotic strains (we called these ωe) and another was estimated from the comparison of the sequences of their free-living bacterial strain relatives (ωf) (Figure 1). Then, we compared ωe with ωf using the ratio R (R=ωe/ωf). In general, R>1 is expected because the efficacy of selection is higher in free-living bacteria (Kuo et al., 2009). Therefore, R is a measure of the relative constraints on endosymbiotic bacterial genes, with R=1, R<1 and R>1 indicating equal constraints, stronger constraints and weaker constraints in endosymbiotic than in free-living bacteria, respectively.
Figure 1.
Determining the relative strength of selective constraints in endosymbiotic bacterial genomes. We calculated the ratio between non-synonymous nucleotide substitutions per non-synonymous site (dN) and nucleotide substitutions per synonymous site (dS) (ω=dN/dS) to estimate the strength of selection on protein-coding genes. This ratio was estimated between pairs of endosymbiotic genomes within each of the five endosymbiotic systems (ωe) and between pairs of relative free-living bacteria (ωf). We used as endosymbiotic gamma-proteobacteria: (1) Buchnera aphidicola (strains from aphids Acyrthosiphon pisum and from Schizaphis graminum), (2) Candidatus Blochmannia (Blochmannia floridanus and Blochmannia pennsylvanicus), (3) Wigglesworthia sp. (Wigglesworthia glossinidia and Wigglesworthia morsitans) and (4) Candidatus Baumannia cicadellinicola (Homalodisca coagulata and Graphocephala atropunctata). We used as endosymbiotic bacteria from the Bacteroidetes group Blattabacterium sp. (Blattabacterium from Blatella germanica and from Periplaneta americana). We used Escherichia coli and Salmonella enterica as the external free-living bacteria pair relatives of gamma-proteobacteria and Flavobacterium branchiophilum and F. psychrophilum as free-living bacterial relatives of Bacteroidetes endosymbionts. We analyzed how the selective constraints on genes varied when comparing endosymbiotic bacteria (in green) with their free-living relatives (in black). The selective constraints on endosymbiotic bacterial genomes relative to their free-living bacterial relatives was calculated as R=ωe/ωf.
Non-parametric bootstrapping
To test the significance of the convergent selective constraints for genes among endosymbiotic bacteria, we performed a test based on non-parametric bootstrap. Briefly, we randomly selected a set of genes from each symbiotic bacterial group genome alignment generated to determine the gene selective constraint. In total, we generated five lists of constrained genes, one for each bacterial group. Then we asked how many genes were found convergently in one, two, three or four of the lists. We repeated this procedure 105 times and drew a distribution that we used as the null distribution from which we calculated the probability of the observed convergences in the real data sets.
Experimental evolution of bacteria under genetic drift
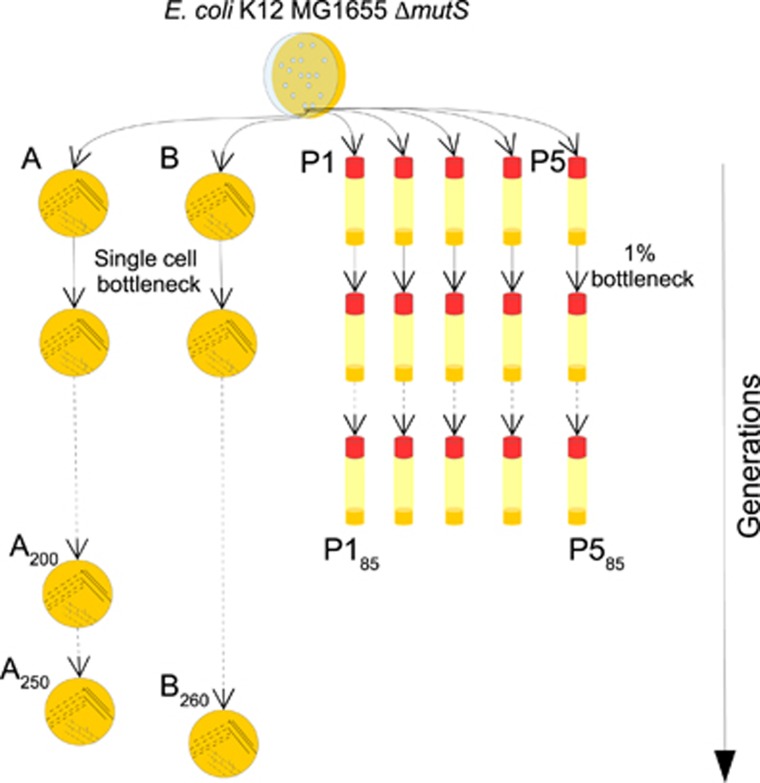
The long-term evolution experiment under strong genetic drift of lines A and B, each of which represent lines of E. coli K12 strain MG1655, is described elsewhere (Alvarez-Ponce et al., 2016). Additionally, from the same ancestral colony, another five independent experimental evolution lines were established in liquid Luria Broth media (LB, Conda laboratory, Madrid, Spain). Each population lineage was serially passaged each 24 h by diluting 1:100 into 10 ml of fresh LB medium in 50 ml Falcon tubes (Corning, Mexico DF, Mexico). Population lineages were passaged 85 times (an estimated 561 generations, 6.6 generations per passage) (Figure 2).
Figure 2.
Experimental evolution of Escherichia coli under two population dynamics. We evolved two independent clonal lines (A and B) and five population lines (P1–P5) derived from a single ancestral population of a hypermutagenic strain of E. coli lacking the repair gene mutS under strong population bottlenecks and rich growth medium. Evolution proceeded with daily passaging a single colony to a new plate for 250 days in line A and 260 days in line B or by daily passaging 1% (100 μl) of populations P1–P5 to fresh LB broth (10 ml). Genomes were sequenced at the end of the evolution experiment.
Whole-genome sequencing
Genome sequences for lines A and B were obtained from our recent work (Alvarez-Ponce et al., 2016). For the evolution experiment in liquid media, we performed paired-end Illumina whole-genome sequencing (TrueSeq DNA PCR-free HT, Illumina Inc., San Diego, CA, USA) at final time (561 generations). Sequencing was performed in a MiSeq benchtop sequencer (Illumina Inc.), using a 2 × 150 bp with 300 cycles configuration. Libraries and sequencing were performed at ValGenetics SL sequencing facility (Valencia, Spain). Single-nucleotide polymorphisms (SNPs) and indels were identified with the breseq v 0.24rc4 (version 4) pipeline (Deatherage and Barrick, 2014) using our E. coli parental genome reported previously (Sabater-Muñoz et al., 2015). We identified radical and non-radical amino-acid substitutions in the evolved populations by classifying substitutions into two categories: (a) radical substitutions: are those changes that involve a change in the charge, polarity or polarity and volume of the original amino acids, and (b) non-radical substitutions: are those replacements that occur between physically and chemically equivalent amino acids. We used the classification of amino acids according to their physical–chemical properties following a previous study (Wernegreen, 2011).
RNA sequencing and analysis
Triplicate cultures were set for the ancestral population and clonal lines at time points 200 and 250 from freshly recovered glycerol stocks in LB at 37 °C with continuous shaking (220 r.p.m.) for ~4 h (until achieving an OD600≃0.6). Cultures were stopped with RNAprotect bacterial reagent (Qiagen, Valencia, CA, USA). Total RNA was extracted from 1.5 ml of stopped–harvested cells using the RNeasy Mini Kit (Qiagen) following the manufacturer's protocol. RNA (integrity number >8) was depleted of ribosomal RNA using the Ribo-Zero rRNA Gram-Negative Removal Kit MRZGN126 (Epicentre-Illumina, Madison, WI, USA). Indexed RNAseq libraries were constructed using strand-specific cDNA synthesis (TruSeq RNA Library Preparation Kit, Illumina), pooled in equimolar concentration and subjected to single-end 50 bp Illumina sequencing in an Illumina HiSeq2000 platform using a 2 × 100 cycles configuration. RNA ribosomal depletion, library construction and sequencing were carried out at LifeSequencing S.L. (Valencia, Spain).
Raw sequences were processed with the RobiNA (Lohse et al., 2012) and Rockhopper v.2.0.3 (McClure et al., 2013) software to determine differentially expressed genes among time points. Briefly, fastq files were subjected to quality trimming, removal of short sequences (<20 pb) and mapped against the E. coli K-12 str. MG1655 NCBI reference genome NC_000913 using Bowtie2 with 2 bp mismatch option. After gene counts, edgeR (Robinson et al., 2010) or DEseq (Anders and Huber, 2010) were used to identify significant differential expression once corrected for multiple testing using the Benjamini–Yekutieli method (Benjamini and Yekutieli, 2005). Each list of differentially expressed genes was subjected to gene ontology (GO) term classification using the PANTHER classification System (http://www.pantherdb.org/), including a GO enrichment analysis, with a P-value cutoff of <0.05. A semantic similarity score, simRel (Schlicker et al., 2006), was used to summarize and remove redundant GO terms in each list, as implemented in the REVIGO software with medium (0.7) allowed similarity (Supek et al., 2011).
Files containing reads for the nine RNA libraries have been deposited in the Sequence Read Archive (http:/ncbi.nlm.nih.gov/sra) under accession number SRP074670.
Results
Signatures of drift and selection in endosymbiotic bacteria of insects
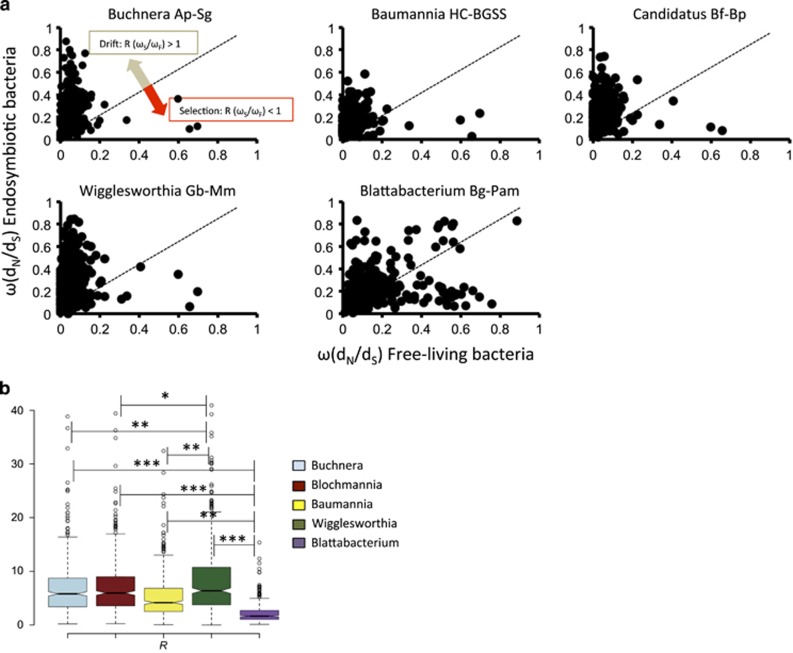
Endosymbiotic bacterial genomes evolve under more relaxed selective constraints when compared with their closest free-living relatives (Moran, 1996; Wernegreen, 2002; Moran et al., 2008; Wernegreen, 2011). However, selection has been proposed to be stronger upon endosymbiotic bacterial genes that are key in producing metabolites for the insect host than on other genes unrelated to the metabolism of the host (Clark et al., 2001; Hansen and Moran, 2014; Price et al., 2014; Bennett and Moran, 2015). To determine the strength of selection on endosymbiotic bacterial genes relative to their free-living bacterial orthologs, we estimated the non-synonymous-to-synonymous rates ratio (ω=dN/dS) for genes of five independent groups of endosymbiotic bacteria (ωe) and two independent groups of free-living bacteria (ωf) (see Material and methods section; Figure 1). To compare ωe with ωf, we calculated R (R=ωe/ωf).
Most of the genes in endosymbiotic bacterial genomes (Table 1) exhibited relaxed constraints compared with their free-living relatives (R>1) (Figure 3a). Comparison of endosymbiotic genomes (Buchnera A. pisum and A. kondoi) that were phylogenetically closer did not change the results (Supplementary Table S1), suggesting that saturation of synonymous sites was not affecting our observations. The more relaxed constraints in endosymbiotic than their free-living bacterial relatives is consistent with reduced efficacy of natural selection in endosymbionts. However, a substantial number of genes in endosymbiotic bacteria (Table 1) showed stronger selective constraints than in their free-living relatives (R<1). The endosymbionts Blattabacterium sp. showed the lowest median R-value (median R=1.65), followed by Ca. Baumannia (median R=4.17), Buchnera (median R=5.81), Ca. Blochmannia (median R=5.95) and Wigglesworthia (median R=6.39) (Figure 3b). Blattabacterium sp. showed significantly stronger relative constraints than the Buchnera–Ca. Blochmannia group (Wilcoxon's rank test: P<2.2 × 10−16), Ca. Baumannia (Wilcoxon's rank test: P<2.2 × 10−16) and the Wigglesworthia endosymbiont (Wilcoxon's rank test: P<2.2 × 10−16). Wigglesworthia endosymbionts showed more relaxed constraints than Ca. Baumannia (Wilcoxon's rank test: P=3.16 × 10−15), Buchnera (Wilcoxon's rank test: P=0.003) and Ca. Blochmannia (Wilcoxon's rank test: P=0.02). Buchnera and Ca. Blochmannia showed more relaxed constraints than Ca. Baumannia (Wilcoxon's rank test: P=1.99 × 10−7), but there was no difference in the relative constraints between Ca. Blochmannia and Buchnera (Wilcoxon's rank test: P=0.37). The stronger constraints in Blattabacetrium sp. stem from a greater proportion of this endosymbiont's genes being involved in the urea metabolism of the host (Gonzalez-Domenech et al., 2012; Patino-Navarrete et al., 2013), with these genes evolving under strong constraints. All together, these results indicate shifts in the selective constraints and, perhaps, changes in the encoded functions, of some bacterial genes in endosymbionts compared with free-living bacteria.
Table 1. Relative selective constraints in endosymbiotic bacterial genomes.
| Endosymbiotic group | No. of genes with R⩽1 (%) | No. of genes with R>1 (%) |
|---|---|---|
| Buchnera | 33 (6.8%) | 454 (93.2%) |
| Candidatus Blochmannia | 45 (9.6%) | 422 (90.4%) |
| Wigglesworthia | 34 (6.6%) | 484 (93.4%) |
| Candidatus Baumannia | 17 (3.8%) | 431 (96.2%) |
| Blattabacterium | 99 (28.1%) | 253 (71.9%) |
Figure 3.
Signatures of natural selection and genetic drift in endosymbiotic bacteria of insects. The strength of selection was determined as the ratio between non-synonymous nucleotide substitutions per non-synonymous site (dN) and synonymous nucleotide substitutions per synonymous site (dS) (ω=dN/dS) for each genome pair. (a) To determine the relative strength of selection on endosymbiotic genomes, we divided the ω of each symbiotic gene (ωe) by that of its ortholog in its free-living bacterial relatives (ωf) and compared this ratio (R) with 1. Values of R>1 imply that ωe>ωf, hence endosymbiotic genes evolved under relaxed selective constrains or under increased genetic drift. Conversely, R<1 implies stronger constrains on endosymbiotic genes than on their free-living bacterial orthologs. (b) The relative efficiency of natural selection, or genetic drift, for each of the endosymbiotic genomes of this study was compared. Differences were tested using Wilcoxon's rank test with significant values being indicated with *P<0.05, **P<0.01 and ***P<10−6.
Convergent host-independent evolution in endosymbiotic bacteria of insects
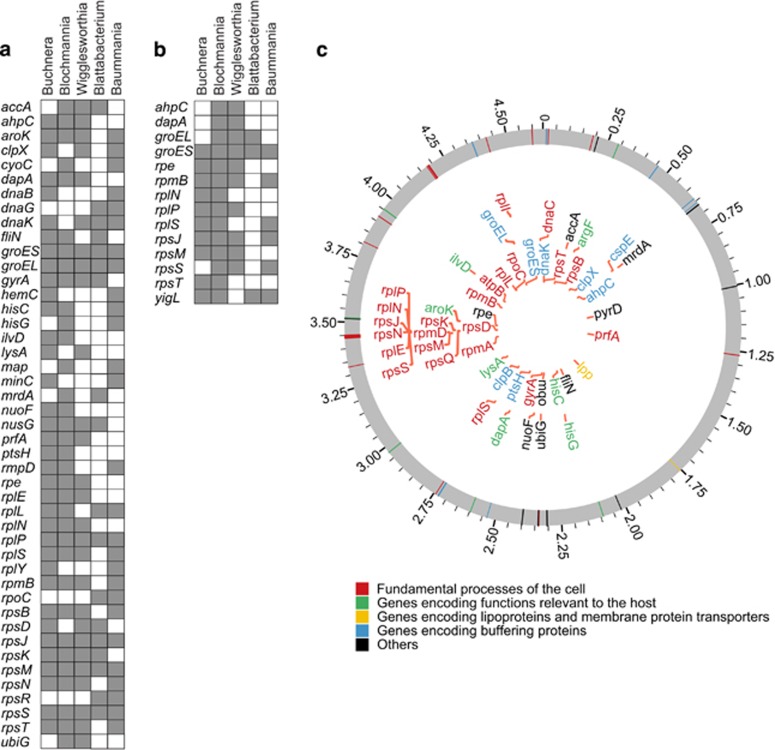
A number of endosymbiotic bacterial genes evolved under relatively moderate selective constraints (that is, 1<R<2) or under stronger selective constraints than in their free-living bacterial relatives (R<1) (Table 1 and Supplementary Table S1). Six of the genes were highly constrained in all the 5 endosymbiotic bacterial groups, 11 in four, 17 in three and 17 in two (Figure 4a). The number of genes constrained convergently in different endosymbiotic bacteria was significantly higher than expected (Material and methods section) (Randomization test: P<10−6, P<10−5 and P=0.001 for convergences in five, four and three endosymbionts, respectively). The set of strongly constrained genes (R<1) (Supplementary Table S1) included one, five and eight genes found in five, four and three independent endosymbiotic bacteria (Figure 4b), respectively, with these convergences being significant (P<10−3, P<10−3 and P<10−3, respectively). Genes constrained in endosymbiotic bacteria encoded chaperones and proteins involved in transcription and translation (Figure 4c).
Figure 4.
Endosymbiotic bacteria of insects converge in their selective constraints at genes that are unrelated to the insect host. We analyzed the distribution of the selective constraints among endosymbiotic genes and studied the convergences among the five independent endosymbiotic groups. We studied two sets of genes, (a) one in which the ratio between the symbiotic and free-living bacterial non-synonymous-to-synonymous rates ratio (R=ωe/ωf) is R<2, hence this set includes genes with strong selective constraints (R<1) and slightly relaxed constraints (1<R<2), and (b) a set of strongly constrained symbiotic genes when compared with their free-living bacterial orthologs R<1. Gray-colored squares in the matrix indicate genes convergently constrained between two or more endosymbiotic groups; white squares are genomes in which such genes are under relaxed constraints. (c) Convergently constrained genes in endosymbiotic genomes. These genes are color-coded according to their functional classification using GO terms. Only seven genes were relevant to the metabolism of the bacterial host (green-colored genes).
Among all the constrained genes, 7 (lysA, argF, hisC, hisG, ilvD, aroK and dapA) were involved in the synthesis of amino acids, perhaps important to supplementing host diets, while at least 35 of them were host unrelated and linked to translation (ribosomal-coding genes rplP, rplN, rpsJ, rpsN, rplE, rpsS, rpsK, rpsM, rpsQ and rpmD; Hypergeometric test with Bonferroni's correction: P=1.70 × 10−12) and protein-binding or stress-related functions (chaperones and chaperonins clpX, dnaK, groES, groEL and ahpC; enrichment of the category ‘binding': P=1.53 × 10−8) (Figure 4c). Some of the constrained genes (dnaK, groES and groEL) have been previously reported to buffer the effects of deleterious mutations (Fares et al., 2002b; Bogumil and Dagan, 2010; Williams and Fares, 2010; Sabater-Muñoz et al., 2015; Aguilar-Rodriguez et al., 2016; Kadibalban et al., 2016) (Figure 4c). The strong selective constraints in genes overlapping among endosymbionts from hosts with different diet requirements support host-independent, however contextual to endosymbiosis, selective constraints of such genes.
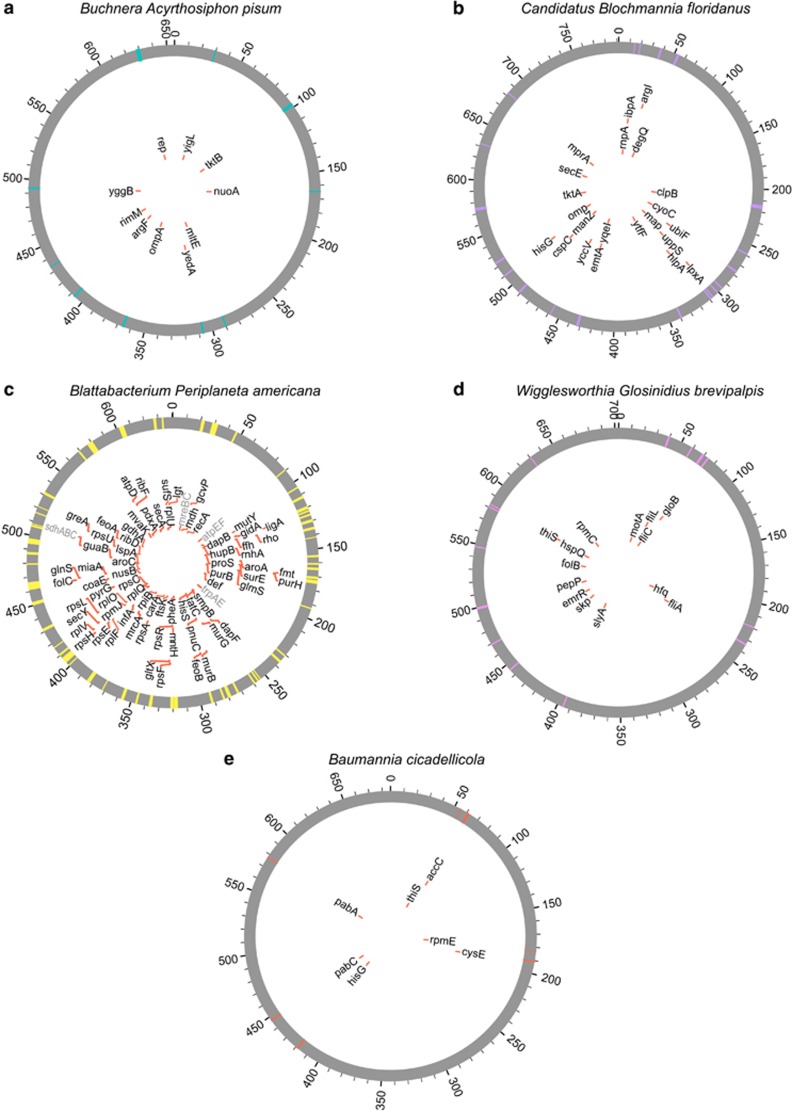
Some of the genes specifically constrained (R<1) in a single endosymbiont lineage but not in others were associated with bacterial pathways that are key in supplementing the metabolism of the insect host (Figures 5a–e). This includes genes from Buchnera involved in amino-acid metabolism (tktB, mltE, ompA, argF) and export of amino acids to the host (yedA, yggB) (Figure 5a) or genes involved in nitrogen metabolism in the symbionts Blochmannia, Wigglesworthia and Blattabacterium (Figures 5b–d). Other symbiont-specific constrained genes were, nevertheless, host unrelated, being involved in stress response in the Blochmannia (clpB, yccV, cspC, ibpA) and Wigglesworthia (hfq, skp, hspQ) endosymbionts or the flagellum biosynthesis pathway in Wigglesworthia endosymbiont (fliC, fliL, fliA).
Figure 5.
Genome distribution of genes with strong selective constraints in endosymbiotic bacteria. We identified genes with strong constraints in each of the five endosymbiotic bacterial lineages: (a) Buchera aphidicola (from Acyrthosiphon pisum); (b) Candidatus Blochmannia (Blochmannia floridanus); (c) Blattabacterium from Periplaneta americana; (d) Wigglesworthia glossinidia; and (e) Ca. Baumannia cicadellinicola. Genes indicated in the circle-representation of the endosymbiotic genomes are those that were specifically identified in that endosymbiotic genome and not the others from the same group. These genes are mostly related to the metabolism of the bacterium that interacts with the metabolism of the insect host.
The mutational spectrum of experimentally evolving bacteria under drift
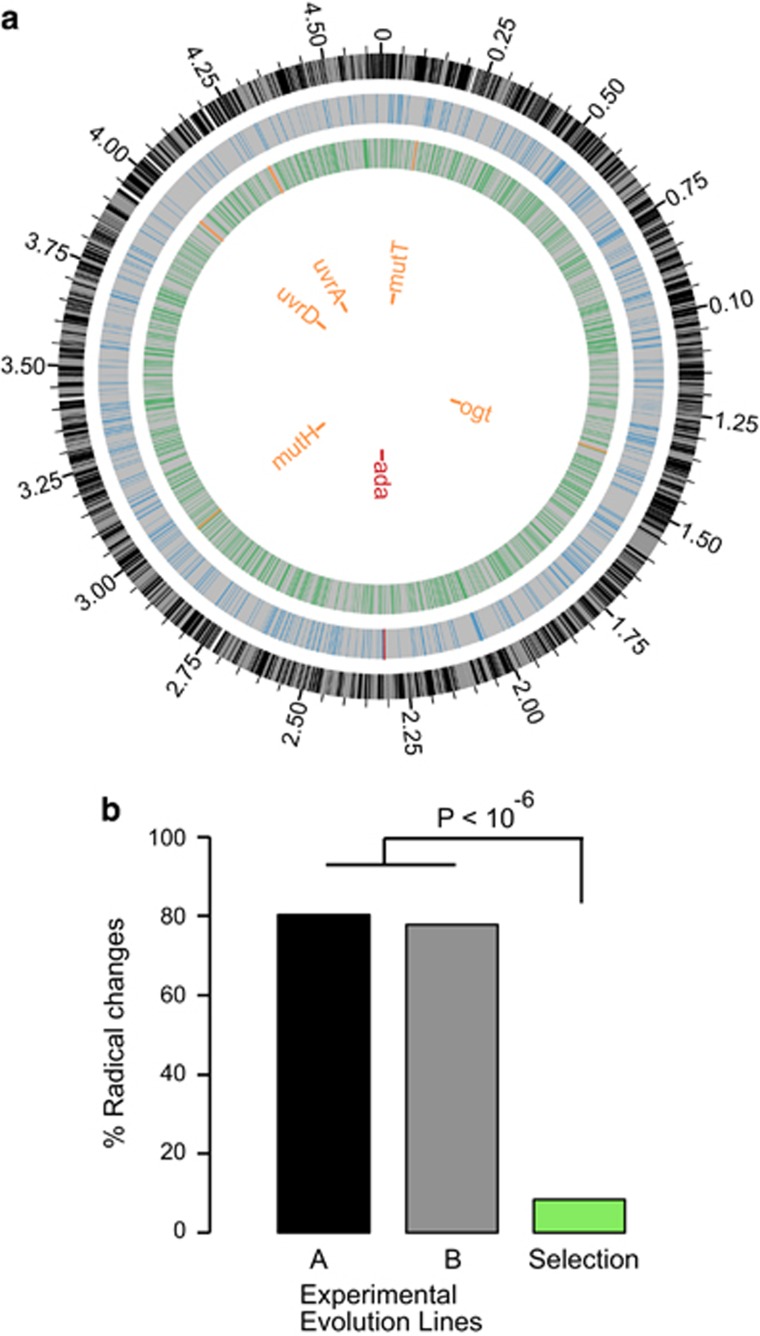
To determine how genetic drift alone affects the genome evolution of bacteria, we examined the mutational dynamics of E. coli bottlenecked populations through an evolution experiment conducted in our laboratory (Alvarez-Ponce et al., 2016). The fact that endosymbiotic bacteria of insects are uncultivable implies that our experimental setup does not emulate the metabolic flux from the host to the endosymbiotic bacteria in nature but allows emulating the population dynamics of endosymbiotic bacteria. Genome sequencing after 5500–5750 generations of evolution and comparison of these genomes with the ancestral population identified 723 and 1268 mutations in lines A and B, respectively (Supplementary Tables S2 and S3). The differences in the mutational profiles found between lines A and B (Supplementary Table S4) were likely due to a greater number of repair genes affected by mutations in line B (including genes ogt, mutH, uvrD, uvrA, mutT) than in line A (ada) (Figure 6a). Importantly, repair genes that mutated in line B are absent from the genomes of the primary symbiotic bacteria of aphids (Dale et al., 2003; Moran et al., 2008; Moran et al., 2009).
Figure 6.
Experimental evolution of Escherichia coli reveals the contribution of the selection–drift balance to the evolution of endosymbiotic genes. (a) Distribution of mutations of lines A and B at the end of the evolution experiment. The outermost circle refers to the genome of E. coli K12 MG1655, used as reference for mapping the mutations in the evolution experiment. The blue circle refers to line A, whereas the green circle represents line B. Genes are indicated with vertical lines to each of the circles. Mutated repair genes for line A (red) and line B (orange) are indicated. (b) Proportion of radical mutations during the evolution experiment of E. coli under strong genetic drift (black and grey columns) and mild genetic drift (green column). Roughly 80% of the mutations in lines A and B were radical amino-acid changes, such that the original amino acid underwent a replacement to an amino acid with different charge, polarity or volume and polarity. The population under milder drift exhibited a significantly lower proportion of its amino-acid replacements being radical (about 8%).
To compare the spectrum of mutations of our experimentally evolved lines to that of endosymbiotic bacteria of insects, we classified proteins mutated with non-synonymous SNPs in line B, which presented the greatest number of mutations, into the different GO categories (Carbon et al., 2009) as provided by AmiGO2 (http://amigo.geneontology.org). Enzymes (catalytic activities), transmembrane transporters and receptors and binding proteins (Supplementary Table S5), most of which are involved in the bacterial–environment interface and likely dispensable in a rich stable environment, were enriched for genes that mutated in our evolution experiment. Importantly, genes that are present in Buchnera were under-represented among the set of mutated genes in line A (39 out of the 408 mutated genes, 9.55%, Fisher's exact test: odds ratio F=0.27, P<2.2 × 10−16) and line B (73 out of the 820 mutated genes, 8.9%, F=0.47, P=5.17 × 10−10).
Three hundred and twenty-eight (80.39%) of the mutations of line A and 638 (77.80%) in line B were radical in terms of amino-acid charge, polarity or polarity and volume (Figure 6b). In a different evolution experiment of E. coli populations under moderate genetic drift for 85 passages (that is, ~6.6 generations per passage; Figure 2), we found 298 polymorphisms, of which 147 were non-synonymous (Supplementary Table S6), and of these, only 85 (57%) were radical. Among the mutations identified in 100% of the reads (that is, mutations fixed in the population) (Supplementary Table S7), only 12 were non-synonymous mutations, of which only 1 mutation (Asp613 to Gly in the gene bcsB) was radical (8.33%). This population thus exhibited fewer radical SNPs than lines A and B (Figure 6b, Fisher's exact test: F=44.61, P=2.96 × 10−7 for line A, and F=38.37, P=7.99 × 10−7 for line B), consistent with its larger effective population size, and hence its higher efficacy of natural selection in removing radical amino acid mutations. Therefore, this experiment suggests that, as in endosymbiotic bacteria of insects, most of the mutations accumulated in lines A and B were deleterious and were accumulated owing to the strong genetic drift imposed during their evolution.
Genome reduction in experimentally evolved bacterial populations
The estimated rate of nucleotide loss in B. aphidicola endosymbionts is 2.9 × 10−8 nucleotide losses per site per year (Gomez-Valero et al., 2004). The number of endosymbiotic bacterial generations per year varies between 15 and 50 (Humphreys and Douglas, 1997). This means that the rate of nucleotide loss in Buchnera ranges between 1.9 × 10−9 and 5.8 × 10−10 nucleotide losses per site per generation. In our experimentally evolved lines, we found a total of 41 and 62 events of gene deletion affecting 38 355 and 3106 base pairs in lines A and B, respectively. The ancestral E. coli strain used in our evolution experiments has a genome size of 4.64 Mb (Sabater-Muñoz et al., 2015), which, taking into account the number of deleted base pairs, yields rates of 1.58 × 10−6 and 1.28 × 10−7 nucleotides deleted per site per generation for lines A and B, respectively. These rates are 831 and 67 times greater, respectively, than the fastest loss rate reported for Buchnera (Gomez-Valero et al., 2004; Moran et al., 2009). Moreover, we identified the deletion of a 35 590 bp region in line A, encompassing 42 genes located in the E. coli chromosome between the pseudogene yoeG and the IS5 transposase and transactivator gene wbbL. As most deletion events only affected a few nucleotides, genome reduction through evolution by genetic drift is likely a gradual process with punctuated events of big deletions, as has also been demonstrated in B. aphidicola (Gomez-Valero et al., 2007). In total, we found more insertion events (83 insertions) than deletion events (41 deletions) in line A (binomial test: P=2 × 10−4), despite a greater number of deleted nucleotides than inserted nucleotides (binomial test: P<2.2 × 10−16). By contrast, in line B we found equivalent deletion events (62 deletions) and insertion events (56 insertions) (binomial test: P=0.32) but a greater number of nucleotides deleted than inserted (binomial test: P<2.2 × 10−22).
There were differences in the rates of nucleotide loss and gain between protein-coding and intergenic regions in the experimentally evolved lines. The protein-coding regions of line A exhibited a total of 38 355 lost nucleotides vs 62 inserted nucleotides, while intergenic regions exhibited 15 nucleotides deleted and 35 inserted. Therefore, while coding regions were shrinking (binomial test: P<2.2 × 10−22), intergenic regions were expanding in size (binomial test: P=0.007). Line B exhibited a similar pattern, with protein-coding regions bearing more deletions (3085 nucleotides) than insertions (36 nucleotides); hence these regions were shrinking (binomial test: P<2.2 × 10−22), while intergenic regions did not reveal significant differences between insertions (24 insertions) and deletions (21 deletions) (binomial test: P=0.76). The difference in the gene deletion–insertion pattern between coding and intergenic regions was significant for lines A (Fisher's exact test: F=1370.99, P<2.2 × 10−16) and B (F=96.69, P<2.2 × 10−16).
Regulatory evolution of experimentally evolved bacteria
We compared the transcriptome of E. coli from line A at different times of the evolution experiment with that of its ancestral, non-evolved line. We observed a genome-wide deregulation along the evolution experiment, affecting >65% of all the genes (Supplementary Table S8). We identified 1303 overexpressed genes and 1251 repressed genes at 200 passages and 1171 overexpressed and 1097 repressed genes at 250 passages. An additional 200 overexpressed genes and 181 repressed genes were observed between the time points 200 and 250. Therefore, during the first 200 single-colony passages, gene regulation was altered at an average rate of 12.8 genes per passage (that is, 2554 genes were deregulated during the 200 passages of evolution: rate of deregulation=2554/200=12.8), while this rate was 7.6 between 200 and 250 (381 deregulated genes/50 passages). Therefore, most regulatory changes took place at the beginning of the evolution experiment.
Genes that became upregulated during the evolution experiment participated in metabolic and regulation processes, while downregulated genes were enriched for cell localization, cellular components and biogenesis processes. Taking the category of molecular functions, upregulated genes were mainly classified in the categories of regulation of translation, electron carrier activity, nucleic acid binding, protein-binding transcription, catalytic and receptor activities and antioxidant activities (Supplementary Figure S1a). Downregulated genes on the other hand were mainly involved in transporter activity (Supplementary Figure S1b). Interestingly, in the transcriptomic analyses at different time points, the enrichment of regulatory activities changed with genes being downregulated during the first 200 passages and upregulated from 200 to 250 passages.
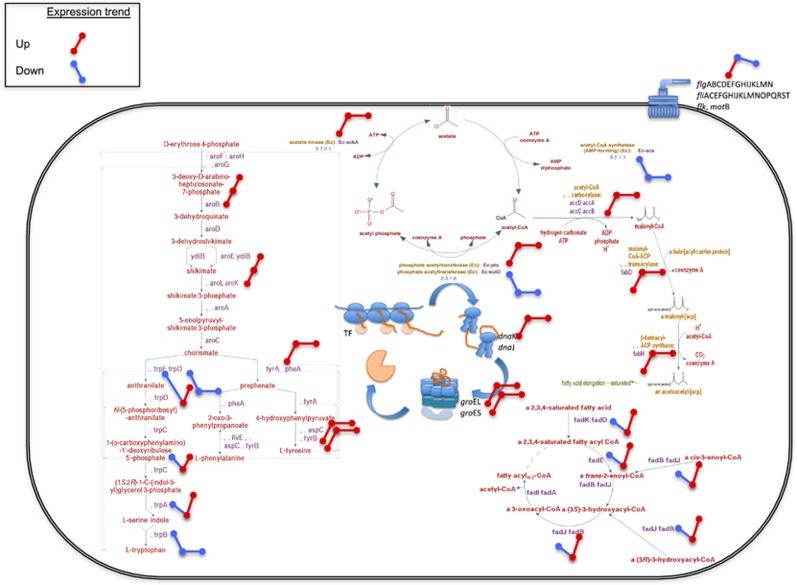
Differentially expressed genes were distributed in a total of 129 pathways (Supplementary Figure S2). Of relevance among these pathways is the one involved in acetate utilization, with many of its genes exhibiting upregulation during the experimental evolution. Strikingly, fadB, a central gene in the acetate production pathway encoding a 729 amino acid-long protein, fixed a nonsense mutation (Tyr539*) and a non-synonymous mutation (Cys494Gly) very close to the substrate-binding site (residue 500) that has very likely affected the function of this gene. The upregulation of other genes in this pathway may have represented a compensatory response to fadB mutations. Regulation was also altered for genes involved in amino-acid biosynthesis (including chorismate, isoleucine, leucine and tryptophan) and synthesis of vitamins biotin, B6 and D (Figure 7). Noticeably, amino-acid biosynthesis pathway genes underwent downregulation during the first stage of the evolution experiment but recovered their expression at the end of the experiment (Figure 7), perhaps resulting from the silencing of the regulatory genes of the operons leuABCD and trpEDCBA during the evolution experiment, an event paralleled by some endosymbiotic bacteria of insects (Moran et al., 2005). Moreover, some chaperones (groESL, dnaK), transporters and transcription factors increased their transcriptional levels along the evolution experiment, in concordance with observations in endosymbiotic bacteria of insects (Baumann et al., 1996; Moran, 1996; Douglas, 2003).
Figure 7.
Transcriptional changes due to genetic drift in experimentally evolved E. coli bacteria resembles main transcriptional changes in some endosymbiotic bacteria. In the final cell of E. coli subjected to experimental evolution under strong genetic drift, some important pathways show overexpression (in red) or downregulation (in blue) when comparing evolved transcriptomes against the ancestral transcriptome. These pathways resemble those observed in B. aphidicola, some of which are involved in the host–bacterium interaction, including synthesis of essential amino acids, and others are more linked to functions unrelated with the host, including the chaperone systems GroEL and DnaK, and those corresponding to the flagellum and bacteria motility.
Among the fully silent genes, that is, genes present in the genome but for which we obtained no RNA reads throughout our evolution experiments, 60% comprised transposons and prophages, known to have been lost soon after the establishment of endosymbiosis between bacteria and insects (van Ham et al., 2003). Noticeable is also the missing coverage of eight tRNA genes, involved in the transfer of anticodons for alanine, glutamate and isoleucine, which represents 10% of all tRNA genes in the genome. In these genes, we detected no silencing mutations, hence the absence of reads for them may be due to the silencing of their regulators. Other genes involved in central metabolism (sgrT: inhibitor of glucose uptake), regulatory genes (sdsR: stationary phase sRNA, mutS regulator; esrE: putative sRNA essential for aerobic growth; rdlB: antisense sRNA toxic peptide LdrB) and detoxification-related genes (iroK, ralA) were silenced during the evolution experiment. Among these, esrE is interesting in that it codes for an essential sRNA postulated to complement growth defects of ubiJ (yipP) deletion strains (Chen et al., 2012; Aussel et al., 2014). Silencing of this gene may therefore lead to a declined growth rate, a feature characteristic of endosymbiotic bacteria of insects housed within the limited space of insect bacteriocytes.
Discussion
As heritable symbionts are clonal, being transmitted through matrilines, the population structure and size results in much less efficient selection acting on the genomes compared with their free-living relatives (Moran, 1996; Wernegreen, 2002; Pettersson and Berg, 2007; Wernegreen, 2011). Against this general pattern, we observed higher selective pressures in some of the endosymbiotic bacterial genes compared with their free-living relatives. Such constrained genes mainly encode functions contributing to buffering the deleterious effects of mutations. It is possible, however, that stronger constraints at some of these genes, such as groE (Henderson et al., 2013), may result from the usage of alternative, previously un-exploited, functions that compensate those that were lost after symbiosis. Moreover, the range of functions of protein interaction partners increases with decreased genome size (Kelkar and Ochman, 2013). This increase in the number of functions of a gene would lead to increased protein functional density and selective constraints on symbiotic genes (that is, dN/dS would decrease as the number of functions increases). Moonlighting proteins have been identified among chaperones, transcription and translation proteins (Henderson et al., 2013; Liu et al., 2014; Gancedo et al., 2016), categories that include the strongly constrained proteins found in our study. This supports a possible shift in the function of such constrained genes after symbiosis.
Has the genome dynamics of the endosymbiont been driven by selection imposed by the host? There is extensive genomic and metabolic integration between the host and the endosymbiotic bacterium. Roughly, 10% of the genes in B. aphidicola, the symbiotic bacteria of aphids, are devoted to the synthesis of essential amino acids needed by the insect host (Moran et al., 2005), many of which are being regulated by proteins from the aphid (Price et al., 2014). An aphid-encoded protein has been shown to localize within Buchnera cells (Nakabachi et al., 2014), and the aphid host has allowed bacteria by the loss of genes underlying immune responses to Gram-negative bacteria (Gerardo et al., 2010; International Aphid Genomics, 2010). Finally, host developmental age seems to impact the transcriptome of the endosymbiotic bacterium in aphids (Bermingham et al., 2009). Despite this integration, we find that signatures of sequence evolution are unrelated to the host, with evidence for strong constraints being found in genes encoding proteins that buffer the consequences of genetic drift.
In support of the predominant role of bacterial population dynamics on the evolution of their genomes, we found that the genomic and transcriptomic evolutionary trajectories of experimentally evolved E. coli populations exhibit striking coincidences with the evolution of Buchnera and many other endosymbiotic bacteria. Given the short evolutionary time of our evolution experiment, such similarities between experimentally evolved and endosymbiotic bacteria support that most events of gene loss and evolution may have taken place during the first stages of bacterial symbiosis with insects and are the product of chance. The role of the host in these genome evolutionary dynamics would therefore be limited to the provisioning of a stable and rich cellular environment to the bacterium, hence relaxing the selective constraints on most endosymbiotic bacterial genes. Therefore, the successful relationship between the aphids and their bacteria is likely the result of three main events: (a) the maintenance after the infection of bacterial genes essential for the host, (b) the evolution in the bacterium of mechanisms for mutational buffering (Moran, 1996; Fares et al., 2002b; Sabater-Muñoz et al., 2015), and (c) an increase in the functional complexity of retained proteins in endosymbionts to compensate for their irreversible degenerative functional evolution.
Genome size reduction is symptomatic of all known symbiotic bacteria (Moran, 1996; Wernegreen, 2002). The compensation of bacterial functions by the host has been proposed to facilitate gene or functional loss in symbiotic bacteria over time, forcing a vertiginous fall of the lineage into what some authors call ‘symbiosis rabbit hole' (Bennett and Moran, 2015). This hypothesis predicts a faster gene loss in endosymbionts than in host-devoid systems in which bacteria evolve under genetic drift. Our observation of a faster rate of genome reduction in experimentally short-time evolved bacteria suggests faster rates of gene loss in endosymbiotic bacteria at the beginning of the symbiosis, which may have slowed down as the density of essential genes for sustaining minimal bacterial life, the host or both increased (Tamas et al., 2002).
The finding in our experimentally evolved lines of genome-wide deregulatory dynamics similar to those of endosymbiotic bacteria supports a prominent role for chance in the evolution of endosymbiotic bacteria. Under this view, chance would lead to convergent patterns of gene evolution and loss in bacteria while the survival of bacteria–host associations would be possible if such patterns were compatible with the metabolisms of the host. This view does not require invoking the necessity generated by the host of having a balanced diet but has likely emerged neutrally as a result of the irreversible genomic decay of endosymbiotic bacteria (Bennett and Moran, 2015).
Acknowledgments
This work was supported by Science Foundation Ireland (12/IP/1637) and grants from the Spanish Ministerio de Economía y Competitividad (MINECO-FEDER; BFU2012-36346 and BFU2015-66073-P) to MAF. DAP and CT were supported by Juan de la Cierva fellowships from MINECO (references: JCI-2011-11089 and JCA-2012-14056, respectively). DAP is supported by funds from the University of Nevada, Reno, NV, USA.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Aguilar-Rodriguez J, Sabater-Munoz B, Montagud-Martinez R, Berlanga V, Alvarez-Ponce D, Wagner A et al. (2016). The molecular chaperone DnaK is a source of mutational robustness. Genome Biol Evol 8: 2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Ponce D, Sabater-Munoz B, Toft C, Ruiz-Gonzalez MX, Fares MA. (2016). Essentiality is a strong determinant of protein rates of evolution during mutation accumulation experiments in Escherichia coli. Genome Biol Evol 8: 2914–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald J. (2014) One Plus One Equals One: Symbiosis and the Evolution of Complex Life. Oxford University Press: Oxford, UK. [Google Scholar]
- Aussel L, Loiseau L, Hajj Chehade M, Pocachard B, Fontecave M, Pierrel F et al. (2014). ubiJ, a new gene required for aerobic growth and proliferation in macrophage, is involved in coenzyme Q biosynthesis in Escherichia coli and Salmonella enterica serovar Typhimurium. J Bacteriol 196: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Baumann L, Clark MA. (1996). Levels of Buchnera aphidicola chaperonin groEL during growth of the aphid Schizaphis graminum. Curr Microbiol 32: 7. [Google Scholar]
- Benjamini Y, Yekutieli Y. (2005). False discovery rate controlling confidence intervals for selected parameters. J Am Stat Assoc 100: 10. [Google Scholar]
- Bennett GM, Moran NA. (2015). Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci USA 112: 10169–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham J, Rabatel A, Calevro F, Vinuelas J, Febvay G, Charles H et al. (2009). Impact of host developmental age on the transcriptome of the symbiotic bacterium Buchnera aphidicola in the pea aphid (Acyrthosiphon pisum. Appl Environ Microbiol 75: 7294–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogumil D, Dagan T. (2010). Chaperonin-dependent accelerated substitution rates in prokaryotes. Genome Biol Evol 2: 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S et al. (2009). AmiGO: online access to ontology and annotation data. Bioinformatics 25: 288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Y, Li Y, Li Y, Fu N, Ye J et al. (2012). Esre: a novel essential non-coding RNA in Escherichia coli. FEBS Lett 586: 1195–1200. [DOI] [PubMed] [Google Scholar]
- Clark JW, Hossain S, Burnside CA, Kambhampati S. (2001). Coevolution between a cockroach and its bacterial endosymbiont: a biogeographical perspective. Proc Biol Sci 268: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C, Wang B, Moran N, Ochman H. (2003). Loss of DNA recombinational repair enzymes in the initial stages of genome degeneration. Mol Biol Evol 20: 1188–1194. [DOI] [PubMed] [Google Scholar]
- Deatherage DE, Barrick JE. (2014). Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol 1151: 165–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AE. (2003). The nutritional physiology of aphids. Adv Insect Physiol 31: 68. [Google Scholar]
- Fares MA, Barrio E, Sabater-Munoz B, Moya A. (2002. a). The evolution of the heat-shock protein GroEL from Buchnera, the primary endosymbiont of aphids, is governed by positive selection. Mol Biol Evol 19: 1162–1170. [DOI] [PubMed] [Google Scholar]
- Fares MA, Ruiz-Gonzalez MX, Moya A, Elena SF, Barrio E. (2002. b). Endosymbiotic bacteria: groEL buffers against deleterious mutations. Nature 417: 398. [DOI] [PubMed] [Google Scholar]
- Gancedo C, Flores CL, Gancedo JM. (2016). The expanding landscape of moonlighting proteins in yeasts. Microbiol Mol Biol Rev 80: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo NM, Altincicek B, Anselme C, Atamian H, Barribeau SM, de Vos M et al. (2010). Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol 11: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Valero L, Latorre A, Silva FJ. (2004). The evolutionary fate of nonfunctional DNA in the bacterial endosymbiont Buchnera aphidicola. Mol Biol Evol 21: 2172–2181. [DOI] [PubMed] [Google Scholar]
- Gomez-Valero L, Silva FJ, Christophe Simon J, Latorre A. (2007). Genome reduction of the aphid endosymbiont Buchnera aphidicola in a recent evolutionary time scale. Gene 389: 87–95. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Domenech CM, Belda E, Patino-Navarrete R, Moya A, Pereto J, Latorre A. (2012). Metabolic stasis in an ancient symbiosis: genome-scale metabolic networks from two Blattabacterium cuenoti strains, primary endosymbionts of cockroaches. BMC Microbiol 12(Suppl 1): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AK, Moran NA. (2011). Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci USA 108: 2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AK, Moran NA. (2014). The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol 23: 1473–1496. [DOI] [PubMed] [Google Scholar]
- Henderson B, Fares MA, Lund PA. (2013). Chaperonin 60: a paradoxical, evolutionarily conserved protein family with multiple moonlighting functions. Biol Rev Camb Philos Soc 88: 955–987. [DOI] [PubMed] [Google Scholar]
- Humphreys NJ, Douglas AE. (1997). Partitioning of symbiotic bacteria between generations of an insect: a quantitative study of a Buchnera sp. in the pea aphid (Acyrthosiphon pisum reared at different temperatures. Appl Environ Microbiol 63: 3294–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Aphid Genomics Consortium. (2010). Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol 8: e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadibalban AS, Bogumil D, Landan G, Dagan T. (2016). DnaK-dependent accelerated evolutionary rate in prokaryotes. Genome Biol Evol 8: 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar YD, Ochman H. (2013). Genome reduction promotes increase in protein functional complexity in bacteria. Genetics 193: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga R, Meng XY, Tsuchida T, Fukatsu T. (2012). Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface. Proc Natl Acad Sci USA 109: E1230–E1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CH, Moran NA, Ochman H. (2009). The consequences of genetic drift for bacterial genome complexity. Genome Res 19: 1450–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CH, Ochman H. (2009). Deletional bias across the three domains of life. Genome Biol Evol 1: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R, Lewis DH. (1983). Biotic environments and the maintenance of sex-some evidence from mutualistic symbioses. Biol J Linnean Soc 20: 28. [Google Scholar]
- Liu XD, Xie L, Wei Y, Zhou X, Jia B, Liu J et al. (2014). Abiotic stress resistance, a novel moonlighting function of ribosomal protein RPL44 in the halophilic fungus Aspergillus glaucus. Appl Environ Microbiol 80: 4294–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M, Bolger AM, Nagel A, Fernie AR, Lunn JE, Stitt M et al. (2012). RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res 40: W622–W627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald SJ, Lin GG, Russell CW, Thomas GH, Douglas AE. (2012). The central role of the host cell in symbiotic nitrogen metabolism. Proc Biol Sci 279: 2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA et al. (2013). Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41: e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. (2012). Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol 10: 13–26. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Loso T, Douglas AE et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA 110: 3229–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira A, Ochman H, Moran NA. (2001). Deletional bias and the evolution of bacterial genomes. Trends Genet 17: 589–596. [DOI] [PubMed] [Google Scholar]
- Moran NA. (1996). Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc Natl Acad Sci USA 93: 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Dunbar HE, Wilcox JL. (2005). Regulation of transcription in a reduced bacterial genome: nutrient-provisioning genes of the obligate symbiont Buchnera aphidicola. J Bacteriol 187: 4229–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, McCutcheon JP, Nakabachi A. (2008). Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42: 165–190. [DOI] [PubMed] [Google Scholar]
- Moran NA, McLaughlin HJ, Sorek R. (2009). The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science 323: 379–382. [DOI] [PubMed] [Google Scholar]
- Nakabachi A, Ishida K, Hongoh Y, Ohkuma M, Miyagishima SY. (2014). Aphid gene of bacterial origin encodes a protein transported to an obligate endosymbiont. Curr Biol 24: R640–R641. [DOI] [PubMed] [Google Scholar]
- Nilsson AI, Koskiniemi S, Eriksson S, Kugelberg E, Hinton JC, Andersson DI. (2005). Bacterial genome size reduction by experimental evolution. Proc Natl Acad Sci USA 102: 12112–12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Navarrete R, Moya A, Latorre A, Pereto J. (2013). Comparative genomics of Blattabacterium cuenoti: the frozen legacy of an ancient endosymbiont genome. Genome Biol Evol 5: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson ME, Berg OG. (2007). Muller's ratchet in symbiont populations. Genetica 130: 199–211. [DOI] [PubMed] [Google Scholar]
- Price DR, Feng H, Baker JD, Bavan S, Luetje CW, Wilson AC. (2014). Aphid amino acid transporter regulates glutamine supply to intracellular bacterial symbionts. Proc Natl Acad Sci USA 111: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Prieto M, Vargas-Chavez C, Latorre A, Moya A. (2015). SymbioGenomesDB: a database for the integration and access to knowledge on host-symbiont relationships. Database 2015: bav109 (1–8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabater-Muñoz B, Prats-Escriche M, Montagud-Martinez R, Lopez-Cerdan A, Toft C, Aguilar-Rodriguez J et al. (2015). Fitness trade-offs determine the role of the molecular chaperonin groel in buffering mutations. Mol Biol Evol 32: 2681–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker A, Domingues FS, Rahnenfuhrer J, Lengauer T. (2006). A new measure for functional similarity of gene products based on Gene Ontology. BMC Bioinformatics 7: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. (2000). Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407: 81–86. [DOI] [PubMed] [Google Scholar]
- Supek F, Bosnjak M, Skunca N, Smuc T. (2011). REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6: e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas I, Klasson L, Canback B, Naslund AK, Eriksson AS, Wernegreen JJ et al. (2002). 50 million years of genomic stasis in endosymbiotic bacteria. Science 296: 2376–2379. [DOI] [PubMed] [Google Scholar]
- Toft C, Fares MA. (2008). The evolution of the flagellar assembly pathway in endosymbiotic bacterial genomes. Mol Biol Evol 25: 2069–2076. [DOI] [PubMed] [Google Scholar]
- van Ham RC, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U et al. (2003). Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci USA 100: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernegreen JJ. (2002). Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet 3: 850–861. [DOI] [PubMed] [Google Scholar]
- Wernegreen JJ. (2011). Reduced selective constraint in endosymbionts: elevation in radical amino acid replacements occurs genome-wide. PLoS One 6: e28905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TA, Fares MA. (2010). The effect of chaperonin buffering on protein evolution. Genome Biol Evol 2: 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (2007). PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.