Abstract
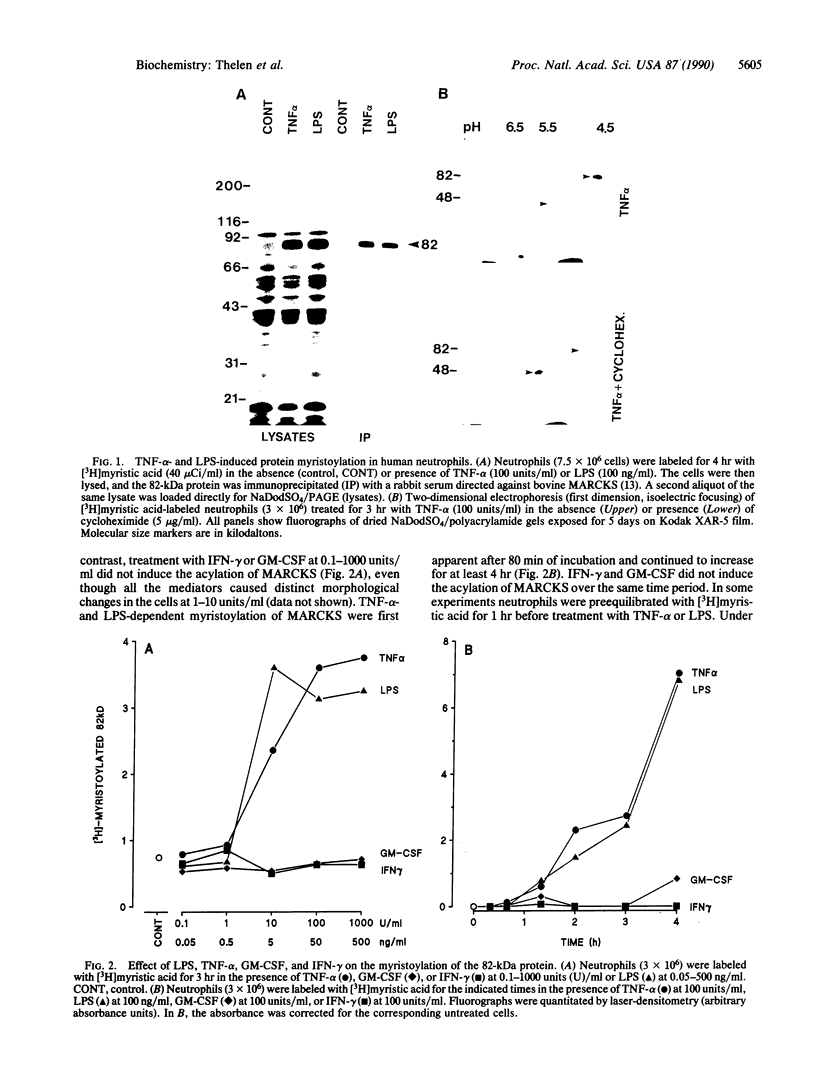
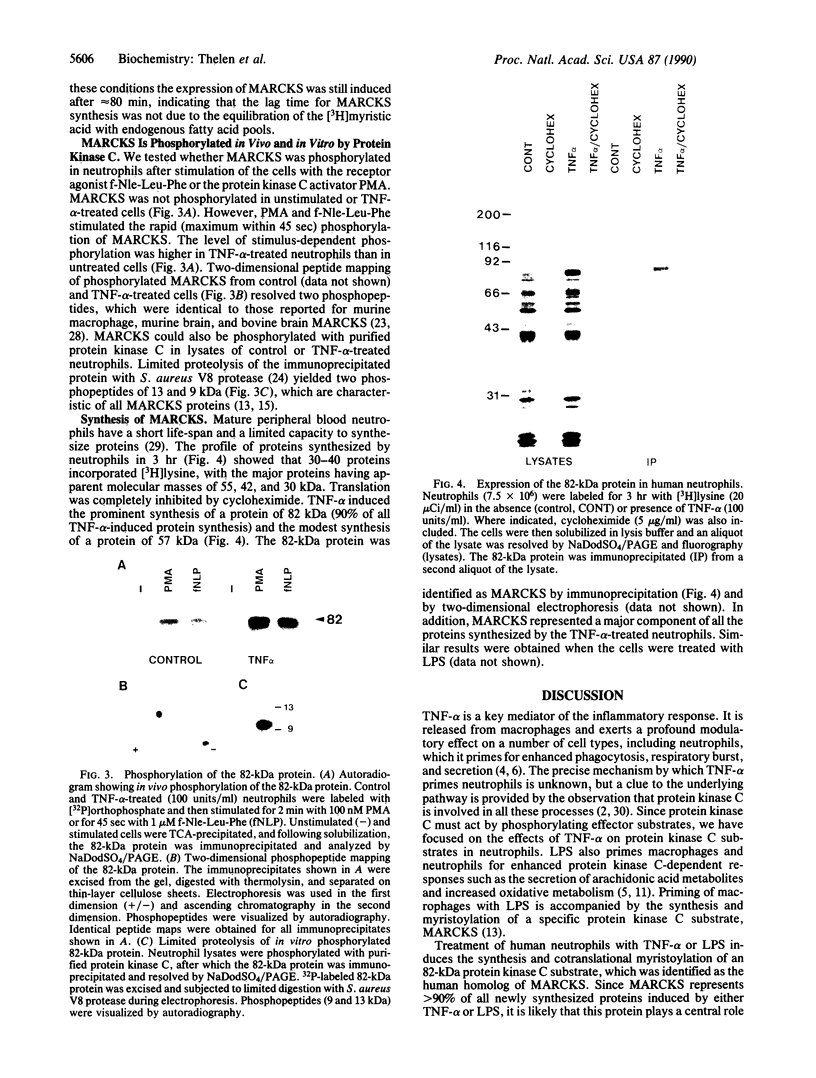
Tumor necrosis factor alpha (TNF-alpha) and bacterial lipopolysaccharide (LPS) induce the synthesis and cotranslational myristoylation of an 82-kDa specific protein kinase C substrate in human neutrophils. The myristic acid is covalently bound via a hydroxylamine-resistant amide linkage to the N-terminal glycine of the protein. The isoelectric point of the protein is at pH 4.6. The protein is rapidly phosphorylated when neutrophils are stimulated with chemotactic agonists or with phorbol 12-myristate 13-acetate, an activator of protein kinase C, and displays two characteristic phosphopeptides in one- and two-dimensional separation systems. Identical phosphopeptides were detected when the 82-kDa protein was phosphorylated in vitro with purified kinase C. The 82-kDa protein was immunoprecipitated by a polyclonal antiserum raised against the 87-kDa specific protein kinase C substrate from bovine brain. From these biochemical and immunological criteria it is concluded that the 82-kDa protein is the human neutrophil homolog of MARCKS, the myristoylated, alanine-rich C kinase substrate previously described in bovine and rat brain and in murine fibroblasts and macrophages. TNF-alpha and LPS prime human neutrophils for potentiated protein kinase C-dependent responses such as the respiratory burst and exocytosis. Consistent with this, these mediators do not induce the phosphorylation of MARCKS but prime the neutrophils for enhanced phosphorylation of this protein when the cells subsequently encounter activators of protein kinase C. This increase in MARCKS phosphorylation can be explained by the elevated levels of the protein observed in TNF-alpha- or LPS-treated neutrophils. Indeed, MARCKS constitutes 90% of all proteins synthesized in response to TNF-alpha or LPS. These data strongly suggest that MARCKS acts as a critical effector molecule in the transduction pathway of these important inflammatory mediators.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. A., Keum M. M., Pure E., Cohn Z. A. Bacterial lipopolysaccharides, phorbol myristate acetate, and zymosan induce the myristoylation of specific macrophage proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5817–5821. doi: 10.1073/pnas.83.16.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Wu W. C., Nairn A. C., Greengard P. Inhibition by calmodulin of calcium/phospholipid-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3622–3625. doi: 10.1073/pnas.81.12.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Berridge M. J. Ernst Klenk Lecture, November 1985. Intracellular signalling through inositol trisphosphate and diacylglycerol. Biol Chem Hoppe Seyler. 1986 Jun;367(6):447–456. [PubMed] [Google Scholar]
- Berton G., Zeni L., Cassatella M. A., Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Blake L. A., West B. C., Lary C. H., Todd J. R., 4th Environmental nonhuman sources of leprosy. Rev Infect Dis. 1987 May-Jun;9(3):562–577. doi: 10.1093/clinids/9.3.562. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Cybulsky M. I., McComb D. J., Movat H. Z. Protein synthesis dependent and independent mechanisms of neutrophil emigration. Different mechanisms of inflammation in rabbits induced by interleukin-1, tumor necrosis factor alpha or endotoxin versus leukocyte chemoattractants. Am J Pathol. 1989 Jul;135(1):227–237. [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M. Platelet-activating factor as a stimulus of exocytosis in human neutrophils. Biochim Biophys Acta. 1986 Aug 29;888(1):42–48. doi: 10.1016/0167-4889(86)90069-8. [DOI] [PubMed] [Google Scholar]
- Dewald B., Thelen M., Baggiolini M. Two transduction sequences are necessary for neutrophil activation by receptor agonists. J Biol Chem. 1988 Nov 5;263(31):16179–16184. [PubMed] [Google Scholar]
- Dewald B., Thelen M., Wymann M. P., Baggiolini M. Staurosporine inhibits the respiratory burst and induces exocytosis in human neutrophils. Biochem J. 1989 Dec 15;264(3):879–884. doi: 10.1042/bj2640879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Molecular cloning, sequence, and expression of a cDNA encoding the chicken myristoylated alanine-rich C kinase substrate (MARCKS). Mol Endocrinol. 1989 Nov;3(11):1903–1906. doi: 10.1210/mend-3-11-1903. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D., Reich E. RNA and protein synthesis in human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1979 Jan 1;149(1):284–289. doi: 10.1084/jem.149.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Patel J., Kligman D. Purification and characterization of an Mr 87,000 protein kinase C substrate from rat brain. J Biol Chem. 1987 Dec 5;262(34):16686–16691. [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Nairn A. C., Greengard P., Cohn Z. A., Aderem A. Bacterial lipopolysaccharide regulates the phosphorylation of the 68K protein kinase C substrate in macrophages. J Biol Chem. 1989 Jun 5;264(16):9118–9121. [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Steinbeck M. J., Roth J. A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989 Jul-Aug;11(4):549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Towler D., Glaser L. Acylation of cellular proteins with endogenously synthesized fatty acids. Biochemistry. 1986 Feb 25;25(4):878–884. doi: 10.1021/bi00352a021. [DOI] [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Greengard P. Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependent systems. J Neurosci. 1988 Jan;8(1):281–288. doi: 10.1523/JNEUROSCI.08-01-00281.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Wilson E., Olcott M. C., Bell R. M., Merrill A. H., Jr, Lambeth J. D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem. 1986 Sep 25;261(27):12616–12623. [PubMed] [Google Scholar]
- Wirthmueller U., De Weck A. L., Dahinden C. A. Platelet-activating factor production in human neutrophils by sequential stimulation with granulocyte-macrophage colony-stimulating factor and the chemotactic factors C5A or formyl-methionyl-leucyl-phenylalanine. J Immunol. 1989 May 1;142(9):3213–3218. [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Phorbol esters cause sequential activation and deactivation of complement receptors on polymorphonuclear leukocytes. J Immunol. 1986 Mar 1;136(5):1759–1764. [PubMed] [Google Scholar]