Abstract
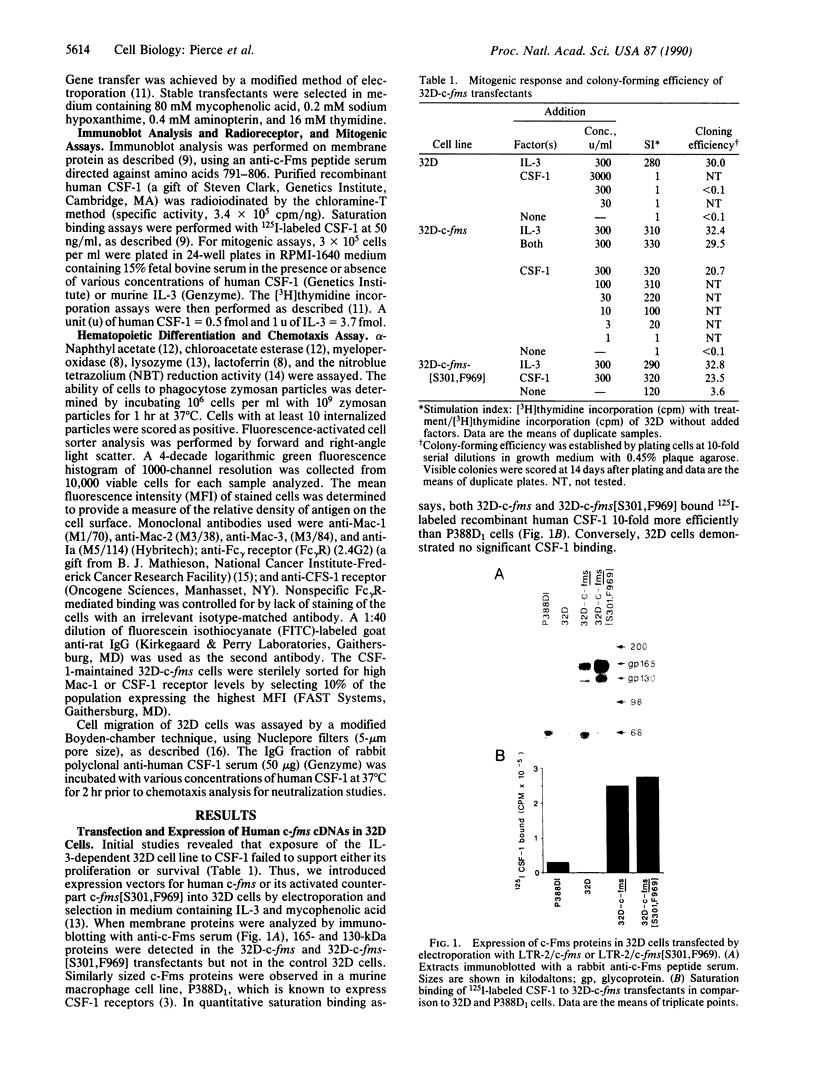
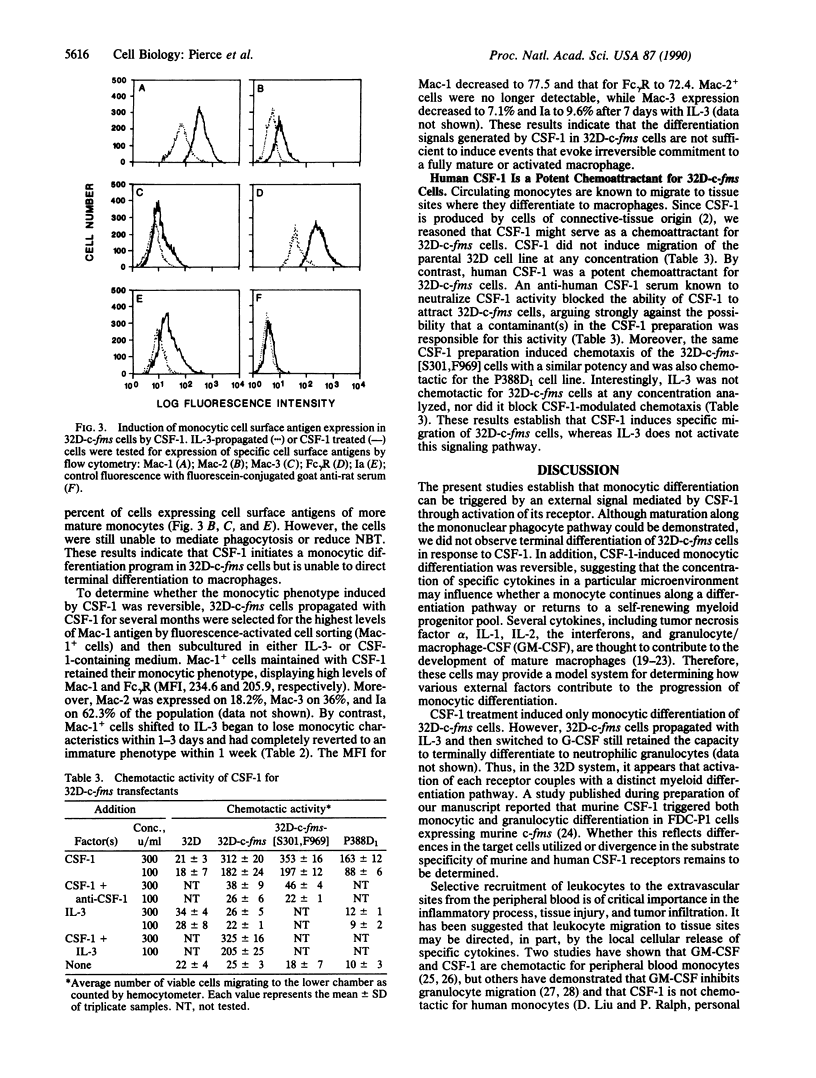
The c-fms protooncogene encodes the receptor for macrophage-colony-stimulating factor (CSF-1). Expression vectors containing either normal or oncogenic point-mutated human c-fms genes were transfected into interleukin 3 (IL-3)-dependent 32D cells in order to determine the effects of CSF-1 signaling in this murine clonal myeloid progenitor cell line. CSF-1 was shown to trigger proliferation in association with monocytic differentiation of the 32D-c-fms cells. Monocytic differentiation was reversible upon removal of CSF-1, implying that CSF-1 was required for maintenance of the monocyte phenotype but was not sufficient to induce an irrevocable commitment to differentiation. Human CSF-1 was also shown to be a potent chemoattractant for 32D-c-fms cells, suggesting that CSF-1 may serve to recruit monocytes from the circulation to tissue sites of inflammation or injury. Although c-fms did not release 32D cells from factor dependence, point-mutated c-fms[S301,F969] (Leu-301----Ser, Tyr-969----Phe) was able to abrogate their IL-3 requirement and induce tumorigenicity. IL-3-independent 32D-c-fms[S301,F969] cells also displayed a mature monocyte phenotype, implying that differentiation did not interfere with progression of these cells to the malignant state. All of these findings demonstrate that a single growth factor receptor can specifically couple with multiple intracellular signaling pathways and play a critical role in modulating cell proliferation, differentiation, and migration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo D., Shirsat N., Wong G. G., Lange B., Clark S., Rovera G. Recombinant human macrophage colony-stimulating factor (M-CSF) requires subliminal concentrations of granulocyte/macrophage (GM)-CSF for optimal stimulation of human macrophage colony formation in vitro. J Exp Med. 1987 Dec 1;166(6):1851–1860. doi: 10.1084/jem.166.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Geissler K., Harrington M., Srivastava C., Leemhuis T., Tricot G., Broxmeyer H. E. Effects of recombinant human colony stimulating factors (CSF) (granulocyte-macrophage CSF, granulocyte CSF, and CSF-1) on human monocyte/macrophage differentiation. J Immunol. 1989 Jul 1;143(1):140–146. [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Kufe D. Expression of the macrophage-specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood. 1987 Apr;69(4):1259–1261. [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Roussel M. F., Ashmun R. A., Sherr C. J. Transduction of human colony-stimulating factor-1 (CSF-1) receptor into interleukin-3-dependent mouse myeloid cells induces both CSF-1-dependent and factor-independent growth. Mol Cell Biol. 1989 Sep;9(9):4069–4073. doi: 10.1128/mcb.9.9.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R., Metcalf D. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol Cell Biol. 1989 Nov;9(11):5081–5092. doi: 10.1128/mcb.9.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Rovera G., Valtieri M., Mavilio F., Reddy E. P. Effect of Abelson murine leukemia virus on granulocytic differentiation and interleukin-3 dependence of a murine progenitor cell line. Oncogene. 1987 Mar;1(1):29–35. [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Griffin J., Kufe D. W. Effects of interferon-gamma on proto-oncogene expression during induction of human monocytic differentiation. J Immunol. 1987 Mar 15;138(6):1954–1958. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtieri M., Tweardy D. J., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J Immunol. 1987 Jun 1;138(11):3829–3835. [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Colella S., Allavena P., Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology. 1987 Mar;60(3):439–444. [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985 Feb;134(2):982–989. [PubMed] [Google Scholar]
- Watson J. D., Eszes M., Overell R., Conlon P., Widmer M., Gillis S. Effect of infection with murine recombinant retroviruses containing the v-src oncogene on interleukin 2- and interleukin 3-dependent growth states. J Immunol. 1987 Jul 1;139(1):123–129. [PubMed] [Google Scholar]
- Weisbart R. H., Billing R., Golde D. W. Neutrophil migration-inhibition activity produced by a unique T lymphoblast cell line. J Lab Clin Med. 1979 Apr;93(4):622–626. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- von Rüden T., Wagner E. F. Expression of functional human EGF receptor on murine bone marrow cells. EMBO J. 1988 Sep;7(9):2749–2756. doi: 10.1002/j.1460-2075.1988.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]