Abstract
Aims
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, and its paroxysmal nature makes its detection challenging. In this trial, we evaluated a novel App for its accuracy to differentiate between patients in AF and patients in sinus rhythm (SR) using the plethysmographic sensor of an iPhone 4S and the integrated LED only.
Methods and results
For signal acquisition, we used an iPhone 4S, positioned with the camera lens and LED light on the index fingertip. A 5 min video file was recorded with the pulse wave extracted from the green light spectrum of the signal. RR intervals were automatically identified. For discrimination between AF and SR, we tested three different statistical methods. Normalized root mean square of successive difference of RR intervals (nRMSSD), Shannon entropy (ShE), and SD1/SD2 index extracted from a Poincaré plot. Eighty patients were included in the study (40 patients in AF and 40 patients in SR at the time of examination). For discrimination between AF and SR, ShE yielded the highest sensitivity and specificity with 85 and 95%, respectively. Applying a tachogram filter resulted in an improved sensitivity of 87.5%, when combining ShE and nRMSSD, while specificity remained stable at 95%. A combination of SD1/SD2 index and nRMSSD led to further improvement and resulted in a sensitivity and specificity of 95%.
Conclusion
The algorithm tested reliably discriminated between SR and AF based on pulse wave signals from a smartphone camera only. Implementation of this algorithm into a smartwatch is the next logical step.
Keywords: Atrial fibrillation, Pulse wave analysis, Rhythm monitoring, Smartphone
What's new?
Photoplethysmographic pulse wave signals from smartphone cameras can be used to screen for atrial fibrillation (AF).
No additional peripheral devices are needed with this App.
Implementation into smartwatches is the next logical step.
Sensitivity and specificity of this retrospective analysis are 95%.
Technical options to detect atrial fibrillation have significantly improved within the past decade. However, they carry the burden of a lack of comfort, invasiveness, and costs. We developed an algorithm that can be used with every smartphone and reliably differentiates between AF and SR in a trial setting. Once integrated into a smartwatch, screening for AF could become as convenient as wearing a watch.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice. Without specific therapy, the risk for stroke and congestive heart failure increases significantly.1 Because of the paroxysmal nature of AF that may be present for years before it becomes persistent, detection is challenging and often unsuccessful. Recent trials support the use of intensified diagnostic strategies to detect AF in selected patients, although the methods used are costly or inconvenient.2,3 Even with the rapidly increasing knowledge in this field, the relevance of subclinical AF and the temporal correlation between AF and stroke remains controversial and is still being addressed in ongoing trials (ARTESiA ClinicalTrials.gov Identifier: NCT01938248).4,5
The use of smartphones and smartwatches in medical practice is currently being evaluated. Most of these devices are equipped with plethysmographic sensors that are able to monitor the heart rate. It is important to recognize, though, that these tools in general are not validated in clinical trials. Based on the hardware available at present, we developed an App that simultaneously processes multiple physiological parameters using a novel pulse wave analysis and nonlinear methods for signal analysis. We specifically aimed at a standalone tool that needs no additional peripheral device except for a smartphone or smartwatch. In this trial, we evaluated the App for its accuracy to differentiate between patients in AF and patients in sinus rhythm (SR).
Methods
Study population
We conducted a case–control study including 80 consecutive in- and outpatients at the University Hospital Basel. Excluded from the study were patients under 18 years of age, patients unable to give informed consent, and patients with either dialysis shunt or lymphedema. The study group required a diagnosis of AF in the patient record and at least one electrocardiogram (ECG) recording showing AF. This group was then compared with a control group of 40 patients in SR at the time of examination.
Signal acquisition
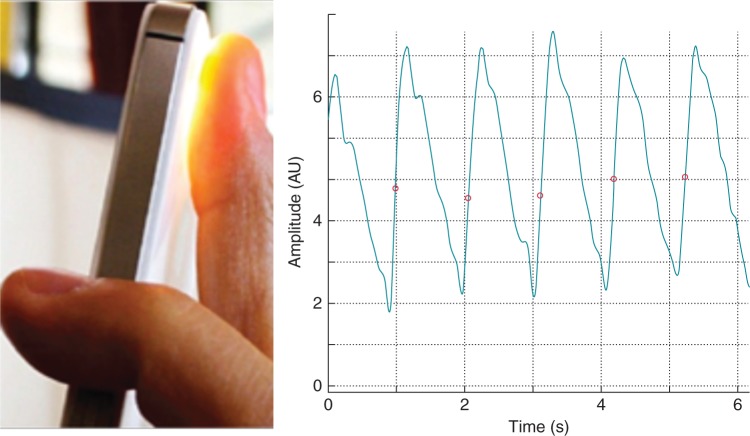
For signal acquisition, we used an iPhone 4S (Apple, Inc., Cupertino, CA, USA). The device was positioned on the index fingertip with the camera lens and LED light placed on the finger. A 5 min video file was recorded (Figure 1, see Supplementary material online, Movie S1). The peripheral pulse wave was extracted from the video signals as explained in detail below.
Figure 1.
iPhone on index finger tip with resulting pulse wave signal of a patient with AF.
As a reference signal, we used a heart rate monitor chest belt (Wahoo TICKR, Model SHRM1G, Wahoo Fitness, 90 West Wieuca Rd NE #110, Atlanta, GA) that was positioned in a standard fashion around the patient's chest. The signal recorded with this belt was transmitted to the iPhone via Bluetooth. This signal provided simultaneous RR intervals and was used as a reference to document the presence of AF at the time of recording.
Pulse waveform analysis
The pulse wave signal was derived from the green light spectrum channel of the recorded video signal. Signals were filtered using a bandpass filter with a lower and upper cut-off frequency of 0.5 and 7 Hz, respectively. A novel heart beat detection algorithm based on a combination of morphology and frequency analysis of the pulse wave was applied to detect all beat-to-beat intervals (BBI). This algorithm was recently validated and yielded an excellent correlation of r > 0.99, compared with RR intervals from standard ECG recordings.6
From the extracted BBI time series, several indices representing the variability of heart rhythm were calculated and analysed regarding their ability to discriminate between AF and SR. For the analysis, premature beats and other disruptions were eliminated and corresponding points on the BBI time series replaced, using an algorithm for adaptive variance estimation.7 Patients in the control group only had a minor number of ectopic beats (<5%). Therefore, the impact of ectopy on variability indices is rather low and is even less after filtering of the tachogram.
Statistics/data analysis
Results were reported as group means and standard deviations. To test for significant differences between AF and SR, we applied the Mann–Whitney U test.
The performance of each index was assessed by estimating the area under the receiver-operating characteristic (ROC) curve. Cut-offs were chosen to achieve a specificity of 90–95%.
Three different statistical tests were examined in their ability to discriminate AF from SR:
Root mean square of successive difference of RR intervals (RMSSD) is a standard index from heart rate variability (HRV) analysis to quantify beat-to-beat alterations.8 In order to adjust for the effect of heart rate on the RR variability, the RMSSD value is normalized to the mean RR interval value. Since in AF the variability is distinctly higher than in SR, normalized RMSSD (nRMSSD) is expected to be higher in patients with AF.
Shannon entropy (ShE) is a statistical method to quantify uncertainty for a random variable and is expected to be higher in patients with AF since the pulse in these circumstances exhibits greater RR interval irregularity compared with pulses recorded from patients with SR.
Both ShE and nRMSSD were used before to discriminate between AF and SR.9
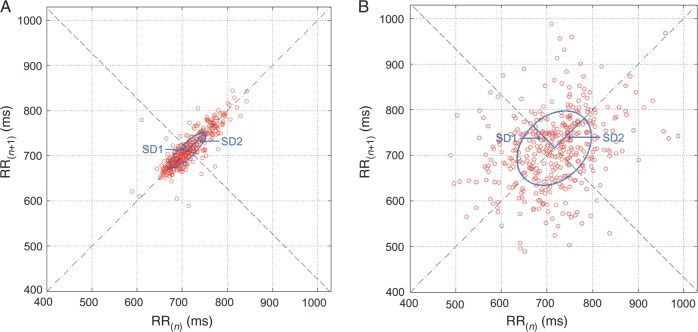
Poincaré plot analysis (PPA) provides a visual tool to characterize the complex nature of time series fluctuations where BBIn is plotted against BBIn−1.10 The Poincaré plot usually displays an elongated cloud of points oriented along the diagonal of the coordinate system. An ellipse is fitted to the cloud of points to characterize its shape. The index SD1/SD2 represents the ratio of the standard deviation of short-term BBI variability (axis vertical to the line of identity, SD1) to the standard deviation of the long-term BBI variability (axis along the line of identity, SD2).11 This index was extracted from 5 min recordings to ensure the formation of the ellipse (Figure 2).
Figure 2.
Poincaré plots of 5 min recordings from patients in SR (A) and patients in AF (B).
Using the statistical tests described above, we compared three different methods to discriminate between AF and SR.
The first method compared nRMSSD and ShE. Both indices were extracted from the pulse wave tachogram. Sensitivity, specificity, and accuracy were calculated for each of these indices separately and for the combination.
For the second method, a filter was applied to the pulse wave tachogram to eliminate premature beats and other disruptions as described above. This improved the applicability of the method and allowed patients with premature beats to be successfully separated from patients with AF.
For the third method, an additional index SD1/SD2 that was extracted from a Poincaré plot of a 5 min recording was tested. SD1/SD2, nRMSSD, and ShE were calculated from a filtered tachogram. Sensitivity, specificity, and accuracy were then calculated for each method separately and for the combination.
Results
Eighty patients were included in the study (40 patients in AF and 40 patients in SR at the time of examination). Patients in the AF group had a mean age of 80 years (SD ± 8) and patients in the SR group 75 years (SD ± 7). Male-to-female ratio was 2.4 in the AF group and 2.5 in the SR group. The average RR interval was higher in the AF group (AF 887 ± 120 ms and SR 784 ± 144 ms, P = 0.0004).
First method
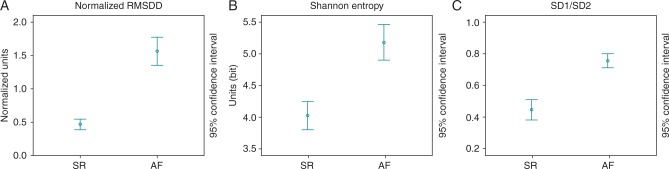
For the discrimination between AF and SR based on a 2-min pulse wave recording, the ShE yielded a sensitivity and specificity of 85 and 95% respectively, applying a cut-off value of 4.9 (Figure 3). This translates into 34/40 patients classified correctly and 2/40 patients classified incorrectly as AF.
Figure 3.
Comparison of nRMSSD (A), ShE (B), and SD1/SD2 (C) in patients with SR and AF.
Second method
The application of the tachogram filter improved sensitivity to 87.5%, while specificity remained stable at 95% using the index nRMSSD with a cut-off of 0.09. This translates into 35/40 patients classified correctly and 2/40 patients classified incorrectly as AF (Figure 3).
Third method
By prolonging the recording time from 2 to 5 min and combining the index SD1/SD2 and nRMSSD, sensitivity and specificity increased to 95% with an area under the curve of 0.93 (Figure 4). The cut-off for classification as AF was an nRMSSD of >0.043 and an SD1/SD2 of >0.6. This translates into 38/40 patients classified correctly and 2/40 patients classified incorrectly as AF.
Figure 4.
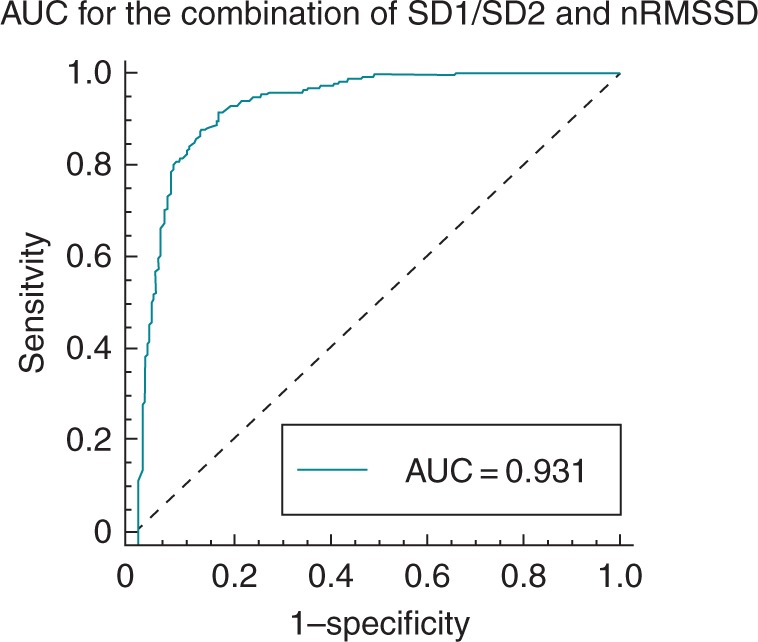
Area under the curve for Test 3, which combined SD1/SD2 analysed from the Poincaré plot and nRMSSD.
Results are presented in detail in Table 1.
Table 1.
Results
| SR (mean ± SD) | AF (mean ± SD) | P-value | AUC | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| Method 1 | ||||||
| nRMSSD | 0.103 ± 0.093 | 0.298 ± 0.121 | <0.001 | 0.892 | 50 | 95 |
| ShE | 3.858 ± 0.711 | 5.350 ± 0.825 | <0.001 | 0.912 | 85 | 95 |
| nRMSSD + ShE | – | – | – | 0.917 | 82.5 | 95 |
| Method 2 | ||||||
| nRMSSD | 0.034 ± 0.026 | 0.146 ± 0.067 | <0.001 | 0.938 | 87.5 | 95 |
| ShE | 3.710 ± 0.643 | 5.007 ± 0.790 | <0.001 | 0.911 | 77.5 | 95 |
| nRMSSD + ShE | – | – | – | 0.926 | 87.5 | 95 |
| Method 3 | ||||||
| nRMSSD | 0.039 ± 0.026 | 0.154 ± 0.070 | <0.001 | 0.942 | 77.5 | 95 |
| ShE | 4.030 ± 0.697 | 5.187 ± 0.885 | <0.001 | 0.872 | 57.5 | 95 |
| SD1/SD2 | 0.447 ± 0.202 | 0.757 ± 0.141 | <0.001 | 0.903 | 77.5 | 90 |
| nRMSSD + ShE | – | – | – | 0.966 | 80 | 95 |
| ShE+SD1/SD2 | – | – | – | 0.959 | 50 | 95 |
| nRMSSD + SD1/SD2 | – | – | – | 0.931 | 95 | 95 |
Comparison and results of the three different methods to differentiate between AF and SR based on the recorded pulse wave signals, using different statistical approaches and combinations thereof.
nRMSSD, normalized root mean square of successive difference of RR intervals; ShE, Shannon entropy; SD1 and SD2 derived from Poincaré plots.
Discussion
Detection of AF for primary prevention and secondary prevention after stroke is a crucial necessity and remains a challenging task. Following the present guidelines, diagnosis of AF has to be confirmed prior to the initiation of anticoagulation therapy.12 We therefore pursued to develop a simple, inexpensive, accessible, and reproducible non-invasive screening method to detect AF.
The principal idea of the method described in this manuscript was demonstrated before by Mc Manus et al. who used a camera and LED light of an iPhone 4S to record pulse waves obtained from the fingertips of 76 patients before and after cardioversion.9 They created an App to transform the optical signal in a wave-shaped curve and tested for distribution of ‘RR’ intervals. With this technique, they could successfully discriminate AF from SR in patients before and after cardioversion with a specificity of 97% and a sensitivity of 96%. We applied the published algorithm to our study data and achieved a reduced sensitivity and specificity of 90 and 85%, respectively. This is most likely due to the fact that we tested consecutive patients and used other patients as controls, whereas Mc Manus et al. tested their algorithm in the same patients before and after cardioversion. We consider our setting closer to the intended use.
While the hardware part of our study is identical, the patients and the software are distinctly different. Processing of the signal follows an extensive algorithm to maximize signal robustness even in case of minor signal quality. This holds particularly true for the accurate calculation of shorter RR intervals during AF, where calculating RR-interval differences is more challenging. It is important to recognize, though, that the absolute heart rate is not part of the presented algorithm. We tested three different methods to differentiate between AF and SR. The highest sensitivity and specificity was achieved using the combination of the indices nRMSSD and SD1/SD2 with the tachogram filter. By prolonging the analyzed interval from 2 to 5 min, we reached a sensitivity and specificity of 95%.
It is important to point out that due to the case–control design of this study, these results most likely overestimate the power of this algorithm in a real-world setting to some extent. Furthermore, signal quality in a trial setting is usually superior compared with real-world use. We therefore see the need to implement a ‘signal quality check’ in the next version of the algorithm and test it prospectively in an unselected patient population.
As this application reliably differentiates between patients in AF and SR, we see a great clinical potential for this technique. It may be used to screen for AF in patients at risk. In addition, it may be utilized in patients who suffer from palpitations and in whom AF is yet to be diagnosed. In case an arrhythmia is detected, a conventional ECG is needed to diagnose AF. It is expected that ECGs can be obtained with the wristband of a smartwatch in the near future. Combined with this App, such a combination would provide an easy and available tool to detect AF.
Limitations
Recording of a pulse wave with a smartphone is not convenient enough for elderly people. The trial was designed as proof of principle before the App can be tested in smartwatches, which are now widely available. The algorithm was evaluated in a retrospective analysis.
Conclusion
We have successfully tested an algorithm that reliably discriminated between SR and AF in individual patients based on pulse wave signals derived from a smartphone camera. Implementation of this algorithm into a smartwatch is the next logical step and will be evaluated in an upcoming trial (WATCH AF).
This App is now available for free for limited testing: www.preventicus.com/afib
Supplementary material
Funding
This trial was funded by grants from the University Hospital Basel. Hardware and Software were provided free of charge by Preventicus. Funding to pay the Open Access publication charges for this article was provided by T. Huebner, Preventicus(R).
Conflict of interest: T.H. is CEO of Preventicus (Developer of the algorithm), A.S. is employed full time as research scientist at Preventicus, and J.E. and S.W. hold virtual Shares of Preventicus.
Supplementary Material
References
- 1. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. . 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 2. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J et al. . Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- 3. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA et al. . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 4. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A et al. . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 5. Ziegler PD, Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD et al. . Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke 2010;41:256–60. [DOI] [PubMed] [Google Scholar]
- 6. Koenig N, Seeck A, Eckstein J, Mainka A, Huebner T, Voss A et al. . Validation of a new heart rate measurement algorithm for fingertip recording of video signals with smartphones. Telemed J E Health 2016[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Voss A, Heitmann A, Schroeder R, Peters A, Perz S. Short-term heart rate variability–age dependence in healthy subjects. Physiol Meas 2012;33:1289–311. [DOI] [PubMed] [Google Scholar]
- 8. Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–81. [PubMed] [Google Scholar]
- 9. McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A et al. . A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 2013;10:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamen PW, Tonkin AM. Application of the Poincare plot to heart rate variability: a new measure of functional status in heart failure. Aust N Z J Med 1995;25:18–26. [DOI] [PubMed] [Google Scholar]
- 11. Lerma C, Infante O, Perez-Grovas H, Jose MV. Poincare plot indexes of heart rate variability capture dynamic adaptations after haemodialysis in chronic renal failure patients. Clin Physiol Funct Imaging 2003;23:72–80. [DOI] [PubMed] [Google Scholar]
- 12. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. . 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.