Abstract
Purpose
Hair cortisol evaluation has been used to help detect patients with suspected Cushing Syndrome. Our goal was to correlate segmental hair cortisol with biochemical testing in patients with Cushing syndrome and controls. This study was a prospective analysis of hair cortisol in confirmed Cushing syndrome cases over 16 months.
Methods
Thirty six subjects (26.5±18.9 years, 75% female, and 75% Caucasian) were analyzed by diurnal serum cortisol, 24 h urinary free cortisol corrected for body surface area (UFC/BSA), and 24 h urinary 17-hydroxysteroids corrected for creatinine (17OHS/Cr). Thirty patients were diagnosed with Cushing syndrome, and six were defined as controls. 3-cm hair samples nearest to the scalp, cut into 1-cm segments (proximal, medial, and distal), were analyzed for cortisol by enzyme immunoassay and measured as pmol cortisol/g dry hair. Hair cortisol levels were compared with laboratory testing done within previous 2 months of the evaluation.
Results
Proximal hair cortisol was higher in Cushing syndrome patients (266.6 ± 738.4 pmol/g) than control patients (38.9 ± 25.3 pmol/g) (p = 0.003). Proximal hair cortisol was highest of all segments in 25/36 (69%) patients. Among all subjects, proximal hair cortisol was strongly correlated with UFC/BSA (r=0.5, p=0.005), midnight serum cortisol (r=0.4, p=0.03), and 17OHS/Cr, which trended towards significance (r=0.3, p=0.06).
Conclusions
Among the three examined hair segments, proximal hair contained the highest cortisol levels and correlated the most with the initial biochemical tests for Cushing syndrome in our study. Further studies are needed to validate proximal hair cortisol in the diagnostic workup for Cushing syndrome.
Keywords: Hair cortisol, Hair, Cortisol, Cushing syndrome, Cushing disease, Children, Adolescence
Introduction
The diagnosis of Cushing Syndrome (CS) is often challenging and inconclusive, despite numerous tests used for the detection of hypercortisolemia and its origin, and is associated with high morbidity and high risk for mortality, if undiagnosed and untreated. The Endocrine Society Clinical Practice Guideline for the diagnosis of this rare disease recommends the following initial tests for CS: 24 h urine free cortisol (UFC), 1-mg overnight or 2-mg 48 h dexamethasone suppression test, late night salivary cortisol, or combination of any of the above (1). Diurnal serum cortisol and 24 h urinary 17-hydroxysteroids (17OHS) have been used in the research setting in the workup of CS.
In some cases, biochemical hypercortisolemia may change daily, requiring repetition of tests to confirm or exclude disease, particularly in patients with so-called “cyclical CS” [1–3]. The selection of diagnostic cutoff values can dramatically alter the sensitivity and specificity of UFC, late night salivary, and serum cortisol tests [1–6]. Furthermore, collecting multiple samples of UFC, 17OHS, diurnal serum cortisol, or salivary cortisol with dexamethasone suppression testing is more reliable than using only one isolated sample to diagnose hypercortisolism [2,7]. Regardless of the cutoff parameters, current testing for hypercortisolemia limits detection to a 24 h period [8,9].
As a potential solution to the limitations of these tests, hair cortisol has been increasingly studied as an additional means to diagnose patients with CS [10]. Much like hemoglobin A1C is a longitudinal marker of blood glucose levels, hair cortisol can be a measure of the body’s glucocorticoid levels over the previous several weeks to months. Hair cortisol has aided in the diagnosis of cyclical CS [11], following post-treatment progress of various presentations of CS [10,11] and monitoring hydrocortisone dosing or patient compliance in adrenal insufficiency [12–15]. Furthermore, moderate correlations of hair cortisol with either serum and salivary cortisol levels or both have been found in elementary school girls, pregnant patients, and non-pregnant participants [16–18]. However, no study to date has compared the serum or urinary cortisol levels for CS patients with segmental hair cortisol data. Comparing hair cortisol with the standard workup for CS should allow further research to investigate the applicability of hair cortisol as an initial or supportive diagnostic test for CS.
In this study, we investigated the reliability of measuring segmental hair cortisol levels of the 3 cm hair samples closest to the scalp in the evaluation for CS. This clinical study was conducted at the National Institutes of Health (NIH) Clinical Research Center (CRC).
Materials and Methods
Study population and biochemical testing
We recruited 49 patients referred to the NIH CRC for the evaluation of CS from September 2013 to January 2015. History and physical examination was performed, and exposure to exogenous steroids was evaluated. CS was diagnosed using clinical assessment, biochemical data, and radiological studies, and confirmed with histopathology (when surgery was performed). Biochemical evidence of CS was evaluated with serial UFC, serial 17OHS corrected for creatinine excretion (17OHS/Cr), serum cortisol and adrenocorticotropic hormone (ACTH) levels, and 48 h low-dose dexamethasone suppression testing. Diurnal serum cortisol and ACTH were collected via a previously placed intravenous catheter at 2330h and 0000h (midnight), repeated only if the patient was awake or in discomfort, and at 0730h and 0800h (early morning) the following day, as previously described [19]. In the final analysis using both diurnal cortisol and ACTH, the average of 2330h and 0000h values represented midnight values, and average of 0730h and 0800h values represented the early morning values. Body mass index (BMI) was calculated in kg/m2, and body surface area (BSA) was calculated using the formula from Dubois and Dubois [20]. For the final analysis, UFC was corrected for BSA (UFC/BSA) to standardize for height among all ages and sizes, as previously described [21]. For comparison to hair cortisol values, we only selected pre-operative laboratory values that occurred within the prior 2 months of hair collection.
Of the 49 patients initially recruited for the study, five patients were excluded because of diagnosis of primary aldosteronism without evidence of cortisol co-secretion, and eight did not have CS but were excluded for midnight serum cortisol levels>49.7 nmol/L. A total of 36 patients were included in the analysis.
All research subjects signed an informed consent. The Institutional Review Boards of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (until 2010) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (2010-present) at NIH approved the research protocols (Clinical Trial Registration Numbers: NCT00005927 and NCT00001595).
Hair sample collection
Hair was collected as previously described [22,23]. In brief, a pencil-width selection of strands of hair was cut with sterile scissors at the vertex of the scalp, capturing at least 3 cm closest to the scalp, without plucking the hair roots to avoid contamination from serum. The 3 cm of hair closest to the scalp was isolated and then further cut into 1-cm lengths (proximal, medial, and distal). The 1 cm samples were individually wrapped in aluminum foil and stored at −20ºC, until transferred to room temperature for shipping to the reference laboratory for processing.
Hair cortisol analysis
Hair samples were processed and analyzed for cortisol according to the methods previously described [23]. Briefly, samples were washed in isopropanol to remove external contamination, dried, and then milled to a fine powder using a Mini-Beadbeater-16 (BioSpec, Bartlesville, OK). Cortisol was extracted overnight into methanol, extracts were dried, and the cortisol was analyzed using a sensitive and specific enzyme immunoassay (Salimetrics, Carlsbad, CA). Assay readouts were converted to picomoles cortisol per gram dry hair weight, and the intra-assay coefficient of variation was <2%. For this assay, estimated cortisol recovery ranges from 97% to 113%. Cross-reactivity with other endogenous steroid hormones is extremely low as reported by the manufacturer, ranging from 0.006% for testosterone to 0.21% for corticosterone. Cross-reactivity for synthetic glucocorticoids is as follows: prednisolone = 0.56%, triamcinolone = 0.08%, and dexamethasone = 19.2%.
Statistical analysis
Calculations were performed with SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Based on a standard deviation (SD) of 25 pmol/g for hair cortisol levels and expected difference of at least 35 pmol/g between cases and controls, a sample size of 35 subjects in a 1:6 ratio was required in order to reach a statistically significant difference with a p-value cutoff of 0.05 and statistical power of 0.8. Results are expressed as mean ± SD unless otherwise indicated. Hair cortisol measurements were logarithmically transformed to induce approximate normality. For group comparisons, the independent Student’s t-test was used to analyze differences in numerical variables; the χ2 test was employed to analyze differences in categorical variables. The Pearson product was used for analysis of correlations between variables. Non-parametric tests were used as appropriate. The p-value for significance was set at <0.05.
Results
Demographics
The mean subject age of the 36 patients in the final analysis was 26.5 ± 18.9 years (CS: 26.2 ± 18.8 years and controls: 27.8 ± 21.2 years) at the time of presentation to the NIH CRC, and 21/36 patients in our cohort were less than 18 years. Thirty patients were diagnosed with CS. The etiology of hypercortisolemia among the CS patients included Cushing Disease (CD; n=20), ectopic CS (n=2), and adrenal (ACTH-independent) CS (n=8). Most subjects in both groups were Caucasian (CS: 70.0%, n=21 and controls: 100%, n=6) and female (CS: 73.3%, n=22 and controls: 83.3%, n=5). Patients were similar in weight, height, BMI, and BSA (Table 1). Complete demographics, laboratory data, and imaging studies and diagnoses for each patient are presented in detail in the Supplemental Table.
Table 1.
Demographic characteristics.
| Variable | All N=36 | CS n=30 (83.33%) | Controls n=6 (16.67%) | p value CS vs. Controls* |
|---|---|---|---|---|
| Age (years) | 26.5±18.9 | 26.2±18.8 | 27.8±21.2 | 0.8 |
| Female, n(%) | 27 (75.0) | 22 (73.3) | 5 (83.3) | 0.6 |
| Ethnicity, n(%) | 0.5 | |||
| Asian | 3 (8.3) | 3 (10.0) | 0 | |
| African-American | 3 (8.3) | 3 (10.0) | 0 | |
| Unknown | 3 (8.3) | 3 (10.0) | 0 | |
| Caucasian | 27 (75.0) | 21 (70.0) | 6 (100.0) | |
| Height (cm) | 151.2±18.2 | 148.9±18.5 | 162.8±11.9 | 0.08 |
| Weight (Kg) | 74.7±26.5 | 74.4±27.8 | 76.7±21.0 | 0.7 |
| BMI (Kg/m2) | 31.9±7.7 | 32.6±7.9 | 28.7±6.3 | 0.3 |
| BSA (m2) | 1.7±0.4 | 1.7±0.4 | 1.8±0.3 | 0.7 |
Data are presented as number (percentage) or mean ± SD.
Mann Whitney U test was performed for continuous variables, and χ2 test was performed for categorical variables.
Biochemical evaluation
Patients diagnosed with CS had higher serum and urinary values than the control group. The CS group, compared to the control group, had significantly greater midnight serum cortisol (435.5±237.5 vs. 33.3±7.7 nmol/L, p<0.001) and early morning cortisol (468.7±192.6 vs. 259.8±176.4 nmol/L, p=0.02), respectively. In the urine studies, the CS group had significantly greater UFC/BSA (599.0±631.8 vs. 24.6±20.6 nmol/24 h/m2, p<0.001) and 17OHS/Cr (5.6±2.9 vs. 1.7±0.5 mmol/24 h/mol Cr, p<0.001) than the control group, respectively.
Hair cortisol evaluation
Proximal hair cortisol values were higher in the CS than control group (266.6±738.4 vs. 38.9±25.3 pmol/g, p=0.003). However, the differences in hair cortisol values in the medial, distal, and average segments between the CS and control groups did not reach statistical significance. The proximal 1 cm hair had the highest median cortisol levels of all segments in all patients (Fig. 1) and controls (Supplemental Fig.) and represented the highest hair cortisol level in 69.4% (25/36) of all patients and 73.3% (22/30) of CS patients.
Fig. 1.
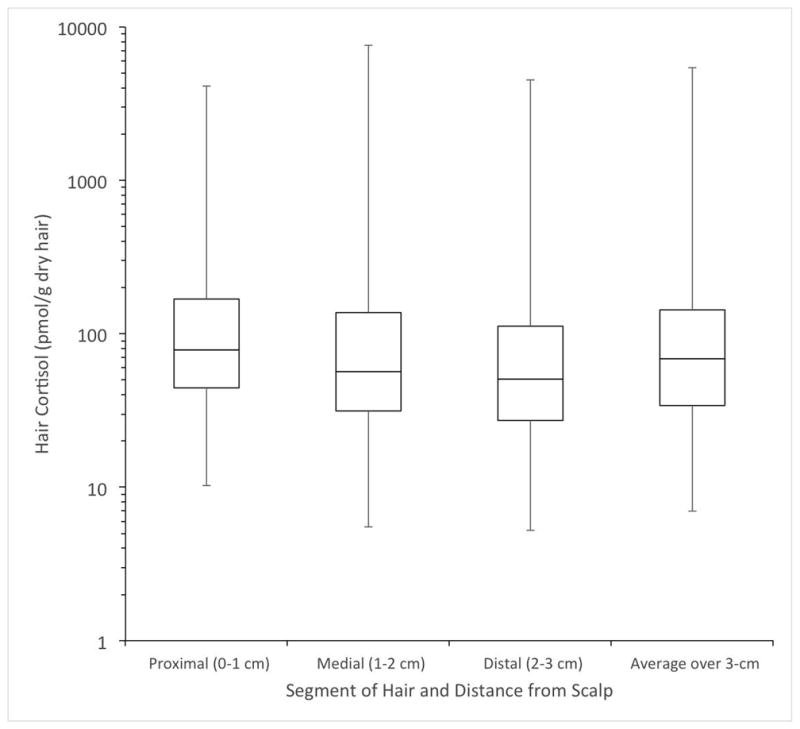
Box plot of hair cortisol segments in the proximal 3 cm of hair of all 36 patients. All 36 patients’ hair cortisol measurements for the proximal, medial, distal, and average hair segments are presented as median and interquartile ranges, with whiskers representing the maximum and minimum.
Correlation analysis
Correlation analysis of the full study population (Table 2) revealed a positive correlation between proximal hair cortisol levels and UFC/BSA (r=0.5, p=0.005, Fig. 2a), midnight serum cortisol (r=0.4, p=0.03, Fig. 2b), and 17OHS/Cr (r=0.3, p=0.06, Fig. 2c), which trended towards significance. Similarly, in all CS patients (Table 3), there was a positive correlation between proximal hair cortisol and UFC/BSA (r=0.5, p=0.009), early morning serum cortisol (r=0.4, p=0.03), and midnight serum cortisol (r=0.3, p=0.07), which trended towards significance. For the medial hair segment, there were mild correlations between medial hair cortisol and UFC/BSA in the entire patient cohort (r=0.3, p=0.05; Table 2) and the CS group (r=0.4, p=0.05; Table 3). For the distal hair segment, there was no statistically significant correlation between distal hair cortisol and any plasma or urinary assessment of hypercortisolemia. Average hair cortisol was mildly correlated with UFC/BSA in all patients (r=0.4, p=0.03; Table 2) and in the CS group (r=0.4, p=0.02; Table 3). In a subgroup analysis of only patients with CD, no correlation of statistical significance was found between any hair cortisol measurement and any of the initial biochemical tests, including diurnal serum ACTH levels (Table 4).
Table 2.
Correlations and levels of significance of biochemical data in all patients (n=36). Hair cortisol values (measured in pmol/g dry hair weight) were log10-transformed for normality when undergoing statistical analysis. All tests represented lab values from all 36 patients, except for the ACTH values, which excluded one patient due to lack of collection. Diurnal ACTH values (n=35) are not presented, because the r-value for all was ≤0.2 with a p-value ≥0.3.
| Hair cortisol segment (distance in cm from scalp) | Average UFC/BSA over D1+D2 (nmol/24h/m2) | Average 17-OHS/Cr over D1+D2 (mmol/24h/mol Cr) | Midnight cortisol (nmol/L) | Early morning cortisol (nmol/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| r | p | r | p | r | p | r | p | ||
| 1 | Proximal | 0.5 | 0.005 | 0.3 | 0.06 | 0.4 | 0.03 | 0.3 | 0.09 |
| 2 | Medial | 0.3 | 0.05 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 |
| 3 | Distal | 0.3 | 0.1 | 0.2 | 0.3 | 0.1 | 0.5 | 0.1 | 0.5 |
|
|
|||||||||
| Average over 3 cm | 0.4 | 0.03 | 0.3 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | |
Statistical significance was considered for p’s<0.05. D1+D2=Day 1 and Day 2.
Fig. 2.
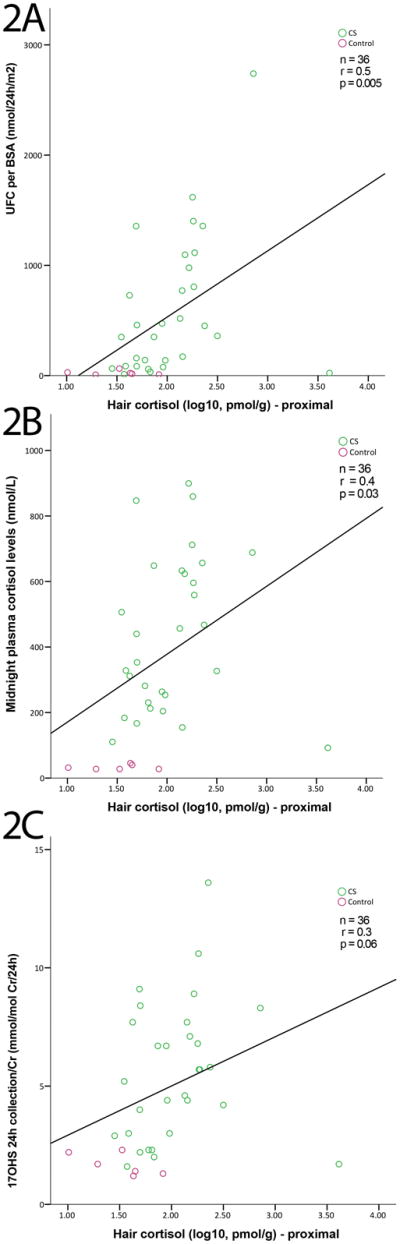
Correlations between proximal hair cortisol and UFC/BSA (A), midnight serum cortisol (B), and 17OHS/Cr (C) among CS patients (green circles) and controls (purple circles). For all graphs, proximal hair cortisol values were log10-transformed for normality. (A) UFC/BSA was strongly and positively correlated with proximal hair cortisol (r=0.5, p=0.005). (B) Midnight cortisol was moderately and positively correlated with proximal hair values (r=0.4, p=0.03). (C) 17OHS/Cr was moderately and positively correlated with proximal hair cortisol but trending toward significance (r=0.3, p=0.06).
Table 3.
Correlations and levels of significance of biochemical data in 30 patients with Cushing Syndrome. Hair cortisol values (measured in pmol/g dry hair weight) were log10-transformed for normality when undergoing statistical analysis.
| Hair cortisol segment (distance in cm from scalp) | Average UFC/BSA over D1+D2 (nmol/24h/m2) | Average 17OHS/Cr over D1+D2 (mmol/24h/mol Cr) | Midnight serum cortisol (nmol/L) | Early morning serum cortisol (nmol/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| r | p | r | p | r | p | r | p | ||
| 1 | Proximal | 0.5 | 0.009 | 0.3 | 0.09 | 0.3 | 0.07 | 0.4 | 0.03 |
| 2 | Medial | 0.4 | 0.05 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.1 |
| 3 | Distal | 0.3 | 0.2 | 0.2 | 0.4 | 0.2 | 0.4 | 0.2 | 0.3 |
|
|
|||||||||
| Average over 3 cm | 0.4 | 0.02 | 0.3 | 0.1 | 0.3 | 0.1 | 0.4 | 0.06 | |
Statistical significance was considered for p’s<0.05. D1+D2=Day 1 and Day 2.
Table 4.
Correlations and levels of significance of biochemical data in 20 patients with Cushing Disease. Hair cortisol values (measured in pmol/g dry hair weight) were log10-transformed for normality when undergoing statistical analysis.
| Hair cortisol segment (distance in cm from scalp) | Average UFC/BSA over D1+D2 (nmol/24h/m2) | Average 17OHS/Cr over D1+D2 (mmol/24h/mol Cr) | Midnight serum cortisol (nmol/L) | Early morning serum cortisol (nmol/L) | Midnight serum ACTH (pmol/L) | Early morning serum ACTH (pmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| r | p | r | p | r | p | r | P | r | p | r | p | ||
| 1 | Proximal | 0.2 | 0.3 | −0.05 | 0.8 | 0.2 | 0.5 | 0.2 | 0.4 | −0.1 | 0.6 | −0.08 | 0.8 |
| 2 | Medial | 0.02 | 0.9 | −0.1 | 0.6 | 0.01 | 1.0 | 0.04 | 0.9 | −0.05 | 0.9 | −0.1 | 0.7 |
| 3 | Distal | −0.05 | 0.8 | −0.2 | 0.5 | −0.04 | 0.9 | −0.2 | 0.5 | −0.05 | 0.8 | −0.1 | 0.7 |
|
|
|||||||||||||
| Average over 3 cm | 0.06 | 0.8 | −0.1 | 0.6 | 0.04 | 0.9 | 0.03 | 0.9 | −0.08 | 0.8 | −0.1 | 0.7 | |
Statistical significance was considered for p’s<0.05. D1+D2=Day 1 and Day 2.
Discussion
We found that proximal hair cortisol directly correlates with midnight serum cortisol and UFC in patients with and without CS. The most proximal 1 cm of hair was the best section of hair for stratifying the two groups of patients in our cohort. Additionally, the proximal hair cortisol correlated best with UFC/BSA among our cohort of CS patients. Other studies reported moderate correlations of hair cortisol with UFC, either as one-time UFC measurement in 39 non-obese subjects (r=0.333, p=0.041) [16] or measuring UFC daily over 63 days in ten healthy participants (r=0.422, p=0.089) with hair collected at the end of 9 weeks [24].
While the growth rate of hair on the vertex is assumed constant, about 1 cm/month [22], hair cortisol concentration is not always consistent. Assuming the rate of hair growth is approximately 1 cm/month at the vertex of the head [25], the most proximal 3 cm of hair may represent the previous 3 months of cortisol exposure. Depending on the cutting technique, at least 0.5 cm of hair, which may represent 2 weeks of potential cortisol exposure, may remain [22]. We chose the initial 3 cm of hair for its reliability compared to distal aspects [26], and our initial goal with segmentation into 1 cm portions was to hopefully identify suspected cases of cyclical CS, as done previously [11].
Both in our study and in the Manenschijn, et al. study with 14 patients with CS [11], segmental hair cortisol stratified CS patients from controls; ectopic CS patients had the highest biochemical measurements of hypercortisolemia than all other CS patients. In our study, with a higher proportion of CD and adrenal CS cases, the differences in hair cortisol levels among all patients and CS subgroups were not as stark. Because of the slight differences between our findings and the study on suspected cyclical CS patients [11], we speculate several confounding factors that can alter hair cortisol measurement in CS patients.
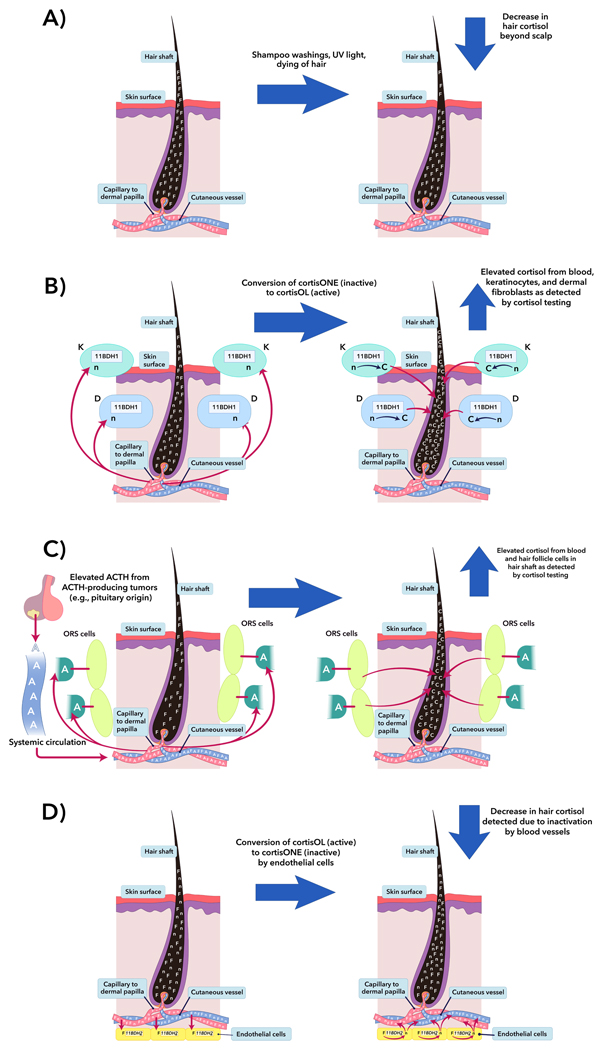
For the measurement of hair cortisol in CS, there are several exogenous and endogenous factors on hair cortisol levels to consider. In several studies [11,14,27], overall hair cortisol values may be overestimated due to lack of isopropanol washings to remove sweat cortisol contamination, and mincing, instead of grinding, may have reduced the yield of actual cortisol from inside the individual hairs. Excessive shampoo washings and hair dyeing [28,29] and prolonged exposure to UV light [30] can diminish quantification in hair samples (Fig. 3a), as supported by findings in a large cohort without psychiatric disease [31]. Even though sweat cortisol has not been found to alter in vivo analysis of hair cortisol levels after short-term exposure to physiological stressors [32], it remains unclear how long term exposure of hair to cortisol from sweat may affect measurements.
Fig. 3.
Potential confounding factors in hair cortisol of CS patients. For simplicity, it is assumed that lipophilic cortisol diffuses from vessel to hair shaft via simple diffusion, similar to how illicit drugs are suspected of being incorporated into hair [45,46]. (A) Excessive shampoo washings and hair dyeing [28,29] can leach cortisol from hair, and prolonged exposure to UV light causes photo-degradation or cross-linking of glucocorticoid molecules [30], decreasing the quantification of cortisol measured in hair. (B) Assuming that skin has local HPA-like activity and appropriate negative feedback loops [33,36], the production and dynamics of locally-synthesized CRH and ACTH should be negligible under systemic suppression in chronic hypercortisolism as in murine models [47]. As may be seen in adrenal tissue in ACTH-dependent CS, in vitro studies of cultured skin sections have shown doubling to tripling of cortisol production after exposure to ACTH [48]. Human epidermal keratinocytes (K) and dermal fibroblasts (D) contain 11-beta-hydroxysteroid dehydrogenase (11BDH) type 1 (11BDH1), which can convert cortisone (inactive) to cortisol (active). In the K and D cells, 11BDH1 is site-dependent and is up-regulated with age, exposure to sunlight, and exposure to glucocorticoids [35,49], and the mechanisms causing increases in cutaneous cortisol in aging persons, such as increased 11BDH1 activity and expression, [49] might also occur in CS patients. The actual change in total hair cortisol by the increase of 11BDH1 kinetics from glucocorticoids in hypercortisolism has not been evaluated. (C) Of the hair follicle components in humans, the keratinocytes of the outer root sheath (ORS) cells possess the ability to produce cortisol after in vitro exposure to CRH and ACTH [33]. Under stimulation from excess systemic ACTH from any cause, ORS cells may locally synthesize cortisol, potentially causing an elevation of total cortisol detected in the hair shaft beyond expected from adrenal-synthesized cortisol. (D) Arterioles feeding the hair bulb express 11BDH type 2 (11BDH2) [37], which may deactivate systemic cortisol to cortisone and potentially lowering the cortisol detected in hair. In hypercortisolemic states, homeostasis is maintained by increased glucocorticoid catabolism, which is probably higher in ectopic CS than CD, likely due to higher cortisol levels in ectopic CS than CD [50]. Therefore, similarly in the skin, cutaneous 11BDH2 might also significantly reduce systemically- and locally-produced cortisol, and this enzyme might have a large role in the expected results in our CS group. This figure was designed using the program, Adobe Illustrator® CS6. F=systemically-produced hydrocortisol, n=hydrocortisone, C=locally-produced hydrocortisol, and A=systemically-produced ACTH.
Cutaneous production of cortisol de novo, activation of cortisone to cortisol, and deactivation of cortisol to cortisone might have significant roles in the discrepancies between hair cortisol levels in CD, adrenal CS, and ectopic CS. In the skin, there are similar synthetic enzymes and feedback mechanisms, which may function like the hypothalamic-pituitary-adrenal (HPA) axis [33] but with additional capabilities for paracrine regulation [34]. Hair follicles and nearby structures have the ability to produce cortisol via 11-beta-hydroxysteroid dehydrogenase (11BDH) type 1 (11BDH1, Fig. 3b) and under ACTH stimulation (Fig. 3c) [33,35,36] and deactivate cutaneous cortisol via 11BDH type 2 (11BDH2), which is expressed in the arterioles supplying the hair bulb (Fig 3d) [37].
With the similar synthetic machinery and feedback mechanisms like the HPA axis, hypercortisolemic states might affect the measured hair cortisol while possibly having minimal to no systemic effect. Similar to ectopic CS and CD, excess systemic ACTH might be expected to hijack the HPA-like axis leading to increased cortisol in hair. Consequently, due to the additive effect of adrenal and cutaneous cortisol synthesis from elevated systemic ACTH, ectopic CS and CD might be expected to have a significantly higher hair cortisol value than in adrenal CS, where this cutaneous axis should suppressed (Fig. 3c). However, a direct linear relationship between ACTH and hair cortisol was not seen in our cohort, and even among the CD patients, there was no significant correlation with any non-hair tests (Table 4). We speculate other factors, like the balance of cortisol activation and deactivation by local 11BDH1 (Fig. 3b) and 11BDH2 (Fig. 3d) might have a significant impact in CS patients, skewing hair measurements. Further studies in hair cortisol evaluation in CS patients should address the effects of 11BDH1, 11BDH2, and response to systemic ACTH on hair cortisol measurements as well as the in vivo glucocorticoid synthetic activity of the hair follicle cells in ACTH-dependent and ACTH-independent CS.
Limitations
Study results should be interpreted with caution: our study was limited by the small control group (n=6), exclusion of patients with midnight serum cortisol > 49.7 nmol/L or hyperaldosteronism, and the majority age of patients less than 18 years. The low number of control patients may confound the interpretation of the hair cortisol cut-off values in the clinical setting. Pseudo-CS patients can have elevated hair cortisol, as in alcoholism [38], obesity [39,40], psychiatric illnesses and chronic stress [41,42], and our exclusion of patients with midnight cortisol > 49.7 nmol/L limited applicability of our study to cases of pseudo-CS and primary aldosteronism without evidence of cortisol co-secretion.
In the current analysis, we did not correct for several covariates, including hair heterogeneity and biometrics, due to sample size limitations. Hence, the results should be taken in the relevant context. Furthermore, most of our patients were able to be evaluated on only one occasion in the 2 months prior to hair sampling before surgery, which limits the correlation of segmental hair cortisol to monthly cortisol levels.
As 58% of our cohort age was less than 18 years, pubertal status on cortisol metabolism may be a factor in hair cortisol measurement. The half-life of cortisol is not significantly different amongst all pubertal stages, even though pubertal status greatly affects clearance and distribution of total and free cortisol [43]. Additionally, puberty is impaired or suppressed in adolescents with CS [44], which could diminish appreciation of differences from various pubertal stages.
However, our study’s strengths are that it is the largest sample so far to analyze segmental hair cortisol in CS and that it is the largest study to compare hair cortisol to any biochemical test for hypercortisolemia in patients with CS. Our study also included a large cohort of CD patients, which has been under-represented in prior studies on hair cortisol. We demonstrated hair cortisol correlates well with some biochemical measures of hypercortisolemia in CS patients, and these findings support further research on the use of this modality in the workup for CS.
Conclusions
We found that, compared with the distal aspects and average of the hair shaft, the most proximal 1-cm hair cortisol is the best marker for reflecting hypercortisolemia in the workup for the rare and diagnostically challenging disease, CS, which if undiagnosed and untreated, has a high associated morbidity and mortality. Our findings support further investigation into the use of the most proximal 1-cm of hair, and hair cortisol in general, as an initial or supportive diagnostic test for CS.
Supplementary Material
Box plot of hair cortisol segments in the proximal 3 cm of hair of 6 control patients. All 6 controls’ hair cortisol measurements for the proximal, medial, distal, and average hair segments are presented as median and interquartile ranges, with whiskers representing the maximum and minimum.
Complete demographics, biochemical evaluation, hair cortisol values, and diagnoses of all patients (n=36). For any values below the lower limit of detection, the value was included as if it was at the lower limit of detection (e.g. if ACTH≤5.0, it was included as 5.0). Patient 9 had surgical resection of the ACTH-producing pituitary adenoma and resolution of hypercortisolemic state 9 months prior to hair collection, and therefore, “symptom duration” of 9 months (*) was actually a surveillance period without evidence of recurrence. For one patient’s pituitary lesion, the pathology reported as “possible (pituitary) adenoma” based on positive staining on small adenohypophysis fragments with Crooke’s hyaline change, which was consistent with ACTH-producing pituitary adenoma. If a procedure was not done or laboratory test was not completed, then it was listed as “Not done.” D1+D2=Day 1 and Day 2.
Acknowledgments
This research was supported in part by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), clinical trials NCT00005927 (Clinical and Molecular Analysis of ACTH-Independent Steroid Hormone Production in Adrenocortical Tissue) and NCT00001595 (A Clinical and Genetic Investigation of Pituitary Tumors and Related Hypothalamic Disorders). We thank the nurses of the NIH Clinical Research Center for assistance with hair cortisol collection. We thank Diane Cooper, MSLS, NIH Library, for assistance in writing this manuscript.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
References
- 1.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The Diagnosis of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman TC, Ghods DE, Shahinian HK, Zachery L, Shayesteh N, Seasholtz S, Zuckerbraun E, Lee ML, McCutcheon IE. High Prevalence of Normal Tests Assessing Hypercortisolism in Subjects with Mild and Episodic Cushing's Syndrome Suggests That the Paradigm for Diagnosis and Exclusion of Cushing's Syndrome Requires Multiple Testing. Horm Metab Res. 2010;42(12):874–881. doi: 10.1055/s-0030-1263128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidambi S, Raff H, Findling JW. Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing's syndrome. Eur J Endocrinol. 2007;157(6):725–731. doi: 10.1530/eje-07-0424. [DOI] [PubMed] [Google Scholar]
- 4.Pecori Giraldi F, Ambrogio AG, De Martin M, Fatti LM, Scacchi M, Cavagnini F. Specificity of First-Line Tests for the Diagnosis of Cushing's Syndrome: Assessment in a Large Series. J Clin Endocrinol Metab. 2007;92(11):4123–4129. doi: 10.1210/jc.2007-0596. [DOI] [PubMed] [Google Scholar]
- 5.Papanicolaou DA, Yanovski JA, Cutler GB, Jr, Chrousos GP, Nieman LK. A Single Midnight Serum Cortisol Measurement Distinguishes Cushing's Syndrome from Pseudo-Cushing States. J Clin Endocrinol Metab. 1998;83(4):1163–1167. doi: 10.1210/jcem.83.4.4733. [DOI] [PubMed] [Google Scholar]
- 6.Newell-Price J, Trainer P, Perry L, Wass J, Grossman A, Besser M. A single sleeping midnight cortisol has 100% sensitivity for the diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf) 1995;43(5):545–550. doi: 10.1111/j.1365-2265.1995.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 7.Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM. Accuracy of diagnostic tests for Cushing's syndrome: A systematic review and metaanalyses. J Clin Endocrinol Metab. 2008;93(5):1553–1562. doi: 10.1210/jc.2008-0139. [DOI] [PubMed] [Google Scholar]
- 8.Grumbach MM, Biller BMK, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JKT, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the Clinically Inapparent Adrenal Mass (“Incidentaloma”) Ann of Intern Med. 2003;138(5):424–430. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 9.Meinardi JR, Wolffenbuttel BHR, Dullaart RPF. Cyclic Cushing's syndrome: A clinical challenge. Eur J Endocrinol. 2007;157(3):245–254. doi: 10.1530/EJE-07-0262. [DOI] [PubMed] [Google Scholar]
- 10.Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SHM. Hair Analysis Provides a Historical Record of Cortisol Levels in Cushing's Syndrome. Exp Clin Endocrinol Diabetes. 2010;118(2):133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manenschijn L, Koper JW, Van Den Akker ELT, De Heide LJM, Geerdink EAM, De Jong FH, Feelders RA, Van Rossum EFC. A Novel Tool in the Diagnosis and Follow-Up of (Cyclic) Cushing's Syndrome: Measurement of Long-Term Cortisol in Scalp Hair. J Clin Endocrinol Metab. 2012;97(10):E1836–E1843. doi: 10.1210/jc.2012-1852. [DOI] [PubMed] [Google Scholar]
- 12.Gow R, Koren G, Rieder M, Van Uum S. Hair cortisol content in patients with adrenal insufficiency on hydrocortisone replacement therapy. Clinic Endocrinol (Oxf) 2011;74(6):687–693. doi: 10.1111/j.1365-2265.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- 13.Staufenbiel SM, Andela CD, Manenschijn L, Pereira AM, van Rossum EF, Biermasz NR. Increased Hair Cortisol Concentrations and BMI in Patients With Pituitary-Adrenal Disease on Hydrocortisone Replacement. J Clin Endocrinol Metab. 2015;100(6):2456–2462. doi: 10.1210/jc.2014-4328. [DOI] [PubMed] [Google Scholar]
- 14.Manenschijn L, Quinkler M, van Rossum EFC. Hair Cortisol Measurement in Mitotane-Treated Adrenocortical Cancer Patients. Horm Metab Res. 2014;46(04):299–304. doi: 10.1055/s-0034-1370961. [DOI] [PubMed] [Google Scholar]
- 15.Noppe G, van Rossum EF, Vliegenthart J, Koper JW, van den Akker EL. Elevated hair cortisol concentrations in children with adrenal insufficiency on hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 2014;81(6):820–825. doi: 10.1111/cen.12551. [DOI] [PubMed] [Google Scholar]
- 16.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30(5):E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 17.D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011;104(2):348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanaelst B, Huybrechts I, Bammann K, Michels N, de Vriendt T, Vyncke K, Sioen I, Iacoviello L, Gunther K, Molnar D, Lissner L, Rivet N, Raul JS, de Henauw S. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49(8):1072–1081. doi: 10.1111/j.1469-8986.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 19.Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, Berthon A, Libe R, Assie G, Espiard S, Drougat L, Ragazzon B, Bertherat J, Stratakis CA. Macronodular Adrenal Hyperplasia Due to Mutations in an Armadillo Repeat Containing 5 (ARMC5) Gene: A Clinical and Genetic Investigation. J Clin Endocrinol Metab. 2014;99(6):E1113–1119. doi: 10.1210/jc.2013-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuBois D, DuBois EF. A Formula To Estimate The Approximate Surface Area If Height and Weight Be Known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 21.Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. Clinical and Genetic Heterogeneity, Overlap with Other Tumor Syndromes, and Atypical Glucocorticoid Hormone Secretion in Adrenocorticotropin-Independent Macronodular Adrenal Hyperplasia Compared with Other Adrenocortical Tumors. J Clin Endocrinol Metab. 2009;94(8):2930–2937. doi: 10.1210/jc.2009-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeBeau MA, Montgomery MA, Brewer JD. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci Int. 2011;210:110–116. doi: 10.1016/j.forsciint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and Analysis of Cortisol from Human and Monkey Hair. J Vis Exp. 2013;(83):e50882. doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ockenburg SL, Schenk HM, van der Veen A, van Rossum EF, Kema IP, Rosmalen JG. The relationship between 63days of 24-h urinary free cortisol and hair cortisol levels in 10 healthy individuals. Psychoneuroendocrinology. 2016;73:142–147. doi: 10.1016/j.psyneuen.2016.07.220. [DOI] [PubMed] [Google Scholar]
- 25.Myers RJ, Hamilton JB. Regeneration and Rate of Growth of Hairs in Man. Ann N Y Acad Sci. 1951;53(3):562–568. doi: 10.1111/j.1749-6632.1951.tb31957.x. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Manenschijn L, Koper JW, Lamberts SWJ, Van Rossum EFC. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10–11):1032–1036. doi: 10.1016/j.steroids.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA. Effects of Shampoo and Water Washing on Hair Cortisol Concentrations. Clin Chim Acta. 2011;412:382–385. doi: 10.1016/j.cca.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman MC, Karban LV, Benitez P, Goodteacher A, Laudenslager ML. Chemical processing and shampooing impact cortisol measured in human hair. Clin Invest Med. 2014;37(4):E252–257. doi: 10.25011/cim.v37i4.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wester VL, van der Wulp NR, Koper JW, de Rijke YB, van Rossum EF. Hair cortisol and cortisone are decreased by natural sunlight. Psychoneuroendocrinology. 2016;72:94–96. doi: 10.1016/j.psyneuen.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Staufenbiel SM, Penninx BW, de Rijke YB, van den Akker EL, van Rossum EF. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology. 2015;60:182–194. doi: 10.1016/j.psyneuen.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Grass J, Kirschbaum C, Miller R, Gao W, Steudte-Schmiedgen S, Stalder T. Sweat-inducing physiological challenges do not result in acute changes in hair cortisol concentrations. Psychoneuroendocrinology. 2015;53:108–116. doi: 10.1016/j.psyneuen.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 34.Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol. 2014;23(6):369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, Age- and Site-Dependent Expression, and Regulation of 11beta-Hydroxysteroid Dehydrogenase Type 1 in Skin. J Invest Dermatol. 2011;131(1):30–36. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 36.Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211(3):199–208. doi: 10.1159/000087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith RE, Maguire JA, Stein-Oakley AN, Sasano H, Takahashi K, Fukushima K, Krozowski ZS. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues. J Clin Endocrinol Metab. 1996;81(9):3244–3248. doi: 10.1210/jcem.81.9.8784076. [DOI] [PubMed] [Google Scholar]
- 38.Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol. 2010;85(3):357–360. doi: 10.1016/j.biopsycho.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Veldhorst MAB, Noppe G, Jongejan MHTM, Kok CBM, Mekic S, Koper JW, van Rossum EFC, van den Akker ELT. Increased Scalp Hair Cortisol Concentrations in Obese Children. J Clin Endocrinol Metab. 2014;99(1):285–290. doi: 10.1210/jc.2013-2924. [DOI] [PubMed] [Google Scholar]
- 40.Wester VL, Staufenbiel SM, Veldhorst MAB, Visser JA, Manenschijn L, Koper JW, Klessens-Godfroy FJM, Van Den Akker ELT, Van Rossum EFC. Long-term cortisol levels measured in scalp hair of obese patients. Obesity. 2014;22(9):1956–1958. doi: 10.1002/oby.20795. [DOI] [PubMed] [Google Scholar]
- 41.Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress. 2012;15(3):348–353. doi: 10.3109/10253890.2011.619239. [DOI] [PubMed] [Google Scholar]
- 42.Herane Vives A, De Angel V, Papadopoulos A, Strawbridge R, Wise T, Young AH, Arnone D, Cleare AJ. The relationship between cortisol, stress and psychiatric illness: New insights using hair analysis. J Psychiatr Res. 2015;70:38–49. doi: 10.1016/j.jpsychires.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Charmandari E, Hindmarsh PC, Johnston A, Brook CG. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: alterations in cortisol pharmacokinetics at puberty. J Clin Endocrinol Metab. 2001;86(6):2701–2708. doi: 10.1210/jcem.86.6.7522. [DOI] [PubMed] [Google Scholar]
- 44.Dupuis CC, Storr HL, Perry LA, Ho JT, Ahmed L, Ong KK, Dunger DB, Monson JP, Grossman AB, Besser GM, Savage MO. Abnormal puberty in paediatric Cushing's disease: relationship with adrenal androgen, sex hormone binding globulin and gonadotrophin concentrations. Clin Endocrinol (Oxf) 2007;66(6):838–843. doi: 10.1111/j.1365-2265.2007.02822.x. [DOI] [PubMed] [Google Scholar]
- 45.Henderson GL. Mechanisms of drug incorporation into hair. Forensic Sci Int. 1993;63:19–29. doi: 10.1016/0379-0738(93)90256-a. [DOI] [PubMed] [Google Scholar]
- 46.Barbosa J, Faria J, Carvalho F, Pedro M, Queirós O, Moreira R, Dinis-Oliveira RJ. Hair as an alternative matrix in bioanalysis. Bioanalysis. 2013;5(8):895–914. doi: 10.4155/bio.13.50. [DOI] [PubMed] [Google Scholar]
- 47.Pang S, Wu H, Wang Q, Cai M, Shi W, Shang J. Chronic stress suppresses the expression of cutaneous hypothalamic-pituitary-adrenocortical axis elements and melanogenesis. PLoS One. 2014;9(5):e98283. doi: 10.1371/journal.pone.0098283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286(12):10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiganescu A, Tahrani AA, Morgan SA, Otranto M, Desmouliere A, Abrahams L, Hassan-Smith Z, Walker EA, Rabbitt EH, Cooper MS, Amrein K, Lavery GG, Stewart PM. 11beta-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J Clin Invest. 2013;123(7):3051–3060. doi: 10.1172/jci64162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart PM, Walker BR, Holder G, O'Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing's syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617–3620. doi: 10.1210/jcem.80.12.8530609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box plot of hair cortisol segments in the proximal 3 cm of hair of 6 control patients. All 6 controls’ hair cortisol measurements for the proximal, medial, distal, and average hair segments are presented as median and interquartile ranges, with whiskers representing the maximum and minimum.
Complete demographics, biochemical evaluation, hair cortisol values, and diagnoses of all patients (n=36). For any values below the lower limit of detection, the value was included as if it was at the lower limit of detection (e.g. if ACTH≤5.0, it was included as 5.0). Patient 9 had surgical resection of the ACTH-producing pituitary adenoma and resolution of hypercortisolemic state 9 months prior to hair collection, and therefore, “symptom duration” of 9 months (*) was actually a surveillance period without evidence of recurrence. For one patient’s pituitary lesion, the pathology reported as “possible (pituitary) adenoma” based on positive staining on small adenohypophysis fragments with Crooke’s hyaline change, which was consistent with ACTH-producing pituitary adenoma. If a procedure was not done or laboratory test was not completed, then it was listed as “Not done.” D1+D2=Day 1 and Day 2.