ABSTRACT
Factors that affect the direction of root growth in response to environmental signals influence crop productivity. We analyzed the root tropic responses of thioredoxin (trxs), thigmotropic (wav2-1), and hydrotropic (ahr1 and nhr1) Arabidopsis thaliana mutants treated with low concentrations of paraquat (PQ), which induces mild oxidative stress, and established a new method for evaluating root waviness (root bending effort, RBE). This method estimates root bending by measuring and summing local curvature over the whole length of the root, regardless of the asymmetry of the wavy pattern under thigmostimulation. In roots of the wav2-1 mutant, but not in those of the trxs and ahr1 mutants, RBE was significantly inhibited under mild oxidative stress. Thigmotropic stimulation of wav2-1 mutant roots, with or without PQ treatment, showed high levels of reactive oxygen species fluorescence, in contrast to roots of the ahr1 mutant. Furthermore, PQ inhibited root growth in all genotypes tested, except in the wav2-1 mutant. In a hydrotropism assay of the trxs and wav2-1 mutants, root growth behavior was similar to the wild type with and without PQ, while the root growth of ahr1 and nhr1 mutants was diminished with PQ. These results indicate that hydrotropic and thigmotropic mutants respond differently to exogenous PQ, depending on the tropic stimulus perceived. Therefore, the mechanisms underlying hydrotropism and thigmotropism may differ.
KEYWORDS: Arabidopsis, gravitropism, hydrotropism, roots, ROS, thigmotropism, thioredoxins
Abbreviations
- PQ
paraquat
- RBE
root bending effort
- ROS
reactive oxygen species
Introduction
Post-embryonic plant development is largely regulated by differential growth movements, or tropisms, which alter the direction and extent of growth in response to environmental cues such as gravity, light, water gradient, obstacles, pathogens, or temperature. The combined effects of stress-specific phenotypes and stimulus-induced morphogenesis define the shape of an adult plant. Changes in root architecture control productivity in crops such as Oryza sativa (rice)1 Zea mays L. (maize),2 and Phaseolus vulgaris (bean)3; thus, studies of root tropic responses are of great interest.
In Arabidopsis thaliana, roots integrate different stimuli such as the gravity vector, moisture gradients, and touch to modulate their growth, avoid drought and obstacles, and fix the plant in the soil.4 Three major forces can redirect root growth: the gravity vector, source of nutrients such as N and P, and a water source. Terrestrial plants direct their root systems downwards into the soil, mainly in response to the gravity vector; however, as the roots search for water and nutrients, they frequently change direction to avoid obstacles (thigmotropism).
Root hydrotropism is a mechanism found in many species to avoid water stress. To date, four hydrotropic response mutants have been isolated in Arabidopsis: nhr1 (no hydrotropic response 1),5 ahr1 (altered hydrotropic response 1),6 miz1 (mizu-kussei 1), and miz2.7 Only two loci (MIZ1 and MIZ2) affecting root hydrotropism have been identified. Genetic redundancy underlying this tropism may have concealed other loci.
Okada and Shimura (1990) reported a gravity-induced touch response in seedlings grown on hard agar in a tilted plate, which resulted in ‘wavy’ roots.8 They isolated a set of mutants with abnormal wavy growth, and found that one of these, wav6, was allelic to pin2.9 PIN2 is an important regulator of gravitropism as it regulates the redistribution of auxin from the stele to the elongation zone of roots.13
In a study of hydrotropic responses using two wav mutants (wav2-1 and wav3-1), increased sensitivity to the moisture gradient was observed after hydrostimulation,10 indicating that common auxin signaling mechanisms might account for the perception and signaling of these two tropisms. Cloning the WAV2 gene revealed that it encodes a protein belonging to the BUD EMERGENCE 46 family. This has an N-terminus transmembrane domain and an α/β-hydrolase domain at the C terminus, which regulates stimulus-induced root bending through inhibition of root tip rotation; however, its molecular function remains elusive.11
Root responses to gravity are well studied in Arabidopsis.12 Amyloplasts are considered key in gravity sensing. Gravitropic responses are mainly regulated by auxin gradients, cytokinin, reactive oxygen species (ROS), pH, and Ca2+ for the transmission of the signal(s), which results in differential growth responses.13-16 There is some evidence to suggest that the mechanism governing root gravitropism is different from that governing hydrotropism; although crosstalk between these two responses has made the elucidation of root tropic responses much more complex than expected.17,18 Nevertheless, when the root perceives a water source or an obstacle, curvature is induced in the root elongation zone.
ROS are also important regulators of differential growth elongation during the development of gravitropic and hydrotropic responses.19,20 However, we are just beginning to understand the functions of ROS during this process. Thus, their participation in tropic responses constitutes an open area for research. Superoxide anions (O2.−) and hydrogen peroxide (H2O2), are generally considered to be metabolic byproducts that participate in the gravitropic response of maize roots.21 However, after 1 h of gravistimulation, the intracellular ROS concentration increased threefold in the primary root tip, indicating that it functions as a second messenger (and/or modulator) in this tropic response.21
The enzymatic pathway for ROS generation probably contributes most of the ROS required for signaling mechanisms. Indeed, in root apical meristems, where cells rapidly proliferate, superoxide anions are found in a greatly enriched zone. The ratio of O2.- to H2O2 determines where cells in the root tip transition from the proliferation to elongation zone, where differentiation occurs.22 Therefore, ROS are important plant growth regulators, and as such, are comparable to a plant hormone.22,23
Accumulation of glutathione and thioredoxins (Trx) in the meristem region is also involved in root development,24 supporting genetic evidence that links thioredoxins, glutathione (GSH), and auxin to the control of shoot and root development.21 Plant Trxs are present in the main cell compartments, including the cytosol and plastids. Trxs act in an antioxidant network, supplying the reducing power necessary for detoxifying lipid hydroperoxides, repairing proteins, regulating enzyme activity, directly detoxifying active ROS, and modulating the redox status of components involved in pathways linked to oxidative stress and the control of gene expression.25
The formation of carbonyl groups on protein amino acid residues as a result of free radical-initiated reactions is also well documented.26 Oxidized proteins are partially denatured, and some hydrophobic regions are exposed to the action of chaperones and the ubiquitin/proteasome system (UPS).26,27 Several reports implicate the proteasome in the degradation of oxidized proteins.29-31 Increased levels of ubiquitin (Ub) conjugates can be detected before changes in other classic oxidative stress markers, such as glutathione disulfide/glutathione and NAD(P)/NAD(P)H ratios, and protein carbonyl content. Thus, the accumulation of Ub conjugates is a sensitive indicator of cellular oxidative stress.32
Root waviness is caused by periodic rotation of the root tip.8 Two methods have been described for estimating root waviness in Arabidopsis. In the first one, the mean wavelength of a root wave and the wave tangent angles are estimated to infer root waviness.11,33 In the second, root waviness is inferred by spectral analysis.34 The first method is best for analyzing roots that have a largely symmetric and periodic wave pattern, while the second is more suitable for randomly wavy roots, and for brief and interrupted time series.
We established a new method to evaluate the root bending effort (RBE) of Arabidopsis seedlings with different root wave intensities. Assuming that root waviness is not symmetrical or sinusoidal, we developed a simple method to obtain an index that describes the effort a root makes for bending under thigmotropic stimulation. Thus, RBE estimates the global waves of a root by measuring and summing its local curvature over the whole length of the root, providing a clear measurement of the thigmotropic phenotype.
The involvement of ROS in thigmotropism has not yet been described; therefore, we compared the effect of ROS produced by paraquat (PQ) on root growth, as well as the effect of PQ on hydrotropic and thigmotropic stimulation in different mutant genotypes of Arabidopsis (i.e., nhr1, ahr1, wav2-1, and several trx mutants). We also analyzed the levels of endogenous Ub conjugates in hydrostimulated roots in response to mild oxidative stress.
Since changes in redox state affect the growth and elongation of plant organs, we hypothesized that, in the presence of PQ, some trx mutants might show altered hydrotropic root curvature and altered waviness compared to hydrotropic and wavy mutants. We found that the mechanisms underlying hydrotropism and thigmotropism differ.
Results
Root growth of Trx and hydrotropic response mutants in the presence of PQ
Root growth is a dynamic process that is modulated by several signaling pathways, including Ca2+ signaling; auxin polar transport; and H+-ATPase activity. Since ROS have emerged as important signaling molecules in roots,39 we applied low concentrations of PQ, a widely used herbicide that induces oxidative stress,36 to Arabidopsis roots; this did not completely block growth of wild-type roots, but increased intracellular ROS levels.
We hypothesized that plants deficient in redox signaling would show altered patterns in tropic responses such as root hydrotropism and thigmotropism. To test this hypothesis, we compared the root growth of trx mutants grown on normal medium versus a medium containing PQ. Seeds were germinated on normal medium containing 0.03 µM PQ and root growth was evaluated after 7 d. Under the PQ treatment, seedling root growth was diminished compared to those grown on normal medium without PQ. In the nhr1, ahr1, and trx h9, trx h1, trx h3, trx h4, trx o, and trx m3 mutants, and in the Col-0 and Ler ecotypes, average root growth was reduced by approximately 50% (Fig. 1). However, root growth of the thigmotropic mutant wav2-1 was inhibited by only 20–30% compared to its respective wild type, suggesting that in this thigmotropic mutant root growth might be negatively influenced by the presence of ROS (Fig. 1).
Figure 1.
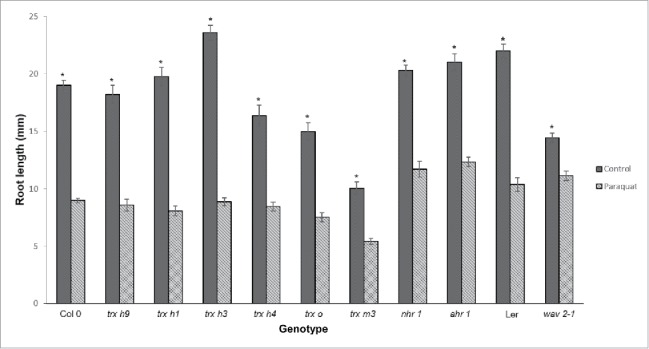
Effect of paraquat on the root growth of trx mutants (trx h9, trx h1, trx h3, trx h4, trx o, trx m3), root hydrotropic response (ahr1 and nhr1), and root waviness (wav2-1) mutants. Seven-day-old seedlings were grown in normal MS medium (without paraquat [PQ], solid bars) or in the presence of 0.03 µM PQ (shaded bars). Root length of PQ-treated roots was compared with controls. PQ reduced root growth by 50% in all genotypes tested, except wav2-1. Vertical bars show mean ± standard error (SE). *Indicates significant differences as determined by two-way ANOVA and Tukey's post-hoc (P < 0.05), n = 50. Data represent one of three biological replicates.
ROS affect root thigmotropism
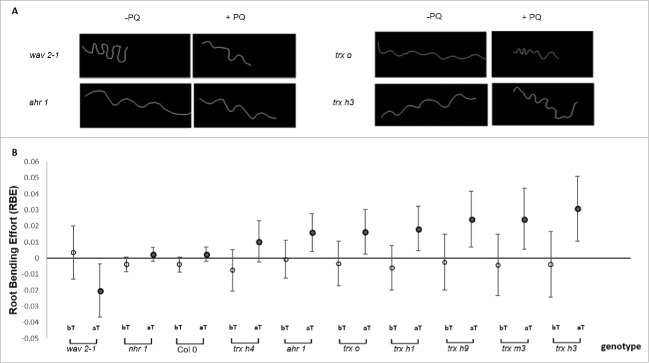
We then calculated the root bending effort (RBE) for each root before and after applying a thigmotropic stimulus to the roots of PQ-treated and control (without PQ) seedlings. The mean RBE of PQ-treated roots was subtracted from the mean RBE of control roots. After applying the thigmotropic stimulus, PQ caused an increase in waviness in the root patterns of ahr1 and trx o, trx h1, trx h9, trx m3, and trx h3 mutants compared to the wild type, nhr1, and trx h4 (Fig. 2C). By contrast, the waviness of wav2-1 roots was significantly decreased under the same conditions (Fig. 2). These observations indicate that thigmotropic behavior requires an appropriate redox state in which thioredoxins participate in root elongation or in differential growth responses.
Figure 2.
Root waviness of different genotypes under oxidative stress. (A) Root images showed the wavy pattern of wav2-1, ahr1, trx o, and trx h3 mutant roots after thigmotropic stimulation in the presence and absence of PQ. wav2-1 roots were less wavy than the other genotypes tested. (B) The mean root bending effort (RBE) of PQ-treated roots was subtracted from the mean RBE of roots not treated with PQ before (bT) and after (aT) thigmotropic stimulation. The RBE values of ahr1, trx o, trx h1, trx h9, trx m3 and trx h3 were higher after exposure to thigmotropic stimuli compared with wav2-1. The RBE of the Columbia-0 (Col-0) genotype was designated as 0 and was calculated as described in Materials and Methods.
In this study, in the presence of PQ, the roots of all trx mutants (independently of its localization: cytosolic or plastidic) had more waves. This effect was specific to thigmotropism because it was only detected when the root encounters an obstacle (in this case, an impenetrable, inclined barrier of hard agar in angled plates (Fig. 2B–C). Since PQ inhibited root growth in wild-type plants and all trx mutants (Fig. 1), differential growth responses of the root are most likely affected by ROS-regulated cell cycle progression.30 Thus, thigmotropic curvature might depend not only on meristem activity and the cell proliferation rate of each genotype, but also on the differential speed of growth elongation in the external or internal curvature of the wave.
Thigmotropism and PQ treatment induce a differential distribution of ROS in the roots
To determine the role of ROS during the thigmotropic response and correlate this with RBE, we compared the intracellular ROS levels in the first root turn or wave of the root tip in roots grown vertically, and in thigmotropic assays of the hydrotropic mutant ahr1 and the wavy mutant wav2-1. The latter two mutants were chosen because they display opposite RBE behaviors (Fig. 2).
Vertically positioned ahr1 roots had very low ROS levels in the elongation zone; however, wild-type roots (Col 0 and Ler) clearly displayed higher ROS levels in this zone (Fig. 3A, C). Wild-type roots in the inclined position had the highest ROS levels in the first root wave (at the root tip) (Fig. 3E, G). This is a noteworthy observation because root tips grown in the vertical position have no physical obstacle, whereas they do (hard agar) in the inclined position, and might require higher ROS levels to develop a root wave.
Figure 3.
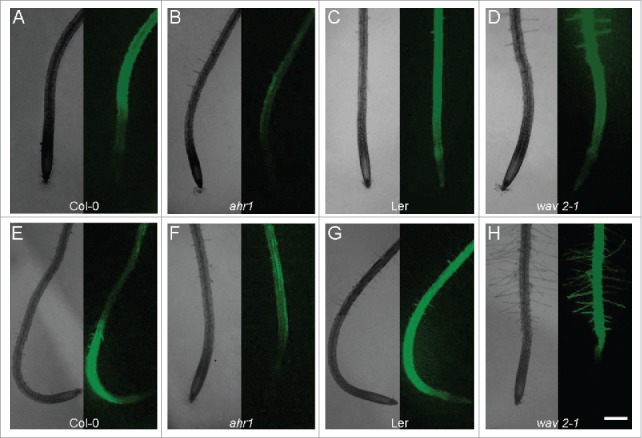
The differential distribution of reactive oxygen species in ahr1 and wav2-1 roots under thigmotropic stimulus. Using a hydrogen peroxide-sensitive fluorescent probe (CM-H2DCFDA), reactive oxygen species (ROS) were localized in roots of ahr1 and wav2-1 mutants and their respective wild-type (Col-0) seedlings in a vertical position (A–D) and under thigmotropic stimulation (E–H). Each panel shows an optical bright field image (left) and a fluorescent image (right). Roots of ahr1 mutant seedlings showed reduced fluorescence in the vertical position compared to Col-0 (A–B). Under thigmotropic stimulus, Col-0 seedling roots (E) showed higher fluorescence in the elongation zone and root tip, while ahr1 roots were less curved and showed less fluorescence in the elongation zone and the root tip. Roots of the wav2-1 mutant and its respective wild type (Ler) had elevated fluorescence in both the vertical position and under thigmotropic stimulus, indicating that the ROS levels in these genotypes are higher (C–D, G–H) compared to Col-0 and the ahr1 mutant (A–B, E–F). Scale bar = 200 µm.
When thigmotropically stimulated, the roots of ahr1 seedlings exhibited very low levels of ROS in the elongation zone compared to Col-0 (Fig. 3E–F). In the vertical position, wav2-1 roots and their respective wild type (Ler) had higher fluorescence in the elongation zone. Under thigmotropic stimulus, Ler had higher levels of fluorescence in the whole root, while wav2-1 lacked fluorescence at the root tip, indicating that in these genotypes, ROS were also differentially localized (Fig. 3C–D, G–H) compared to Col-0 and ahr1 (Fig. 3A–B, E–F).
We also tested the effect of PQ on the distribution of ROS fluorescence in ahr1 and wav2-1 root seedlings grown in the vertical position compared to their respective wild types (Col-0 and Ler). Col-0 and ahr1 roots both displayed considerably higher fluorescence compared to wav2-1 and Ler (Fig. 4). In addition, all genotypes tested showed significantly decreased fluorescence when under the thigmotropic stimulus in the presence of PQ (Table S1).
Figure 4.
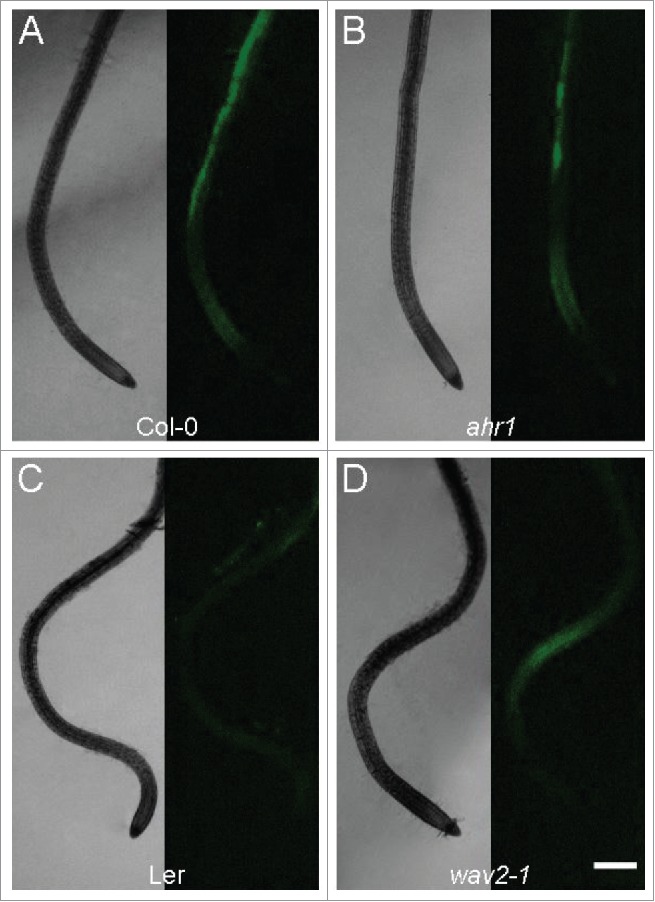
Paraquat treatment changes the distribution of reactive oxygen species in ahr1 and wav2-1 mutants. Root seedlings of Col-0 (A), ahr1 (B), Ler (C), and wav2-1 (D) were grown in the vertical position in the presence of paraquat (PQ). Each panel shows root tips in optical bright field (left) and fluorescent images (right). Roots of the ahr1 mutant and Col-0 had considerably higher fluorescence than the wav2-1 mutant and Ler (A-B). These genotypes (Ler and wav2-1) had a significantly reduced fluorescence signals under thigmotropic stimulus when in the presence of PQ (C-D) (see data in Supplemental Table 1). Scale bar = 200 µm.
Trx mutants displayed hydrotropic responses in the roots
Using square Petri dishes containing normal growth medium in the upper part and medium with a low water potential (the hydrotropic assay medium) in the lower part, 5 different trx mutants (trx h9, trx h1, trx h3, trx h4, trx o and trx m3) were grown on the hydrotropic part of the medium. This was designed to isolate the nhr1 mutant.37 Seedlings were classified as having no hydrotropic response when its root grew beyond the border between the two media when the Petri dish was placed vertically on edge.
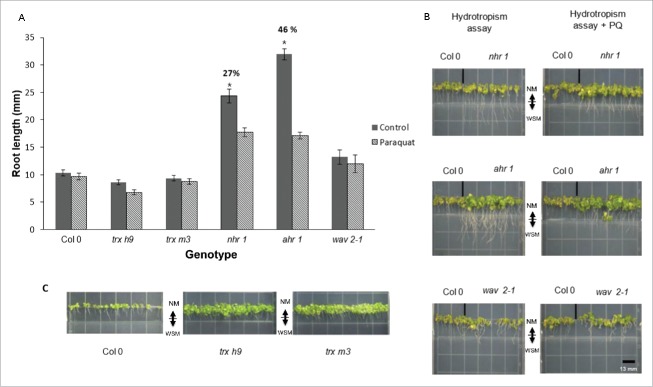
The 9 trx mutants showed a similar root hydrotropic response to the wild type (data not shown); trx h9 and trx m3 were included to comparing their hydrotropic responses with the no-hydrotropism (ahr1 and nhr1) and wavy (wav2-1) mutants (Fig. 5A, C). Only the roots of nhr1 and ahr1 grew into the hydrotropic assay medium, indicating a lack of hydrotropic response (Fig. 5A, B).
Figure 5.
Roots of trxs mutants display a hydrotropic response in the assay medium with or without paraquat. (A–B) Seedlings were grown on hydrotropism assay medium for 11 d, and root length was compared in plates treated (shaded bars) or untreated (solid bars) with paraquat (PQ). Roots of nhr1 and ahr1 mutants showed similar hydrotropic responses in the assay medium with and without PQ (roots crossed the border between the two media); however, growth of nhr1 roots was less inhibited in the presence of PQ. (C) Representative images of wild-type Col-0, trx h9 and trx m3 seedlings are shown in the hydrotropic assay medium only in the presence of PQ. Indicates significant differences compared with its own genotype when treated with PQ (as determined by two-way ANOVA and Tukey's post hoc, P < 0.05, from three biological replicates), n = 50. NM, normal medium; WSM, water stress medium. Arrowhead depicts the border between the NM and WSM. Scale bar = 13 mm.
The effect of adding PQ to each medium in the hydrotropic assay was also tested. Roots of nhr1 and ahr1 elongated and crossed beyond the border between the two media in the presence of the oxidant, whereas roots of other genotypes did not. However, PQ inhibited nhr1 root elongation to a lesser extent (27%) than ahr1 (46%) (Fig. 5A). As expected, wild-type roots did not grow in this assay; neither did the trx mutants trx h9 or trx m3 (Fig. 5). The other trx mutants responded in a similar way to trx h9 and trx m3 (data not shown). Nonetheless, compared to nhr1, ahr1 roots were the most sensitive to PQ-induced oxidation; this might suggest that in these mutants, ROS are handled in different pathways compared to the wild type (Fig. 5A–B). Roots of the wav2-1 mutant did not grow much beyond the border of the two media in the hydrotropic assay medium (Fig. 5A–B).
The gravitropic responses of hydrotropic mutants were unaffected by oxidative stress
ROS positively regulate the gravitropic response of maize roots.21 We examined the root curvature of mutant roots after reorienting them at 90° for 24 h. Under control conditions (without PQ), most trx mutants produced root angles similar to those of the wild type. PQ did not affect the gravitropic curvature of trx h9, trx h3, trx h4, trx o, or trx m3 roots (Fig. 6), suggesting that, in the absence of Trx products, ROS may not be involved in the gravitropic response.
Figure 6.
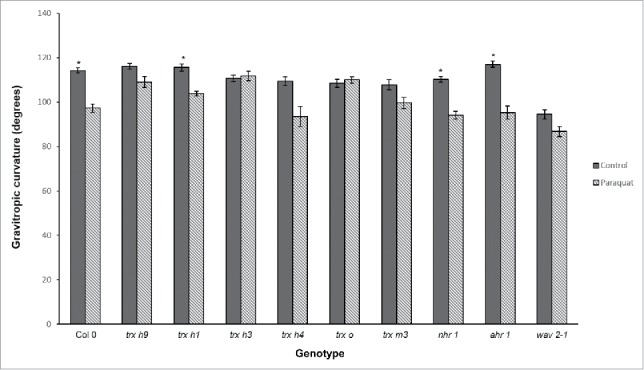
Root gravitropic responses of different genotypes under oxidative stress. Four-day-old seedlings were reoriented by 90° and the root curvature (angle) was measured after 24 h. Gravitropic curvature was diminished by paraquat (PQ) treatment in all genotypes except for trx h3, trx o and wav2-1, in which the angle of curvature was similar in seedlings treated and untreated with PQ. The trx h1, wild type (Col-0), nhr1 and ahr1 genotypes showed significant differences in their gravitropic curvatures when treated with PQ (as determined by two-way ANOVA and Tukey's post-hoc, P < 0.05, from three biological replicates). n = 30
PQ treatment reduced the angle of root curvature of wild-type, txr h1, nhr1, and ahr1 roots (Fig. 6). This is different to observations made in maize roots (Zea mays L. cv. Golden Cross Bantam),21 indicating that PQ-generated ROS have a different effect on the root gravitropic response of these Arabidopsis mutants, and that in Arabidopsis, gravitropism is promoted by ROS (most probably from the dissociation of H2O2).
Lateral root formation in the Trx, wavy, and hydrotropic mutants in the presence of PQ
The branching process required for roots to acquire water and nutrients during root development is sensitive to stress conditions, and is also regulated by the circadian clock and auxin signaling.38 In our hydrotropic assay medium, PQ treatment reduced lateral root development by ∼80% in the wild type, trx m3 and trx h9 mutants, and ahr1. In nhr1, lateral root emergence was repressed by only 53% (Fig. 7). While PQ inhibited the development of lateral roots in almost all seedlings tested, roots of the wild type, trx h9, trx m3, and ahr1 were more sensitive than those of nhr1, indicating a slight resistance of nhr1 to PQ treatment and a lesser susceptibility to ROS in terms of lateral root emergence.
Figure 7.
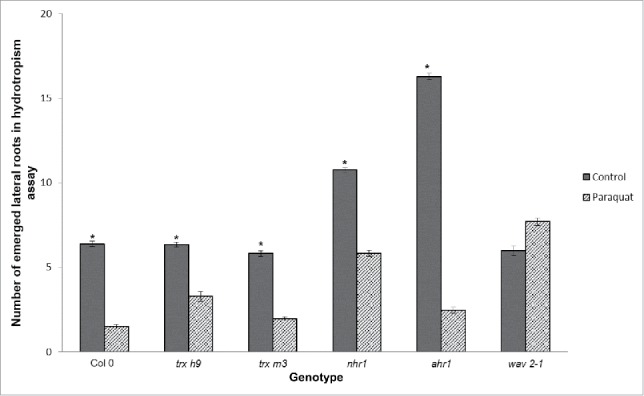
Oxidative stress promotes lateral root emergence in the wav2-1 mutant. Lateral roots of 11-day-old seedlings grown in hydrotropism assay medium were counted. Paraquat (PQ)-inhibited lateral root emergence was observed in all mutants tested, except the wav2-1 mutant. Bars indicate means ± standard error (SE) from three biological replicates. *Indicates significant differences compared to controls and PQ-treated medium using Dunn´s multiple comparison test, Kruskal-Wallis test, and Mann-Whitney U test, from three biological replicates (P < 0.05). n = 30.
Surprisingly, wav2-1 was unaffected by PQ-induced oxidative stress, and in fact produced 21% more lateral roots in the hydrotropic assay medium than the wild type (Fig. 7). This suggests that, in wav2-1, root branching may have a different mechanism to overcome PQ-induced oxidative stress. The mechanism by which ROS affects lateral root emergence has not been elucidated, although there is evidence to suggest that redox signaling regulates lateral root formation.39
The emergence of lateral roots in both nhr1 and wav2-1 was less sensitive to PQ in the low water potential conditions of the hydrotropism assay than the wild-type, indicating that these mutants might possess a common mechanism to cope with water deficit and elevated ROS.
Protein analysis: Protein carbonylation, protein ubiquitination, and 20S analysis
In plant cells, ROS are both emergency signals and cytotoxic molecules. Terrestrial plants have evolved scavenging mechanisms to use these signals to acclimatize to stress conditions. In response to cellular stress, such as mild oxidative stress, levels of endogenous Ub conjugates are increased, which can be detected before changes in other ‘classic’ oxidative stress markers, such as glutathione disulfide/glutathione and NAD(P)/NAD(P)H ratios, and protein carbonyl content. Accumulation of Ub conjugates is therefore one of the most sensitive indicators of cellular oxidative stress.32
The formation of carbonyl groups on protein amino acid residues as a result of free radical-initiated reactions is another good marker of redox imbalance.26,40 The relationship between oxidatively damaged proteins and their degradation via the UPS has been extensively studied. Proteins are partially denatured by oxidation (i.e., carbonylation), exposing some hydrophobic regions to the action of chaperones and the UPS.29-31
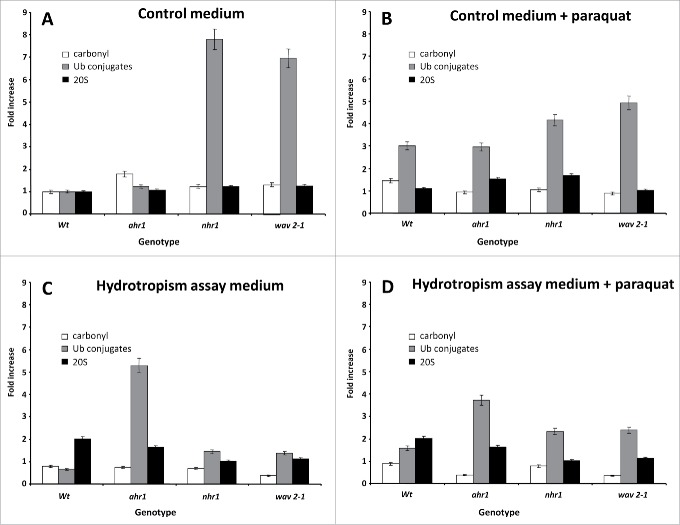
To explore the role of oxidative stress on root hydrotropism, we determined the contents of protein carbonyls, Ub conjugates, and proteasomes in a set of tropic mutants (ahr1, nhr1, wav2-1) grown under four conditions: control medium, control medium with PQ, hydrotropism assay medium, and hydrotropism assay medium with PQ (Fig. 8). All data were standardized to wild-type growth under control conditions (wild type = 1). We determined the reacting carbonyl groups of protein carbonylation with 2,4-dinitro phenylhydrazine (DNPH) that formed protein-bound 2,4-dinitrophenyl hydrazones. Hydrazones were quantified with an anti-hydrazone antibody. Under control conditions, the proportion of oxidized proteins in the three mutants was greater than 1 (approximately 1.2–1.8), and only the wild type had more oxidized proteins in the presence of PQ (1.46) (Fig. 8B).
Figure 8.
Carbonyl, ubiquitin conjugates and 20S content of total proteins extracted from Arabidopsis root seedlings. The roots of eleven-day-old seedlings grown in control medium without (A) and with paraquat (PQ; B), or in the hydrotropic assay medium without (C) or with PQ (D). Equal amounts of total protein (5 mg) were used to measure protein carbonyl, ubiquitin (Ub) conjugates and 20S proteasome content by slot blot, as described in Materials and Methods. Ub conjugate content was the most responsive and sensitive parameter (A–D). Root seedlings of the nhr1 and wav2-1 mutants had higher Ub conjugate content under control conditions (A), while in the presence of paraquat (PQ) or in the hydroptropism assay medium, the same mutants showed a clear decrements (B–C), indicative of more active Ub conjugate-degrading machinery. The ahr1 mutant had the highest Ub conjugate content in the hydrotropism assay medium, whether with or without PQ. Carbonyl and 20S content showed minor but significant variations. Values are expressed as fold increases, where the wild type (Wt) in control medium is considered as 1. Means and standard deviations (SD) were calculated from three biological replicates.
In wav2-1 mutants grown in the hydrotropic assay medium, the carbonyl group content was nearly 50% lower than that of the wild type; similar results were observed in this assay medium in the presence of PQ (Fig. 8C–D). Roots of the ahr1 mutant showed a comparable reduction in carbonyl groups, but only when PQ was present in the hydrotropic assay medium (Fig. 8D). Other genotypes tested showed no changes in carbonyl group content in the hydrotropism assay medium, in both the presence and absence of PQ (Fig. 8C–D). These observations indicate that the change in the level of oxidized proteins might be related to the hydrotropic or thigmotropic phenotypes of these mutants.
Several studies indicate the influence of oxidative stress on the capacity of the UPS to degrade proteins.41 An increase in substrate availability can increase intracellular protein degradation in response to oxidative stress. The activities of the E1 and E2 components of the Ub conjugation system increased in response to mild oxidative stress.42 The different sensitivities of the Ub conjugation machinery and the proteasome to mild oxidative stress may account for the accumulation of Ub conjugates; such accumulation may be a useful indicator of cellular oxidative stress. By contrast, inactivation of the Ub-conjugating enzymes may reduce Ub conjugate levels in cells under severe oxidative stress. In control medium, the basal level of ubiquitination in ahr1 was similar to that of Col-0, while nhr1 and wav2-1 had high levels of Ub conjugates (8- and 7-fold, respectively) (Fig. 8A). In the presence of PQ, this response was reduced in nhr1 and wav2-1 by threefold and twofold, respectively, while in Col-0 and ahr1 the quantity of Ub proteins doubled (Fig. 8B). In the hydrotropism assay medium, the roots of Col-0, nhr1, and wav2-1 seedlings exhibited fewer Ub conjugate levels, though this decrease was fivefold more pronounced in nhr1 and wav2-1 (Fig. 8C), while protein ubiquitination was fivefold greater in ahr1 roots, and was slightly lower in the presence of PQ (Fig. 8C–D). This indicates a clear difference in redox regulation in ahr1, nhr1, and wav2-1.
Available data indicate that the proteasome is important in selectively degrading oxidized proteins, in either a Ub-dependent or Ub-independent manner. The exposure of hydrophobic patches may be a molecular basis for the recognition of oxidized proteins by chaperones, or by the proteasome itself.43,44 Proteasome activity increased upon adaptation to mild oxidative stress. Mild to moderate oxidative stress increases the susceptibility of proteins to degradation and enhances proteolytic capacity, therefore promoting intracellular protein degradation. By contrast, extensive but non-lethal oxidative stress impairs the proteolytic system function, reducing intracellular protein degradation and inducing intracellular accumulation and aggregation of damaged proteins. The proteasome is a target of oxidative stress, and is more susceptible to oxidative inactivation than ubiquitination enzymes.45,46 In many cell types, when UPS function was impaired or inhibited, redox sensitive transcription factors enhanced the expression of proteasome subunits and increased proteasome activity.47-49
In this study, we used slot blot hybridization to compare the proteasome of hydrotropic mutants and wav2-1 by tracing the presence of total 20S core particles. All root seedlings grown in the control medium had similar quantities of 20S; however, the addition of PQ caused a slight increase in ahr1 and nhr1 roots (Fig. 8A, B). Roots of nhr1 and wav2-1 showed a sevenfold higher level of Ub conjugates under control conditions (Fig. 8A), indicating that in the presence of PQ, they degrade more proteins than both Col-0 and ahr1 (Fig. 8B). In the hydrotropism assay medium, 20S levels of wild-type and ahr1 roots doubled, while nhr1 and wav2-1 roots exhibited similar levels to those observed in the control medium. Total 20S was unaltered in all genotypes, despite the presence of PQ in the hydrotropism assay medium (Fig. 8D). In the hydrotropic assay medium, ahr1 roots had higher levels of Ub conjugates, indicating its normal growth pattern in this medium. In the PQ-treated hydrotropic assay plates, ahr1, nhr1, and wav2-1 roots had slightly higher Ub conjugate levels than Col-0. The four mutants tested showed no considerable change in protein carbonylation (Fig. 8D).
Discussion
There is increasing interest in the area of oxidative stress signaling in plants. A recent study reported that ROS are important in integrating stimulus responses, tuning root tropisms by promoting gravitropism and negatively regulating hydrotropism.20 Here, we present evidence to suggest that ROS signaling is involved in the root thigmotropic and hydrotropic responses of different trxs mutants, and hydrotropism (ahr1 and nhr1) and thigmotropism (wav2-1) mutants. In both the wild type and the tested mutants, root growth was diminished in the presence of mild oxidative stress caused by the presence of PQ, indicating that the tested mutants are PQ sensitive despite different percentages of observed growth inhibition. Interestingly, growth of wav2-1 roots was less sensitive to the presence of ROS, suggesting that they are either resistant to the inhibitory effect of PQ, or they have accelerated ROS scavenging mechanisms to maintain growth under oxidative stress (Fig. 1).
We established a new method for evaluating the root bending effort (RBE) of Arabidopsis seedlings that, when grown on a hard agar surface, produce different root wave patterns. Using this method, we analyzed the root waviness of different Arabidopsis genotypes in the presence or absence of PQ. According to Okada and Shimura (1990),8 waviness is quantified by measuring the tangent angle (the angle between a tangent to the direction of root growth and a hypothetical axis at each intersection between that axis and the root), the wavelength, and the root growth rate. Roots of wav2-1 have larger wave tangent angles and shorter wavelengths than the roots of wild-type seedlings. However, their method does not take into account the fact that in some Arabidopsis genotypes exhibited root waves even in the absence of thigmostimulation (data not shown).
The RBE method is not restricted to sinusoidal or symmetrical waves, and considers the accumulated waves in a root, divided by total root length (see equations 1 and 2 in Materials and Methods). Using this method, the RBE of wild-type roots was considered as control value. The waviness of wav2-1 roots was reduced in the presence of PQ (Fig. 2A, C), but was promoted in ahr1 and the trxs h4, trx o, trx h1, trx h9, trx m3 and trx h3, but not nhr1 (Fig. 2C). Roots of nhr1 exhibited no differences in waviness after thigmostimulation in the presence of PQ. These results indicate that the sensitivity of wav2-1 to mild oxidative stress is sufficient to decrease its wavy phenotype. In other words, regulation of the ROS detoxifying system might be different in root thigmotropism.
Using the H2O2−sensitive fluorescent probe H2DCF-DA, we compared the spatial distribution of root ROS levels in seedlings grown in a vertical position or under thigmotropic stimuli in ahr1, wav2-1 and their respective wild types. In seedlings grown vertically, the spatial distribution of ROS fluorescence was considerably diminished in ahr1 compared to wav2-1, Ler and Col-0 (Fig. 3A–D). This pattern was also observed in thigmostimulated roots (Fig. 3E–H). Elevated fluorescence was seen in the roots of wav2-1 and Ler grown in the vertical position, showing that the ROS levels in these genotypes are increased compared to Col-0 and ahr1 (Fig. 3C–D). Roots of ahr1 accumulated significantly less O2•− and proline,50 thus it is not surprising that they also had reduced ROS fluorescence.
The addition of PQ to plates positioned vertically or under thigmotropic stimulation substantially decreased ROS fluorescence and distribution in Col-0, Ler and wav2-1, but not ahr1 roots (Supplemental Table 1), indicating that ahr1 is mostly insensitive to ROS when submitted to thigmotropic stimuli but is sensitive to gravitropic stimuli (Figs. 2, 4, and 6). On the other hand, wav2-1 roots were insensitive to PQ under thigmostimulation, hydrostimulation and gravistimulation (Figs 2, 5, and 6). This indicates that hydrotropic and thigmotropic mutants respond differently to PQ depending upon the tropic stimuli perceived. Endogenous ROS signals might regulate which tropic stimulus is more important to the root for maintaining its explorative function.
The phenotypes of nhr1 and ahr1 was affected by addition of PQ to the hydrotropic assay medium; nonetheless, there were marked differences in the inhibition of their respective root growth (27% and 46%, respectively) compared to the untreated assay medium (Fig. 5). The reduced sensitivity to mild oxidative stress of these hydrotropic response mutants might be related to their differential levels of Ub conjugates (Fig. 8C–D). In the hydrotropic assay medium, root growth of wav2-1 was very similar in both untreated and PQ-treated plates, indicating that wav2-1 is hydrotropic (Fig. 5). All trx mutants tested displayed a hydrotropic response, as did the wild type (data not shown), suggesting that the function of TRX genes is dispensable in the hydrotropic response.
In roots of genotypes tested after being reoriented at 90° for 24 h, gravitropic responses indicated that only trx h3, trx o, and wav2-1 were insensitive to PQ, while wild-type, trx mutants (trx h1, trx h9, trx h4 and trx m3), and the hydrotropic mutants ahr1 and nhr1 were sensitive (Fig. 6). This variability might reflect differences in the normal redox status and redox resistance and/or sensitivity of these seedlings when gravitropically stimulated in the presence of PQ. Hence, it is difficult to generalize how mild oxidative stress regulates the root gravitropic response of different Arabidopsis genotypes. These results differed from those reported recently by Krieger et al. (2016),20 since we applied mild oxidative stress to seedling roots using PQ, while they utilized ROS scavengers such as ascorbate and diphenyliodonium.
Most studies exploring the regulation of lateral root emergence have focused on the role of auxin synthesis or signaling processes. Recent reports shed light on circadian clock-related gene regulation in the formation of lateral roots.38 However, an earlier report on lateral root formation supported the idea that manipulating auxin levels or signaling was insufficient to induce ectopic root branching, and that periodic branching and bending of primary roots required an endogenous mechanism.51 In our study, the number of emerged lateral roots in PQ-treated seedlings was compared with those grown under normal conditions. We found that wav2-1 had more emerged lateral roots in the presence of PQ (Fig. 7), indicating that is stimulated by ROS, and thus might be a good model for studying ROS-regulated lateral root formation.
To determine whether oxidative stress participates in the root hydrotropic response, we examined protein carbonylation, ubiquitin conjugate and proteasome content of two hydrotropic mutants (ahr1 and nhr1) and a wavy mutant (wav2-1) grown under four different conditions (Fig. 8). Under control conditions, the oxidized protein content of the three mutants considerably increased; however, in the presence of PQ only the wild type had increased levels of these proteins (Fig. 8A, B). This suggests that the three mutants are insensitive to PQ.
In the hydrotropic assay medium without PQ (Fig. 8C), the quantity of oxidized proteins slightly decreased in the wild type, and in ahr1 and nhr1, while in the wavy mutant wav2-1, levels of these proteins were significantly diminished (Fig. 8C). In the hydrotropic assay medium with PQ, the levels of these proteins decreased substantially in ahr1 roots only (Fig. 8D).
The hydrotropic mutants and wav2-1 displayed different protein degradation patterns upon application of a hydrotropic stimulus, and also during mild oxidative stress, which is indicative of the complex interactions between root tropisms, ROS, and protein degradation. Future studies should consider these complex interactions in more detail to understand how roots respond to different simultaneous environmental cues.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana seeds of the wild-type ecotypes Columbia-0 (Col-0) and Landsberg erecta (Ler), and wav2-1 and trx mutants, were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus, OH, USA). Thioredoxin (trx) mutants were from the homozygous Salk collection: 006237C (trx h1), 018261C (trx o), z061968C (trx m3), 081049C (trx h9), 111160 (trx h3), and 151722 (trx h4). The hydrotropic mutants nhr1 and ahr1 were isolated in our lab from the Col-0 background.
Seeds were germinated on ‘normal’ medium (half-strength Murashige and Skoog [MS] medium with 0.5% [w/v] sucrose, at pH 5.7), or hydrotropic assay medium containing two media layers (upper: normal medium, lower: water stress medium containing half-strength MS, 0.5% [w/v] sucrose, 0.5% [w/v] alginic acid and 2.5% [w/v] glycerol, as described by Eapen et al., 2003).37 To generate ROS, 0.03 µM paraquat (PQ) (Anaquat, Agricultura Nacional de Jalisco S.A de C.V.) was added to the media. Arabidopsis seedlings were grown for the lengths of time indicated under long day conditions (16 h light/8 h dark) cycle, 80 µM photons m−2 s−1 at 24°C.
Root thigmotropism assay and RBE method
Thigmotropism was evaluated as described by Okada and Shimura (1990),8 with modifications. Three or 6 days (control or PQ-treated plates, respectively) after sowing the seeds on hard MS medium (0.5 x and 1.5% [w/v] agar), the position of the root tip was marked on the Petri dishes (using a marker pen). Then, for 3 or 6 d (control or PQ plates, respectively), Petri dishes were tilted to an angle of 60°. After 3 d in this position, plates were photographed with a Nikon D1 Digital Camera equipped with an AF Nikon 70–300 mm lens, at a fixed distance between the camera and the different Petri dishes. Four digital photographs (2238 × 1468 pixels; 5 × 5 cm; Tiff format) were taken of each Petri dish containing Arabidopsis seedling roots with a wavy pattern, each covering one quarter of the whole square Petri dish. Each image was cropped into a rectangular sub-image (with a variable size fitting the root, but with a fixed resolution of 833 pixels/inch) and saved as a separate file. Using ImageJ software (version 1.48; http://rsb.info.nih.gov/ij/), each sub-image was binary-converted by assigning a white color to the root over a black background. Thus we obtained a 1 pixel-width skeleton of the root. A text file was created containing the x and y coordinates of all points along each root. The RBE analysis was made with at least n = 100 seedling roots from each genotype.
In this study, it was assumed that not all root waves are symmetrical or sinusoidal (Fig. 2). For this reason, we determined a new and simple way to obtain an index demonstrating the effort a root makes to bend under the influence of a thigmotropic stimulus. We defined this as RBE, which measures and cumulates each root wave or bend, and divided this by the total length of the root (see equations 1 and 2). In this way, a completely straight root would have a total normalized RBE of 0, while values increasing from 0 to 1 are applied to larger waveforms.
To measure the local bending effort of each root, we used the procedure described by Bullard et al. (1995)52 to compute mean curvature in two or three dimensions (2D or 3D). In our 2D images, for each point pi along the root, local root curvatures were calculated by computing the portion of the surface enclosed by a small circular template centered on pi lying on one side of the interface (see Fig. 9). The local bending effort for a group of k points (enclosed by the circular template) centered over a point pi within the root was defined as:
| (1) |
Figure 9.
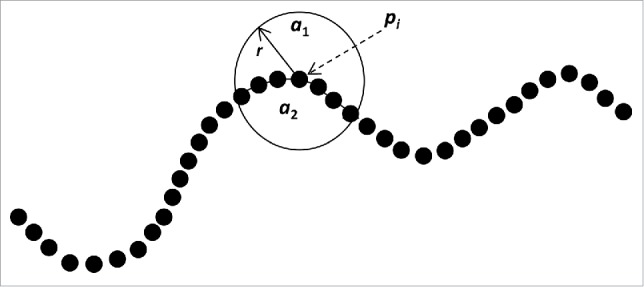
Root bending effort. For each point Pi along the root, mean root bending effort (RBE) is calculated by averaging the ratio of the surfaces a1/a2 are enclosed by the circular mask with radius r, centered sequentially over points pi.
where: ‘pi’ is a point belonging to the root, on which the circle template is centered; ‘a1’ and ‘a2’ are the areas between the segment of the arc template and the root boundary.
For a certain root ‘m’ formed by n points, RBEtotal is then defined as:
| (2) |
where: n is the number of points of a root ‘m’; RootLength = k n (k = calibration constant). RBEtotal ranges from 0 (for a straight line) to value approaching to 1 (for maximal root bending) and shows the total bending effort of the root during its growth.
Treatment of root seedlings with a ROS sensitive dye (CM-H2DCFDA)
The ROS-sensitive probe CM-H2DCFDA [5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester] (catalog no. C6827, Molecular Probes), was dissolved in DMSO (catalog no. D8779, Sigma), and centrifuged for 2 min at 7000 rpm to remove non-dissolved particles. The dye solution was then diluted with water to a final concentration of 30–50 µM and added to plates containing Arabidopsis root seedlings. After 10 min, the medium was replaced with free dye medium to discard any unincorporated dye, and measurements were taken. This procedure was done carefully to avoid any mechanical stress to cells that might otherwise result in the production of ROS.
Acquisition and processing of fluorescent images
All images were acquired with a CCD camera (Sensys, Roper Scientific) attached to a Nikon TE300 inverted microscope with a 40X/1 N.A. water immersion objective lens, and operated with MetaMorph/MetaFluor software (Universal Imaging, Molecular Devices). Dye-treated roots were excited using a xenon illumination source (DG-4, Sutter Instruments) containing a 175-Watt ozone-free xenon lamp (330–700 nm) and a galvanometer-driven wavelength switcher. Seedlings containing the CM-H2DCFDA probe were excited at 484 nm, and emission was collected at 530 nm (20 nm band pass). All filters used were from Chroma Technology, and image acquisition and analysis were carried out using MetaMorph/MetaFluor software. Relative fluorescence intensity levels were calculated using the line scan option from Image J in the region of interest; data were exported to Microsoft Excel to prepare graphs and conduct standard deviation analysis.
Localization of ROS in root seedlings grown in the root thigmotropic assay
In the thigmotropic assay, the final curve or wave produced by the growing root (that closest to the growing tip, which experiences the intracellular ROS required for wave formation) was selected. To collect data indicating fluorescence intensity, several lines were drawn from the internal side of the curve to the external region. This approach was useful to compare the fluorescence intensity at both the internal and external sides of the curve or wave of a root tip.
Analysis of root growth in the hydrotropism assay
Seedlings were grown on hydrotropism assay medium for 11 d, after being photographed with a Nikon D7000 digital camera (Nikon Co.). Lateral roots emerging through the root epidermis were counted under a light microscope (Nikon Eclipse E600; Nikon Co.). Images were analyzed using Adobe Photoshop CS 8.0 (Adobe Systems Inc., San Jose, CA, USA). Root growth was measured using ImageJ.
Root gravitropism
Root gravitropic curvature was measured using 4-day-old seedlings grown vertically on mock or 0.03 µM PQ medium. Petri dishes were reoriented to 90° for 24 h, and images were taken before and after reorientation. Images were analyzed as described above, and root angle was measured using ImageJ.
Analysis of protein carbonylation, protein ubiquitination and 20S content
Total proteins were extracted by adding one volume of 2 × Laemmli sample buffer to liquid nitrogen-frozen plant material. Samples were immediately heated to 95°C for 5 min, and centrifuged at 14000 g for 10 min. Proteins from recovered supernatants were precipitated with methanol/chloroform to remove waxes and pigments. Resultant pellets were re-suspended in 1 × Laemmli buffer for immunoblot or slot blot analysis. Protein content was estimated by Bradford assay. Equal amounts of total protein (5 mg) were loaded into each well for immunoblot or slot blot analysis. Nitrocellulose membranes (Hybond-C Extra, Amersham Biosciences) were blocked in 5% low-fat milk in Tris-buffered saline 0.1% Tween 20 (TBS-T) for 30 min at room temperature. Incubation with primary antibody was for 1 h at room temperature. After washing three times with TBS-T, membranes were incubated with secondary anti-rabbit-HRP antibody or anti-mouse-HRP (Santa Cruz Biotechnology) at a 1:5000 dilution for 1 h at room temperature. Finally, the membrane was washed three times with TBS-T, developed with ECL reagent (Amersham cat. RPN2109), and exposed to X-ray films (Kodak cat. 6040331).
The following primary antibodies were used: rabbit-anti-ubiquitin antibody (cat. # sc-9133 Santa Cruz Biotechnology, Inc.) at 1:1000 dilution, and mouse-anti-proteasome 20S alpha+beta (Abcam ab22673) at 1:5000 dilution.
Protein carbonyl content was estimated using a protein slot blot procedure, followed by an immunochemical protocol (OxyBlot Protein Oxidation detection kit, from Chemicon International) that detects 2,4-dinitrophenylhidrazone after reacting samples with 2,4-dinitrophenylhydrazine (DNPH). Quantification of carbonyl content, 20S proteasome content and total Ub conjugates was made by densitometry of the autoradiograms using NIH ImageJ 1.48 software. All data were standardized to wild-type growth under control conditions (wild type = 1).
Statistical analysis
Data were analyzed using JMP® software, version 11.0 for Windows 7 Home Premium x64 (SAS Institute Inc., 1989–2007), and analyzed with two-way ANOVA and Tukey's post hoc, unpaired Student t-tests, Dunn´s multiple comparison test, Kruskal-Wallis test, and Mann-Whitney U test. Graphs were prepared using Microsoft Excel. In all cases the difference was considered significant when P < 0.05. Images were digitalized for publication using Adobe Photoshop CS 8.0 (Adobe Systems Inc.). All experiments were repeated at least thrice.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are indebted to Y. Sánchez [Instituto de Biotecnología (IBt)-UNAM] for providing the homozygous trx h3 and trx h4 mutant seeds; M. Saucedo (IBt-UNAM) for his technical skills in testing the root hydrotropic response; M.E. Campos (IBt-UNAM) for her project management and technical skills; F. Lara (IBt-UNAM) and R. García (IBt-UNAM) for technical assistance with imaging root tip fluorescence; K. Juárez (IBt-UNAM) for performing the statistical analysis; and A. Almeida (IBt-UNAM) and J. Martínez (IBt-UNAM) for editing the figures. We are grateful to S. Ainsworth and R. Rodríguez for providing library and computer support (IBt-UNAM). Likewise, we are grateful to the Arabidopsis Biological Resource Center (Ohio State University, Columbus, OH, USA) for providing the wav2-1 and all trx mutant seeds used in this study.
Funding
Funding for this study was received from Consejo Nacional de Ciencia y Tecnología (CONACYT), grant numbers 177107 (to G.I. Cassab), and 240595 and 253247 (to L. Cárdenas); and from Dirección General de Asuntos del Personal Académico (DGAPA-UNAM), grant numbers IN226810 (to G. Ponce), IN207814 (to L. Cárdenas), and IN212116 (to F. Lledias).
References
- 1.Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al.. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 2013; 45:1097-102; PMID:; http://dx.doi.org/ 10.1038/ng.2725 [DOI] [PubMed] [Google Scholar]
- 2.Sebastian J, Yee M-C, Goudinho Viana W, Rellán-Álvarez R, Feldman M, Priest HD, Trontin C, Lee T, Jiang H, Baxter I, et al.. Grasses suppress shoot-borne roots to conserve water during drought. Proc Natl Acad Sci USA 2016; 113:8861-6; PMID:; http://dx.doi.org/ 10.1073/pnas.1604021113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch JP. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann Bot 2013; 112:347-57; PMID:; http://dx.doi.org/ 10.1093/aob/mcs293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazan K. Auxin and the integration of environmental signals into plant root development. Ann Bot 2013; 112:1655-65; PMID:; http://dx.doi.org/ 10.1093/aob/mct229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eapen D, Barroso ML, Ponce G, Campos ME, Cassab GI. Hydrotropism: root growth responses to water. Trends Plant Sci 2005; 10:44-50; PMID:; http://dx.doi.org/ 10.1016/j.tplants.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Saucedo M, Ponce G, Campos ME, Eapen D, García E, Luján R, Sánchez Y, Cassab GI. An altered hydrotropic response 1 (ahr1) mutant of Arabidopsis recovers root hydrotropism with cytokinin. J Exp Bot 2012; 63:3587-602; PMID:; http://dx.doi.org/ 10.1093/jxb/ers025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A, Takahashi A, Kakimoto Y, Miyazawa Y, Fujii N, Higashitani A, Takahashi H. A gene essential for hydrotropism in roots. Proc Natl Acad Sci USA 2007; 104:4724-9; PMID:; http://dx.doi.org/ 10.1073/pnas.0609929104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 1990; 250:274-6; PMID:; http://dx.doi.org/ 10.1126/science.250.4978.274 [DOI] [PubMed] [Google Scholar]
- 9.Simmons C, Migliaccio F, Masson P, Caspar T, Soll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant 1995; 93:790-8; PMID:; http://dx.doi.org/ 10.1111/j.1399-3054.1995.tb05133.x [DOI] [PubMed] [Google Scholar]
- 10.Takahashi N, Goto N, Okada K, Takahashi H. Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta 2002; 216:203-11; PMID:; http://dx.doi.org/ 10.1007/s00425-002-0840-3 [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki S, Harada A, Inada S, Sugimoto-Shirasu K, Stacey N, Wada T, Ishiguro S, Okada K, Sakai T. The Arabidopsis WAVY GROWTH 2 protein modulates root bending in response to environmental stimuli. Plant Cell 2005; 17:537-47; PMID:; http://dx.doi.org/ 10.1105/tpc.104.028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol 2010; 61:705-20; PMID:; http://dx.doi.org/ 10.1146/annurev.arplant.043008.092042 [DOI] [PubMed] [Google Scholar]
- 13.Mori IC, Schroeder JI. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 2004; 135:702-8; PMID:; http://dx.doi.org/ 10.1104/pp.104.042069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiol Plant 2010; 138:405-13; PMID:; http://dx.doi.org/ 10.1111/j.1399-3054.2009.01321.x [DOI] [PubMed] [Google Scholar]
- 15.Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 2009; 21:2341-56; PMID:; http://dx.doi.org/ 10.1105/tpc.109.068395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassab GI, Eapen D, Campos ME. Root hydrotropism: An update. Am J Bot 2013; 100:14-24; PMID:; http://dx.doi.org/ 10.3732/ajb.1200306 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi H, Scott TK. Hydrotropism and its interactions with gravitropism in maize roots. Plant Physiol 1991; 96:558-64; PMID:; http://dx.doi.org/ 10.1104/pp.96.2.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi N, Yamazaki Y, Kobayashi A, Higashitani A, Takahashi H. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol 2003; 132:805-10; PMID:; http://dx.doi.org/ 10.1104/pp.018853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Considine MJ, Foyer CH. Redox regulation of plant development. Antioxid Redox Signal 2014; 21:1305-26; PMID:; http://dx.doi.org/ 10.1089/ars.2013.5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger G, Shkolnik D, Miller G, Fromm H. Reactive oxygen species tune root tropic responses. Plant Physiol 2016; 172:1209-1220; PMID:; http://dx.doi.org/ 10.1104/pp.16.00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in. Plant Physiol 2001; 126:1055-60; PMID:; http://dx.doi.org/ 10.1104/pp.126.3.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010; 143:606-16; PMID:; http://dx.doi.org/ 10.1016/j.cell.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 23.Tsukagoshi H. Control of root growth and development by reactive oxygen species. Curr Opin Plant Biol 2016; 29:57-63; PMID:; http://dx.doi.org/ 10.1016/j.pbi.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 24.Considine MJ, Sandalio ML, Foyer HC. Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann Bot 2015; 116:469-73; PMID:; http://dx.doi.org/ 10.1093/aob/mcv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira Dos Santos C, Rey P. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci 2006; 11:329-34; PMID:; http://dx.doi.org/ 10.1016/j.tplants.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 26.Wong CM, Marcocci L, Liu L, Suzuki YJ. Cell signaling by protein carbonylation and decarbonylation. Antioxid Redox Signal 2010; 12:393-404; PMID:; http://dx.doi.org/ 10.1089/ars.2009.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep 2001; 2:1133-8; PMID:; http://dx.doi.org/ 10.1093/embo-reports/kve246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadis S, Atienza C, Finley D. Synthetic signals for ubiquitin-dependent proteolysis. Mol Cell Biol 1995; 15:4086-94; PMID:; http://dx.doi.org/ 10.1128/MCB.15.8.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrington DA, Sun H, Murray KK, Costa J, Williams TD, Bigelow DJ, Squier TC. Selective degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem 2001; 276:937-43; PMID:; http://dx.doi.org/ 10.1074/jbc.M005356200 [DOI] [PubMed] [Google Scholar]
- 30.Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett 2006; 580:2910-6; PMID:; http://dx.doi.org/ 10.1016/j.febslet.2006.03.028 [DOI] [PubMed] [Google Scholar]
- 31.Grune T, Davies KJA. Breakdown of oxidized proteins as a part of secondary antioxidant defenses in mammalian cells. BioFactors 1997; 6:165-72; PMID:; http://dx.doi.org/ 10.1002/biof.5520060210 [DOI] [PubMed] [Google Scholar]
- 32.Taylor A, Shang F, Nowell T, Galanty Y, Shiloh Y. Ubiquitination capabilities in response to neocarzinostatin and H2O2 stress in cell lines from patients with ataxia-telangiectasia. Oncogene 2002; 21:4363-73; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1205557 [DOI] [PubMed] [Google Scholar]
- 33.Buer CS, Masle J, Wasteneys GO. Growth conditions modulate root-wave phenotypes in Arabidopsis. Plant Cell Physiol 2000; 41:1164-70; PMID:; http://dx.doi.org/ 10.1093/pcp/pcd042 [DOI] [PubMed] [Google Scholar]
- 34.Thompson MV, Holbrook NM, Biology E. Root-Gel Interactions and the Root Waving Behavior. 2004; 135:1822-37; https://doi.org/ 10.1016/0927-0256(95)00014-H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Angel M, Linstead P, Costa S, Brownlee C, Jones JDG, et al.. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003; 422:442-6; PMID:; http://dx.doi.org/ 10.1038/nature01485 [DOI] [PubMed] [Google Scholar]
- 36.Kurepa J, Smalle J, MVan Montagu, Inze D. Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J 1998; 14:759-64; PMID:; http://dx.doi.org/ 10.1046/j.1365-313x.1998.00168.x [DOI] [PubMed] [Google Scholar]
- 37.Eapen D, Barroso ML, Campos ME, Ponce G, Corkidi G, Dubrovsky JG, Cassab GI. A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiol 2003; 131:536-46; PMID:; http://dx.doi.org/ 10.1104/pp.011841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voß U, Wilson MH, Kenobi K, Gould PD, Robertson FC, Peer WA, Lucas M, Swarup K, Casimiro I, Holman TJ, et al.. The circadian clock rephases during lateral root organ initiation in Arabidopsis thaliana. Nat Commun 2015; 6:7641; PMID:; http://dx.doi.org/ 10.1038/ncomms8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, Orman-Ligeza B, Van Isterdael G, Beeckman T, Draye X, Casero P, Del Pozo JC. The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol 2014; 165:1105-119; PMID:; http://dx.doi.org/ 10.1104/pp.114.238873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lledías F, Rangel P, Hansberg W. Singlet oxygen is part of a hyperoxidant state generated during spore germination. Free Radic Biol Med 1999; 26:1396-404; PMID:; http://dx.doi.org/ 10.1016/S0891-5849(98)00341-4 [DOI] [PubMed] [Google Scholar]
- 41.Grune T, Reinheckel T, Davies KJA. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem 1996; 271:15504-09; PMID:; http://dx.doi.org/ 10.1074/jbc.271.26.15504 [DOI] [PubMed] [Google Scholar]
- 42.Shang F, Gong X, Taylor A. Activity of ubiquitin-dependent pathway in response to oxidative stress. J Biol Chem 1997; 272:23086-93; PMID:; http://dx.doi.org/ 10.1074/jbc.272.37.23086 [DOI] [PubMed] [Google Scholar]
- 43.Shringarpure R, Grune T, Mehlhase J, Davies KJA. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem 2003; 278:311-8; PMID:; http://dx.doi.org/ 10.1074/jbc.M206279200 [DOI] [PubMed] [Google Scholar]
- 44.Giulivi C, Pacifici RE, Davies KJA. Exposure of hyrophobic moieties promotes the selective degradation of hydrogen peroxide-modified hemoglobin by multicatalytic proteinase complex, proteasome. Arch Biochem Biophys 1994; 311:329-341; PMID:; http://dx.doi.org/ 10.1006/abbi.1994.1245 [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Zhou J, Fernandes AF, Sparrow JR, Pereira P, Taylor A, Shang F. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci 2008; 49:3622-30; PMID:; http://dx.doi.org/ 10.1167/iovs.07-1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J 1998; 335(3):637-42; PMID:; http://dx.doi.org/ 10.1042/bj3350637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CS, Tee LY, Warmke T, Vinjamoori A, Cai A, Fagan AM, Snider BJ. A proteasomal stress response: Pre-treatment with proteasome inhibitors increases proteasome activity and reduces neuronal vulnerability to oxidative injury. J Neurochem 2004; 91:996-1006; PMID:; http://dx.doi.org/ 10.1111/j.1471-4159.2004.02813.x [DOI] [PubMed] [Google Scholar]
- 48.Kapeta S, Chondrogianni N, Gonos ES. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem 2010; 285:8171-84; PMID:; http://dx.doi.org/ 10.1074/jbc.M109.031575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang TL, Lin SW, lun Wu S, Hong CM. Regulation of ubiquitin and 26S proteasome mediated by phenolic compounds during oxidative stress. J Nutr Biochem 2013; 24:1970-81; PMID:; http://dx.doi.org/ 10.1016/j.jnutbio.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 50.Salazar-Blas A, Noriega-Calixto L, Campos ME, Eapen D, Cruz-Vázquez T, Castillo-Olamendi L, Sepulveda-Jiménez G, Porta H, Dubrovsky JG, Cassab GI. Robust root growth in altered hydrotropic response1 (ahr1) mutant of Arabidopsis is maintained by high rate of cell production at low water potential gradient. J Plant Physiol 2017; 208:102-14; PMID:; http://dx.doi.org/ 10.1016/j.jplph.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 2010; 329:1306-11; PMID:; http://dx.doi.org/ 10.1126/science.1191937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bullard JW, Garboczi EJ, Carter WC, Fuller ER. Numerical methods for computing interfacial mean curvature. Comput Mater Sci 1995; 4:103-16; http://dx.doi.org/ 10.1016/0927-0256(95)00014-H [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.