Abstract
This review summarizes recent evidence concerning hormonal and sex chromosome effects in obesity, atherosclerosis, aneurysms, ischemia/reperfusion injury, and hypertension. Cardiovascular diseases (CVD) occur and progress differently in the two sexes, because biological factors differing between the sexes have sex-specific protective and harmful effects. By comparing the two sexes directly, and breaking down sex into its component parts, one can discover sex-biasing protective mechanisms that might be targeted in the clinic. Gonadal hormones, especially estrogens and androgens, have long been found to account for some sex differences in CVD, and molecular mechanisms mediating these effects have recently been elucidated. More recently the inherent sexual inequalities in effects of sex chromosome genes have also been implicated as contributors in animal models of CVDs, especially a deleterious effect of the second X chromosome found in females but not in males. Hormonal and sex chromosome mechanisms interact in the sex-specific control of certain diseases, sometimes by opposing the action of the other.
Graphical Abstract

Introduction: The importance of studying sex differences in cardiovascular diseases
Cardiovascular diseases (CVDs) manifest differently in men and women.1, 2 The overall lifetime risk of cardiovascular disease is similar in the two sexes, but men develop cardiovascular disease earlier than women (Figure 1).3, 4 At age 55, the lifetime risk of a first incident coronary heart disease is higher in men than women, but the risk of first incident cerebrovascular disease or heart failure is higher in women than men.3 These sex differences suggest that biomedical principles, learned from the study of males, may not apply equally to females. CVDs should be studied in both sexes. However, separate study of both sexes is not enough. Rather, the two sexes must be directly compared, with the purpose of finding factors that cause sex differences and therefore prevent or alleviate disease in one sex more than the other. The present review summarizes limited evidence about the causes of sex differences in CVDs in humans, and recent investigations of rodent models in which causal factors can be isolated and studied in a more controlled manner. Although there is no expectation that mice and other model organisms are equivalent to humans, studies of diverse organisms, which are similar to and different from humans, is the main strategy for formulating basic biological concepts that guide our understanding of human physiology. In the present case, studies of mice have recently uncovered new ideas that have yet to be applied to humans. Investigation of animals offers two distinct advantages. The first is the ability to independently manipulate diverse sex factors (those that cause sex differences in physiology and disease, including hormones and numerous specific sex chromosome genes) to judge their separate effects, as well as their interactions. The second advantage is the ability to discover downstream molecular mechanisms controlled by sex factors, which might be targets for therapy. The ideas presented here include an emerging field of research based on study of animals. The new ideas will require validation and application to humans, to achieve better understanding of human physiology and disease.
Figure 1.
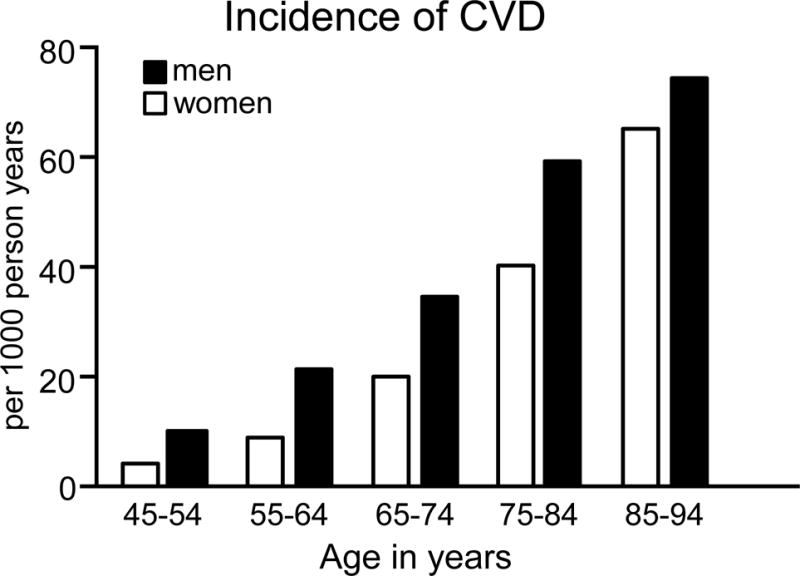
Incidence of cardiovascular disease in men and women as a function of age. Data on the incidence of cardiovascular disease defined as coronary heart disease, heart failure, stroke and intermittent claudication in women (white bars) and men (black bars) from 45 to 94 years of age. Data are derived from the Framingham Heart Study as reported by the National Heart, Lung and Blood Institute138 and adapted from reference4 with permission of the publisher.
Conceptual framework
Although sex differences in CVDs are caused in part by environmental or social differences between men and women (e.g., occupational hazards, habits, social stresses),5–7 we focus here on biological factors that are important and tractable for study in animal models.
Where do sex differences start? At the moment of conception, XX and XY zygotes differ only in their sex chromosomes. Thus, all sex differences arise from the inherent sexual inequality of these two chromosomes.8 We list here four classes (two Y-linked and two X-linked) of sex chromosome mechanisms that could conceivably cause sex differences in phenotype:
(1) Y chromosome effects
(a) Gonadal effects
The most important sex-differentiating effect is caused by the Y-linked gene Sry, which acts within gonadal primordia to cause differentiation of testes in males.9 Genes that are present in both sexes cause differentiation of ovaries unless Sry is present, so Sry expression is the root cause of sex differences in the type of gonads. The differentiation of testes rather than ovaries establishes a lifelong difference in the levels of gonadal hormones, especially testosterone, estradiol, and progesterone, which act directly on cardiovascular and other tissues to make them function differently in the two sexes. Some effects of testosterone (or its metabolite 17β-estradiol) are permanent (“organizational” effects), for example the hormone-induced male pattern of differentiation of the genitals or brain. Others are reversible (“activational” effects) and may last only as long as the hormone is present.10
(b) Non-gonadal effects
Y chromosome genes act outside of the gonads to have male-specific effects. For example, Sry is expressed in the brain and other tissues, especially in catecholaminergic cells and neurons, where its effects may include effects on hypertension.11–13 Studies of XY mice with different versions of the Y chromosome show remarkable differences in the severity of autoimmune disease.14 Effects of the Y chromosome must be specific to males, but the implied sex difference could be compensated for by other factors in females. These effects have so far been infrequently studied because historically it has been difficult to target the Y chromosome, or manipulate it without also changing gonadal hormone levels. Moreover, this chromosome encodes relatively few genes, and except for Sry, none has been specifically implicated yet in sex differences in physiology.
(2) X chromosome effects
(a) X gene dosage
Most X chromosome genes do not show large sex differences in their level of expression, because one X chromosome is transcriptionally silenced in XX adult somatic cells. Thus, most X genes are expressed about equally from the single active X chromosome in both XX and XY cells. Accordingly, concepts of sexual differentiation have long ignored X chromosome genes as potential inherent causes of sex differences in phenotype. However, some genes escape inactivation and are expressed from each X chromosome so that they have higher levels in XX than XY cells.15–18 Among these are a set of X chromosome genes known to be highly dosage sensitive,19 implying that one vs. two doses of the genes are expected to cause phenotypic differences. In mice and humans, the X escapees include two histone demethylases, Kdm5c and Kdm6a, which are likely to have widespread effects on autosomal gene expression. Other X escapees also have fundamental effects on cell function, and include the translation initiation factor Eif2s3x and RNA helicase Ddx3x, which is involved in transcriptional regulation, pre-mRNA splicing, mRNA export, cellular signaling, and viral replication. The X escapees are candidates for causing sex differences in CVD, but none has been specifically implicated to date.
(b) X gene imprinting
XY cells receive a maternal imprint on X genes, but XX cells receive imprints from both parents. The inherent imbalance in parental imprinting is a potential cause of sex differences in expression of specific X genes that affect CVD phenotypes. To date, this difference in imprinting has not been specifically implicated as the cause of any sex difference in phenotype, possibly because this class of genes is not widely studied, and proving their role in sexual differentiation is experimentally complex.
In addition to the effects listed here, there may be non-genic effects (i.e., not requiring gene expression) of the X or Y chromosomes that alter the epigenetic status of the autosomes. These mechanisms are speculative and are discussed elsewhere.20
Mouse models to discriminate hormonal and sex chromosome effects that cause sex differences
In recent years, a major goal has been to distinguish sex differences caused by gonadal hormones vs. sex chromosome complement (XX vs. XY). Hormonal effects are most often detected by manipulating hormone levels, synthesis, or action to find which hormones account for sex differences.21 Detecting non-gonadal effects of sex chromosome complement involves manipulating the number of X and Y chromosomes, holding gonadal hormones as constant as possible. The most frequently used model is the Four Core Genotypes mouse model (FCG).22 In this model Sry is a transgene on chromosome 3, and is not present on the Y chromosome, so that XX and XY mice can each have either testes (with Sry, XXM or XYM) or ovaries (without Sry, XXF or XYF) (Figure 2). This model can discriminate between sex differences determined by gonadal type (XXM and XYM differ from XXF and XYF) vs. those determined by the effects of sex chromosomes (XXF and XXM differ from XYF and XYM).
Figure 2.
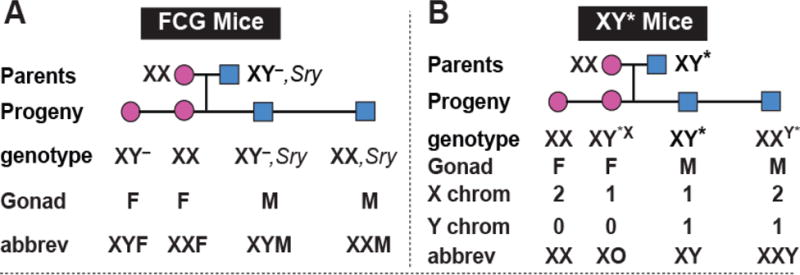
Two mouse models for measuring sex chromosome effects. A, In the Four Core Genotypes (FCG) model, the testis determining gene (Sry) is removed from Y chromosome to make the “Y–” chromosome, and an Sry transgene is inserted into chromosome 3. XY– mice have ovaries and are called XY females (XYF) here. Mice with an Sry transgene have testes and are called males, XXM or XYM. B, The XY* model is useful for figuring out whether a difference between XX and XY is due to effects of the X chromosome or Y chromosome. It compares groups that differ in the number of X chromosomes (female mice with XX or XO and male mice with XY or XXY) or compares mice that differ in the presence/absence of Y chromosome (XO vs. XY and XX vs. XXY), to determine which causes XX vs. XY differences. Adapted from18
Once a sex difference is found to be caused by a sex chromosome effect, the next step is to figure out if the effect is caused by the X or Y chromosome, using the XY* mouse model (Figure 2). The Y* chromosome has a variant pseudoautosomal region that recombines abnormally with the X chromosome, producing mice with unusual complements of sex chromosomes. For example, some progeny have a fusion of an X and Y chromosome whereas others lack most of one X chromosome.23, 24 The progeny are the near-equivalents of XO, XX, XY, and XXY. This model allows detecting an effect of one vs. two X chromosomes, by comparing XO vs. XX (gonadal females), or XY vs. XXY (gonadal males). One can also detect an effect of the Y chromosome by comparing XO vs. XY, and XX vs. XXY.25 Localizing the effect to the X or Y chromosome then leads to studies of candidate X or Y genes that might cause the sex chromosome effect.
Previous reviews discuss methods and interpretation of these models, including methods to avoid hormonal confounds of sex chromosome complement.22, 26 Below we review studies of gonadal hormone and sex chromosome influences on obesity, atherosclerosis, aneurysms, cardiac ischemia/reperfusion, and hypertension.
Obesity
Many CVDs are associated with obesity. Men and women differ in the development of obesity, and the manifestation of obesity-related conditions such as hyperlipidemia, insulin resistance, and type 2 diabetes. Men generally have greater body weight than women, but the proportion of body weight as fat is greater in women.27–29 In the mouse, males also have greater body weight, but the degree of body fat is dependent on diet and strain. In some strains, a high fat diet leads to similar adiposity, whereas in others one sex has greater adiposity than the other.30, 31 Multiple mechanisms contribute to sex differences in fat storage, including ability to expand different anatomic depots (e.g., greater subcutaneous fat storage in women), or ability to mobilize fat stores (e.g., greater capacity for adipose tissue lipolysis in men).27–29 Additional sex differences include greater insulin sensitivity and higher adiponectin and leptin levels in women and female mice, and lower dietary fatty acid oxidation and greater circulating triglyceride levels in men.30, 32 Some of the metabolic differences between men and women are reduced after menopause, possibly caused by the decline in ovarian hormones.33 However, hormonal differences cannot fully account for all differences between men and women in lipid metabolism.34
Genome-wide genetic studies in humans have identified genetic loci that influence body mass index and/or waist-hip-ratio specifically in one sex, or to a different extent in the two sexes. These include loci on the autosomes as well as on the X chromosome.35, 36 In the mouse, numerous genetic loci with differential effects in males and females have been described, including several loci on chromosome X.30, 37 The mechanisms underlying sex-specific effects of genetic polymorphisms on obesity merit further study.
The use of experimental models such as the FCG and XY* mice (Figure 2) have informed us about genetic and hormonal mechanisms underlying sex differences in obesity.17 In gonad-intact FCG mice, male mice (with testes, either XX or XY) have greater body weight than female mice (with ovaries, XX and XY) (Figure 3A). In addition, XX mice weigh more that XY mice of the same gonadal sex. After gonadectomy (GDX) of adult mice, male-female sex differences are reduced, indicating that acute effects of gonadal hormones contribute to male-female differences in body weight. Weeks or months after GDX, however, the body weight of XX mice increases more than that of XY mice, and XX mice eventually have nearly twice as much body fat.17 These results suggest that the presence of two X chromosomes, and/or the absence of the Y chromosome, leads to enhanced body fat. The XY* model resolves this question in favor of the X chromosome dose. Mice with two X chromosomes (XX or XXY) have greater body weight than mice with one X chromosome (XY or XO)(Figure 3B).17 XX mice after GDX also have accelerated weight gain on a high fat diet, with increased food intake, relative to XY, during the light phase of the diurnal cycle, but with no differences in energy expenditure or locomotor activity.17, 38 XX mice also develop more profound fatty liver, greater evidence of insulin resistance, and higher circulating cholesterol levels than XY mice when stressed with fat- or cholesterol-enriched diets.17, 38 Thus, the number of X chromosomes has a substantial effect on obesity and related morbidities.
Figure 3.

Summary of effects of gonadal hormones and sex chromosome complement on body weight and adiposity in FCG and XY* mice. A, Male mice (with testes) weigh more than female mice (with ovaries). XX mice also weigh more than XY mice, although the magnitude of effect is less than that due to gonads.17 Four weeks after gonadectomy (GDX), all four genotypes reach a similar weight. Thereafter XX mice weigh more than XY mice, whether they originally had ovaries or testes. B, In XY* mice, males weigh more than females when gonads are intact, irrespective of sex chromosome complement. After GDX, the effect of gonadal hormones is abolished, and mice with two X chromosomes (XXY, XX) weigh more than mice with one X chromosome (XY, XO).17
Localizing the sex chromosome effect to the X chromosome opens the door to identifying novel X-linked molecular determinants of sex differences in obesity. A leading hypothesis suggests a contribution from X escapee genes, which are expressed at higher levels in XX compared to XY tissues.17 Testing this hypothesis further will involve assessing the effects on obesity when expression levels of individual X escapee genes are modulated in a controlled manner.
Atherosclerosis
Among all cardiovascular diseases, coronary artery disease is the leading cause of death. Over two centuries ago, clinicians began to report sex differences in the prevalence of angina pectoris.39 In general, women have a delay in the development of atherosclerosis compared to men, with both sexes increasing in incidence with age.40 A recent meta-analysis suggests that increased total serum cholesterol is a significant risk factor for coronary artery disease in both sexes, with a small, but significantly stronger effect in men compared to women.41 Women have higher HDL cholesterol levels and lower bile acid and cholesterol synthesis than men throughout adult life.42 Although sex differences in lipoprotein levels persist after menopause, endogenous sex hormone levels influence lipoprotein levels. For example, post-menopausal women have higher LDL cholesterol than premenopausal women or men of the same age.43–45 Furthermore, ovariectomy increases LDL cholesterol levels.46 Among individuals, the level of plasma estrone correlates with HDL cholesterol levels, and correlates inversely with total cholesterol.47, 48
Testosterone may influence atherosclerosis in both sexes. In postmenopausal women of the Atherosclerosis Risk in Communities Study who were not taking hormone therapy, the level of sex hormone binding globulin (SHBG), which binds approximately 60% of circulating testosterone, correlated with a more favorable lipid profile (lower total and LDL cholesterol, higher HDL cholesterol). Thus, relative androgen excess during menopause, rather than loss of estrogen, may adversely affect lipid status of women.49 Conversely, testosterone has been suggested to protect males from atherosclerosis.50–53 Males with higher circulating testosterone concentrations had higher HDL cholesterol levels.54,55 In studies tracking serum lipids and lipoproteins from childhood to adulthood, levels of HDL cholesterol dropped in boys, but were not changed in girls after puberty.56, 57 These results suggest effects of endogenous testosterone during development to regulate HDL cholesterol. Moreover, the decline in testosterone levels with age58 is associated with age-dependent increases in atherosclerosis in males,59–61 although other age-related factors could be causal. Serum SHBG concentrations correlate inversely with atherosclerosis in men.62, 63 These studies offer tantalizing suggestions that endogenous androgens may have opposite or divergent effects in males and females, but further research is needed.
Even more controversial than these effects of endogenous sex hormones are the effects of exogenous sex hormone therapy on coronary artery disease. In women with established coronary artery disease, administration of combined conjugated equine estrogen plus medroxyprogesterone acetate therapy increases the risk of heart disease events at one year.64 In the large Women’s Health Initiative, continuous treatment with conjugated equine estrogen plus medroxyprogesterone acetate resulted in significantly increased risk of coronary artery events and stroke.65, 66 Finally, in women with hysterectomies administered unopposed estrogen (specifically, oral conjugated equine estrogen, 0.625 mg/d) for the prevention of chronic postmenopausal conditions, there were no benefits on coronary heart disease, with an increased risk for venous thromboembolism and stroke.67 It is important to note that the number of risk factors present in the study population and other differences in study design (e.g., use of statins) may have influenced the cardiovascular outcomes of these studies. Recent results from the Early versus Late Intervention Trial with Estradiol (ELITE) highlights the potential importance of timing of initiation of 17β-estradiol therapy relative to menopause.68 Oral 17β-estradiol therapy was associated with less progression of subclinical atherosclerosis when therapy was initiated within 6 years after menopause, but not when therapy was initiated 10 or more years after menopause. These differential effects of estradiol therapy might be related to changes in estrogen receptor (ER) expression in pivotal target tissues such as the vasculature. Current guidelines continue to caution against use of postmenopausal hormone therapy to prevent cardiovascular events in women. However, given the conflicting conclusions reached from these various studies, an understanding of cardiovascular risk in women requires further research on hormone treatment in terms of type and delivery method of estrogen and progesterone, as well as the timing and duration of treatment.
Inbred mouse strains are valuable for varying sex and other variables when genetic background is held constant. Mice and humans differ in lipid profile, with mice carrying the majority of blood cholesterol in HDL particles, whereas humans have much greater levels of LDL cholesterol.69 These species differences can be diminished by using mice with elevated LDL or VLDL cholesterol levels caused by genetic manipulations (e.g., LDL receptor (Ldlr) or apolipoprotein E (ApoE) deficiency) or diet.69, 70 Sex differences in atherosclerotic lesions have been examined in mouse models. In apoE-deficient mice, some reports describe larger aortic root lesions in female compared to male mice (for example,71, 72), but others suggest that this is true only in younger mice, with older apoE-deficient mice show lesions of similar size and composition.73 Mouse models have demonstrated a relatively consistent protective effect of estrogens to reduce atherosclerosis.74 In Ldlr-deficient and apoE-deficient female mice, ovariectomy increases atherosclerosis, which is reversed by administration of 17β-estradiol.75–77 Interestingly, protective effects of estradiol are independent of changes in plasma cholesterol and do not occur in mice lacking ERα globally or in endothelial cells.78, 79, 80 Estradiol may be protective because of its effects to increase endothelial nitric oxide,81, 82 prostacyclin,83, 84 and macrophage cholesterol efflux85, and to reduce macrophage inflammation86–88 and smooth muscle cell proliferation.89, 89, 90 Nevertheless, a great deal more information is needed to understand estrogen effects on lesion development, progression and regression.
Similar to exogenous estrogen, there is considerable controversy over the benefits of testosterone replacement therapy for aging males.91 In 2015, the US Food and Drug Administration (FDA) issued a caution regarding use of testosterone products in aging males based on increased risk of heart attack and stroke. The caution was based on a review of five retrospective cohort studies and two meta-analyses of controlled testosterone trials. Although results were not entirely consistent across these studies, the FDA concluded that there was sufficient concern that testosterone administration may increase adverse cardiovascular outcomes. To date, however, there have been no randomized controlled trials to evaluate cardiovascular outcomes with testosterone use.
Studies of animal models of atherosclerosis indicate that testosterone regulates levels of apolipoprotein B-containing lipoproteins92, HDL-cholesterol93, 94, vascular cell adhesion molecule-1,95 foam cell formation96 and vascular calcification97. However, some of these studies were performed in genetically manipulated mice (e.g., ApoE-deficient mice) with lower HDL cholesterol, raising concern about their applicability to testosterone regulation of these lipoproteins in humans.
In addition to sex hormones, sex chromosomes have been linked to the regulation of serum lipid profiles and coronary artery disease. Polysomy of the Y chromosome (primarily 47, XYY karyotype) is associated with increased cardiovascular mortality.98 Europeans with a common inherited haplotype of the Y chromosome have a 50% increased risk of coronary artery disease that is associated with an altered immune response.99 In contrast, women with monosomy X (Turner syndrome) have an atherogenic lipid profile (increased LDL cholesterol level and particle size)100 and exhibit an increased risk for ischemic heart disease.101 Most sex chromosome aneuploidy conditions (e.g., XO, XXY) in humans, however, involve concomitant changes in gonadal hormone levels, making it difficult to separate sex chromosome and hormonal effects. Studies in the FCG mouse model have revealed both gonadal sex and sex chromosome contributions to the regulation of serum lipid profiles, with particularly robust effects of the X chromosome dosage on HDL cholesterol levels.38 Further studies will be required to determine the mechanisms by which sex chromosome complement influences lipid profiles and coronary artery risk.
Aneurysms
Sex is the largest non-modifiable risk factor for abdominal aortic aneurysms (AAAs), with males having 4–10 fold increased risk of AAA development.102, 103 This pronounced sex difference has resulted in current screening guidelines for AAA diagnosis that only apply to males.104, 105 The lack of screening in women is a serious concern. Although the prevalence of AAAs is much lower in women, the rate of expansion and propensity to rupture is more aggressive in females.106, 107, 108 Recent studies estimate that AAA deaths in women (45% of all prevalent cases) are higher than would be expected based on an overall lower incidence of AAA in females.109 Regardless, there are no effective therapies that slow the progression of AAAs in either sex, much less therapies that address potential sex differences in AAA growth mechanisms.
Studies of animal models have increased understanding of mechanisms, mostly hormonal, underlying sex differences in AAAs. In general, endogenous and exogenous estrogens have protective effects on experimentally induced AAAs, with results differing dependent on the model under study. In elastase-induced AAAs, ERα expression in aorta is greater in females than males,110 and estrogen synthesis in ovaries and non-ovarian tissues contributes to protection of female mice.111 When AAAs are induced by infusion of angiotensin II (AngII) in female ApoE-deficient mice, ovariectomy has no effect on AAAs,94 but treatment of males with estrogen attenuates AAA development.112 Moreover, administration of exogenous 17β-estradiol to ovariectomized females abolishes progressive increases in abdominal aortic diameter and AAA size.113 Collectively, these results support a potential effect of exogenous estrogens to reduce experimental AAA expansion.
Testosterone has also been implicated as a contributor to experimental AAA formation and progression in male and female mice. In the AngII model of AAAs, castration of male mice markedly reduces AAA formation, which is restored by administration of dihydrotestosterone.94, 114 These effects are attributed to testosterone’s effect to increase expression of abdominal aortic type 1a angiotensin receptor.114 Notably, whereas female hypercholesterolemic mice are resistant to AngII-induced AAAs, adult Ldlr−/− female mice previously treated with testosterone as neonates are highly susceptible to AngII-induced AAAs even though they have low levels of testosterone in adulthood.115 Finally, castration of adult male ApoE−/− mice, after an AAA has formed from AngII infusion, halts progressive aortic dilations caused by continued AngII infusions.116 Although evidence from animal models suggests a clear role for testosterone to promote AAAs, there is little known regarding a role for endogenous or exogenous testosterone on human AAAs.
The interesting dichotomy between males and females in the duration of testosterone exposure required to increase AAA susceptibility suggests that other mechanisms, potentially sex chromosomes, contribute to sex differences in AngII-induced AAAs. This idea is supported by a recent comparison of Ldlr−/− XX female vs. XY female mice from the FCG model infused with AngII to induce AAA formation.117 XY females had much greater AAA formation than XX females, an effect that was independent of ovarian hormone levels, and exacerbated by treatment with androgens. As in other measures of CVDs, both sex chromosome complement and gonadal hormones interact to affect disease outcomes. In this case, a male complement of sex chromosomes (XY) and male gonadal hormones may synergize in their effects to cause sex differences.
Cardiac Ischemia/Reperfusion Injury
Women prior to menopause have lower risk of ischemic heart disease than age-matched men.118 The lower incidence of coronary artery disease in women during reproductive age is believed to be caused in part by ovarian hormones because loss of ovaries in young women increases coronary artery disease.119 Similarly, when mice receive ischemia/reperfusion (I/R) injury to mimic heart attack, infarct size in ovariectomized mice is larger than in gonadally intact mice.120 Among the gonadal sex hormones, estradiol is most convincingly reported to be protective in animal studies of myocardial I/R injury.121 Estradiol pre-treatment reduces the infarct size in ovariectomized mice122 as well as in male mice subjected to in vivo I/R injury.123
If estrogens were the only factors accounting sex differences in ischemic heart disease in humans, one might expect an increase of disease after menopause in women because of the reduction in plasma estrogens, and that estrogenic hormone replacement therapy might help prevent heart disease. The Women’s Health Initiative study, however, failed to find that hormone replacement therapy reduced the risk of ischemic heart disease of postmenopausal women.64, 65 Moreover, a recent large population-based prospective study found only a minor increase in the risk of ischemic heart disease after menopause at age 55–65. Indeed, the risk of ischemic heart disease increases in both men and women with age until the age of 65–69 and more rapidly up to 95, and the sex difference in incidence persists throughout life with perhaps a modest reduction in the size of the sex difference after 85.124,125 This pattern suggests that ovarian hormones do not alone account for the sex differences, and we should look for factors in males that make them different from females, and/or other factors in females that contribute to their incidence of disease. Recent studies of gondectomized mice with altered sex chromosomes (FCG and XY* models, Figure 2) found sex differences in I/R caused by the number and type of sex chromosomes.18 GDX adult mice with two X chromosomes (XX or XXY) have ~50% larger infarct size, compared to mice with one X chromosome (XY or XO). The presence vs. absence of the Y chromosome had no effect, so Y genes do not contribute to the sex difference in I/R injury under these conditions. Thus, the presence of a second X chromosome has deleterious effects both on adiposity17 (reviewed above) and on I/R injury. However, the effects on I/R injury are not secondary to greater adiposity because they exist before the effects on adiposity are evident.126 Again, as for effects on adiposity, the X escapee genes are top candidates for causing these effects because of their higher expressed dose in mice with two X chromosomes.
The X chromosome effect in mice seems paradoxical because it goes in the “wrong” direction to explain the reduced susceptibility of women to I/R injury. In GDX female mice, the second X chromosome promotes rather than prevents injury. This observation nevertheless has potential clinical significance in humans because female-specific factors (e.g., estrogens and the second X chromosome) may interact with one another such that the protective effect of estradiol is mitigated by the presence of a second X chromosome. I/R disease occurs mostly in older women, when estrogen levels are low. Thus, women may be protected by estrogens early in life, but after menopause they may be more susceptible to I/R injury especially because of the deleterious effects of the second X chromosome. These ideas, stemming from animal research, will be easier to test in humans once the X gene(s) causing the effect have been discovered in mice.
Hypertension
According to the World Health Organization, hypertension is a major cause of cardiovascular disease leading to death throughout the world.127 The onset of hypertension occurs earlier in men than women in diverse human populations. The finding of male susceptibility and female resilience is mirrored in the vast majority of animal models of hypertension, regardless of whether it is induced or genetic.128
Many studies suggest that estradiol is a major factor in female resilience to developing hypertension. The prevalence of hypertension is greater in women with ovarian hormone deficiency (e.g., premature ovarian failure or ovariectomy prior to menopause) as well as in postmenopausal women compared to age-matched premenopausal women. Furthermore, animal studies show that blood pressure is increased in females that are ovariectomized or lack ERα.128
Animal models of testosterone deficiency (e.g., castration) suggest that the presence of testosterone can contribute to male susceptibility to hypertension, and anabolic steroid use in men is associated with increased blood pressure. However, the prevalence of hypertension is greater in men with clinically low testosterone levels.128 Thus, more investigation into the effects of sex steroids in men is necessary to gain a clearer understanding of their role.
One exciting area of sex difference research in hypertension involves the immune system. Whereas male mice have higher blood pressure than females after AngII infusion, this sex difference disappears in mice deficient in T-cells.129, 130 Adoptive transfer of male T-cells into T-cell deficient mice exacerbates hypertension in male but not female recipient mice, indicating that the sex of the host plays a key role in resilience and susceptibility to immune modulation of hypertension.129 Furthermore, T-cell modulation of blood pressure depends on the sex of the donor because adoptive transfer of male but not female T-cells can exacerbate the degree of AngII-induced hypertension in the male host.130
Experiments using FCG mice have enabled dissection of the role of the sex chromosomes from the gonadal hormones. The magnitude of hypertension induced by AngII infusion is greater in GDX XX mice compared to the XY, regardless of whether the mice were born with ovaries or testes.131 Further studies using this model suggest that females with intact ovaries are protected from hypertension due to vasodilatory mechanisms mediated by endothelium-derived hyperpolarizing factors and the AngII receptor. Although endothelium-derived hyperpolarizing factors only require estradiol, AngII-receptor-mediated vasodilation requires both estradiol and the XX sex chromosome complement.132 Thus, ovarian hormones and sex chromosomes appear to have complex effects on susceptibility to hypertension, similar to I/R injury.
Although the risk of CVD increases with higher blood pressures in both sexes, the risk of developing CVD is greater in men than women at equivalent age and blood pressure (Figure 4).133, 134 Understanding why men are at greater risk or why women are at lower risk may lead to the development of novel therapeutics that would be beneficial for treating or preventing CVD in both men and women. Most animal studies of hypertension-associated CVD including ischemic heart disease, stroke, heart failure, atrial fibrillation and cardiac sudden death have been conducted in males. In the few that have directly compared markers of CVD end organ damage between males and females, most show that males exhibit greater injury than females.
Figure 4.
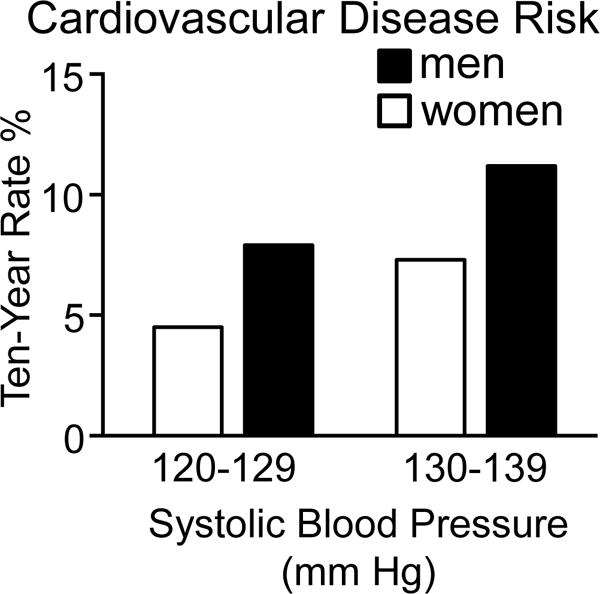
Cardiovascular disease risk in middle aged men and women as a function of blood pressure. Shown are data from the Framingham Heart Study and adapted from reference134 with permission of the publisher on estimated ten year rates of cardiovascular disease risk defined as a composite of coronary heart disease, cerebrovascular events, peripheral artery disease, and heart failure in normotensive (systolic blood pressure: 120–129 mm Hg) and pre-hypertensive (systolic blood pressure: 130–139 mm Hg) women (white bar) and men (black bar) 50 to 54 years of age who were non-smokers and did not have diabetes.
For those studies that investigated the cause of the sex differences, estradiol was shown to be a major contributor to female protection. For example, in a study of endothelial damage in the spontaneously hypertensive rat, estradiol protected the vasculature by increasing the generation of the vasodilator nitric oxide in the endothelium, and reduced superoxide anion concentrations by inhibiting AngII-receptor-mediated production of superoxide anion via NAD(P)H oxidase.135 No studies to date have rigorously examined the cause of sex differences in the direct relationship between blood pressure and magnitude of injury in models of CVD or in the mechanisms underlying these sex differences. Thus, we do not understand why the risk of developing CVD is far greater in men than women at equivalent blood pressures.
Conclusion: The future of sex differences in CVD
The studies summarized here reveal pervasive sex differences in CVDs, and document diverse mechanisms that contribute to sex differences in CVD. To understand the mechanisms underlying sex differences, the biological variable of sex must be broken down into its component parts. At the outset, we listed four categories of sex-biasing mechanisms: gonadal (hormone-mediated) effects of Sry, non-gonadal effects of Y genes, X gene dosage, and imprinting of X genes. There is strong support that sex differences are created by some of these mechanisms, especially effects of gonadal hormones, but others are known (sex chromosome effects) but have not yet been rigorously explained by dosage or imprinting effects of specific X or Y genes. The vast majority of studies, in humans and animal models, has focused on the role of gonadal hormones. As reviewed within specific sections above, estrogens are the most frequently studied factors, and have protective effects in all types of CVDs discussed here. The relative dearth of studies of androgens points to an important area for further research. The potential importance of sex chromosome complement, especially X chromosome number, has only recently been discovered in research on mice. However, the X genes involved, and their sites and mechanisms of action, are completely unknown. Moreover, hormonal and sex chromosome mechanisms clearly influence the same disease outcomes, sometimes in opposite directions,136 but the mechanistic nature of this interaction is completely unstudied. Finally, the sex-biased protective mechanisms of sex hormones and sex chromosome genes, discovered by manipulating specific sex-biasing factors in animal research, have yet to be fully translated to the clinic. Evidence suggests already that the effectiveness of specific drug therapies may depend on sex or levels of sex-biasing factors such as estrogens.137 We anticipate a rich series of studies in the near future that will make progress on these exciting questions.
Highlights.
-
–
Study of sex differences in cardiovascular physiology and disease in mice allows one to break down biological sex into its component parts, so that harmful or protective effects of hormones and sex chromosome genes can be identified.
-
–
In mice, estrogens are generally protective in various models of cardiovascular and associated diseases.
-
–
XX and XY animals differ in cardiovascular physiology and disease, independent of the type of gonadal hormones.
-
–
The biological factors protecting one sex from disease, more than the other, may potentially be targeted in novel therapies.
Acknowledgments
Sources of Funding
NIH grants R01DK083561 (K.R. and A.P.A.), P01HL028481 (K.R.), R56HL119886 and R01HL131182 (M.E. and A.P.A.), R01HL107326 (L.A.C.), R01HL119380 (K.S.), American Heart Association grant 16GRANT27760058 (M.E.).
Many thanks to Grégoire Ruffenach for creating the graphic abstract.
Abbreviations
- AAA
abdominal aortic aneurysm
- AngII
Angiotensin-II
- ApoE
apolipoprotein
- AAA
abdominal aortic aneurysm
- CVD
cardiovascular disease
- ERα
estrogen receptor alpha
- FCG
Four Core Genotypes mouse model
- FDA
US Food and Drug Administration
- GDX
gonadectomized
- HDL
high-density lipoprotein
- I/R
ischemia/reperfusion
- LDL
low-density lipoprotein
- Ldlr
LDL receptor
- SHBG
steroid hormone-binding globulin
- Sry
Sex determining region on the Y chromosome, the testis-determining gene
- XXF
XX mouse that developed ovaries
- XXM
XX mouse that developed testes
- XYF
XY mouse that developed ovaries
- XYM
XY mouse that developed testes
- XY*
Male mouse with mutant Y* chromosome, father of XY* model mice
Footnotes
Disclosures
The authors declare no competing interests.
Reference List
- 1.US National Institute of Medicine Committee on Understanding the Biology of Sex and Gender Disorders. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington D.C.: National Academy Press; 2001. [Google Scholar]
- 2.Maric-Bilkan C, Arnold AP, Taylor DA, Dwinell M, Howlett SE, Wenger N, Reckelhoff JF, Sandberg K, Churchill G, Levin E, Lundberg MS. Report of the National Heart, Lung, and Blood Institute Working Group on Sex Differences Research in Cardiovascular Disease: Scientific Questions and Challenges. Hypertension. 2016;67:802–807. doi: 10.1161/HYPERTENSIONAHA.115.06967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leening MJ, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, Heeringa J, Portegies ML, Hofman A, Ikram MA, Hunink MG, Franco OH, Stricker BH, Witteman JC, Roos-Hesselink JW. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. doi: 10.1136/bmj.g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.Shi A, Tao Z, Wei P, Zhao J. Epidemiological aspects of heart diseases. Exp Ther Med. 2016;12:1645–1650. doi: 10.3892/etm.2016.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puckrein GA, Egan BM, Howard G. Social and Medical Determinants of Cardiometabolic Health: The Big Picture. Ethn Dis. 2015;25:521–524. doi: 10.18865/ed.25.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering TG. The effects of environmental and lifestyle factors on blood pressure and the intermediary role of the sympathetic nervous system. J Hum Hypertens. 1997;11(Suppl 1):S9–18. [PubMed] [Google Scholar]
- 8.Arnold AP. The end of gonad-centric sex determination in mammals. Trends Genet. 2011;28:55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development. Nat Rev Endocrinol. 2014;10:673–683. doi: 10.1038/nrendo.2014.163. [DOI] [PubMed] [Google Scholar]
- 10.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Loke H, Harley V, Lee J. Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol. 2015;65:139–150. doi: 10.1016/j.biocel.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Ely D, Underwood A, Dunphy G, Boehme S, Turner M, Milsted A. Review of the Y chromosome, Sry and hypertension. Steroids. 2010;75:747–753. doi: 10.1016/j.steroids.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Case LK, Teuscher C. Y genetic variation and phenotypic diversity in health and disease. Biol Sex Differ. 2015;6:6. doi: 10.1186/s13293-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disteche CM. Dosage compensation of the sex chromosomes. Annu Rev Genet. 2012;46:537–560. doi: 10.1146/annurev-genet-110711-155454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balaton BP, Brown CJ. Escape Artists of the X Chromosome. Trends Genet. 2016;32:348–359. doi: 10.1016/j.tig.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, Arnold AP, Eghbali M. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res. 2014;102:375–394. doi: 10.1093/cvr/cvu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellott DW, Hughes JF, Skaletsky H, et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–499. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011;27:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinol. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 22.Arnold AP. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: Why compensation changes the game. Exp Neurol. 2014;259:2–9. doi: 10.1016/j.expneurol.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT, Washburn IL. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of thepseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- 24.Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinol. 2013;154:1092–1104. doi: 10.1210/en.2012-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23:1345–1352. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parks BW, Sallam T, Mehrabian M, et al. Genetic architecture of insulin resistance in the mouse. Cell Metab. 2015;21:334–346. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parks BW, Nam E, Org E, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santosa S, Jensen MD. The Sexual Dimorphism of Lipid Kinetics in Humans. Front Endocrinol (Lausanne) 2015;6:103. doi: 10.3389/fendo.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law J, Bloor I, Budge H, Symonds ME. The influence of sex steroids on adipose tissue growth and function. Horm Mol Biol Clin Investig. 2014;19:13–24. doi: 10.1515/hmbci-2014-0015. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler TW, Justice AE, Graff M, et al. The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study. PLoS Genet. 2015;11:e1005378. doi: 10.1371/journal.pgen.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung YJ, Perusse L, Sarzynski MA, et al. Genome-wide association studies suggest sex-specific loci associated with abdominal and visceral fat. Int J Obes (Lond) 2016;40:662–674. doi: 10.1038/ijo.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett BJ, Davis RC, Civelek M, et al. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLoS Genet. 2015;11:e1005711. doi: 10.1371/journal.pgen.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, Arnold AP, Reue K. Increased High-Density Lipoprotein Cholesterol Levels in Mice With XX Versus XY Sex Chromosomes. Arterioscler Thromb Vasc Biol. 2015;35:1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heberden W. Commentaries on the history and cure of diseases. In: Willis FA, Keys TE, editors. Cardiac Classics. St. Louis: C.V. Mosby; 1941. p. 224. [Google Scholar]
- 40.McGill HC, Stern MP. Sex and atherosclerosis. Atherosclerosis Rev. 1979;4:157–242. [Google Scholar]
- 41.Peters SA, Singhateh Y, Mackay D, Huxley RR, Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis. 2016;248:123–131. doi: 10.1016/j.atherosclerosis.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Galman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Intern Med. 2011;270:580–588. doi: 10.1111/j.1365-2796.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 43.Middelberg RP, Spector TD, Swaminathan R, Snieder H. Genetic and environmental influences on lipids, lipoproteins, and apolipoproteins: effects of menopause. Arterioscler Thromb Vasc Biol. 2002;22:1142–1147. doi: 10.1161/01.atv.0000022889.85440.79. [DOI] [PubMed] [Google Scholar]
- 44.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 45.Guthrie JR, Taffe JR, Lehert P, Burger HG, Dennerstein L. Association between hormonal changes at menopause and the risk of a coronary event: a longitudinal study. Menopause. 2004;11:315–322. doi: 10.1097/01.gme.0000094208.15096.62. [DOI] [PubMed] [Google Scholar]
- 46.Oliver MF, Boyd GS. Effect of bilateral ovariectomy on coronary-artery disease and serum-lipid levels. Lancet. 1959;2:690–694. doi: 10.1016/s0140-6736(59)92129-4. [DOI] [PubMed] [Google Scholar]
- 47.Cauley JA, Gutai JP, Glynn NW, Paternostro-Bayles M, Cottington E, Kuller LH. Serum estrone concentrations and coronary artery disease in postmenopausal women. Arterioscler Thromb. 1994;14:14–18. doi: 10.1161/01.atv.14.1.14. [DOI] [PubMed] [Google Scholar]
- 48.Kumagai S, Kai Y, Sasaki H. Relationship between insulin resistance, sex hormones and sex hormone-binding globulin in the serum lipid and lipoprotein profiles of Japanese postmenopausal women. J Atheroscler Thromb. 2001;8:14–20. doi: 10.5551/jat1994.8.14. [DOI] [PubMed] [Google Scholar]
- 49.Mudali S, Dobs AS, Ding J, Cauley JA, Szklo M, Golden SH. Endogenous postmenopausal hormones and serum lipids: the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2005;90:1202–1209. doi: 10.1210/jc.2004-0744. [DOI] [PubMed] [Google Scholar]
- 50.Farias JM, Tinetti M, Khoury M, Umpierrez GE. Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:4698–4703. doi: 10.1210/jc.2014-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shores MM, Matsumoto AM. Testosterone, aging and survival: biomarker or deficiency. Curr Opin Endocrinol Diabetes Obes. 2014;21:209–216. doi: 10.1097/MED.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–2079. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- 53.Lee WC, Kim MT, Ko KT, Lee WK, Kim SY, Kim HY, Yang DY. Relationship between Serum Testosterone and Cardiovascular Disease Risk Determined Using the Framingham Risk Score in Male Patients with Sexual Dysfunction. World J Mens Health. 2014;32:139–144. doi: 10.5534/wjmh.2014.32.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutai J, LaPorte R, Kuller L, Dai W, Falvo-Gerard L, Caggiula A. Plasma testosterone, high density lipoprotein cholesterol and other lipoprotein fractions. Am J Cardiol. 1981;48:897–902. doi: 10.1016/0002-9149(81)90356-8. [DOI] [PubMed] [Google Scholar]
- 55.Heller RF, Miller NE, Lewis B, Vermeulen A, Fairney A, James VH, Swan AV. Associations between sex hormones, thyroid hormones and lipoproteins. Clin Sci (Lond) 1981;61:649–651. doi: 10.1042/cs0610649. [DOI] [PubMed] [Google Scholar]
- 56.Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133:884–899. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- 57.Eissa MA, Mihalopoulos NL, Holubkov R, Dai S, Labarthe DR. Changes in Fasting Lipids during Puberty. J Pediatr. 2016;170:199–205. doi: 10.1016/j.jpeds.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 59.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corona G, Monami M, Boddi V, Rastrelli G, Melani C, Balzi D, Sforza A, Forti G, Mannucci E, Maggi M. Pulse pressure independently predicts major cardiovascular events in younger but not in older subjects with erectile dysfunction. J Sex Med. 2011;8:247–254. doi: 10.1111/j.1743-6109.2010.01966.x. [DOI] [PubMed] [Google Scholar]
- 61.Ruige JB, Mahmoud AM, De BD, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97:870–875. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 62.Hak AE, Witteman JC, De Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 63.Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007;19:176–182. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 64.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 65.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 66.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 67.US Preventative Services Task Force. Hormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the US Preventative Services Task Force. Ann Intern Med. 2005;142:855–860. [PubMed] [Google Scholar]
- 68.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med. 2016;374:1221–1231. doi: 10.1056/NEJMoa1505241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith DD, Tan X, Tawfik O, Milne G, Stechschulte DJ, Dileepan KN. Increased aortic atherosclerotic plaque development in female apolipoprotein E-null mice is associated with elevated thromboxane A2 and decreased prostacyclin production. J Physiol Pharmacol. 2010;61:309–316. [PMC free article] [PubMed] [Google Scholar]
- 72.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 73.Caligiuri G, Nicoletti A, Zhou X, Tornberg I, Hansson GK. Effects of sex and age on atherosclerosis and autoimmunity in apoE-deficient mice. Atherosclerosis. 1999;145:301–308. doi: 10.1016/s0021-9150(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 74.Hodgin JB, Maeda N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinol. 2002;143:4495–4501. doi: 10.1210/en.2002-220844. [DOI] [PubMed] [Google Scholar]
- 75.Marsh MM, Walker VR, Curtiss LK, Banka CL. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in LDL receptor-deficient mice. J Lipid Res. 1999;40:893–900. [PubMed] [Google Scholar]
- 76.Elhage R, Arnal JF, Pieraggi MT, Duverger N, Fievet C, Faye JC, Bayard F. 17 beta-estradiol prevents fatty streak formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- 77.Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 80.Villablanca AC, Tenwolde A, Lee M, Huck M, Mumenthaler S, Rutledge JC. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res. 2009;2:289–299. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arnal JF, Fontaine C, Billon-Gales A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 82.Chow RW, Handelsman DJ, Ng MK. Minireview: rapid actions of sex steroids in the endothelium. Endocrinology. 2010;151:2411–2422. doi: 10.1210/en.2009-1456. [DOI] [PubMed] [Google Scholar]
- 83.Mikkola T, Turunen P, Avela K, Orpana A, Viinikka L, Ylikorkala O. 17 beta-estradiol stimulates prostacyclin, but not endothelin-1, production in human vascular endothelial cells. J Clin Endocrinol Metab. 1995;80:1832–1836. doi: 10.1210/jcem.80.6.7775630. [DOI] [PubMed] [Google Scholar]
- 84.Mikkola T, Ranta V, Orpana A, Ylikorkala O, Viinikka L. Effect of physiological concentrations of estradiol on PGI2 and NO in endothelial cells. Maturitas. 1996;25:141–147. doi: 10.1016/0378-5122(96)01057-2. [DOI] [PubMed] [Google Scholar]
- 85.Badeau RM, Metso J, Wahala K, Tikkanen MJ, Jauhiainen M. Human macrophage cholesterol efflux potential is enhanced by HDL-associated 17beta-estradiol fatty acyl esters. J Steroid Biochem Mol Biol. 2009;116:44–49. doi: 10.1016/j.jsbmb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Frazier-Jessen MR, Kovacs EJ. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995;154:1838–1845. [PubMed] [Google Scholar]
- 87.Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 88.Vegeto E, Ghisletti S, Meda C, Etteri S, Belcredito S, Maggi A. Regulation of the lipopolysaccharide signal transduction pathway by 17beta-estradiol in macrophage cells. J Steroid Biochem Mol Biol. 2004;91:59–66. doi: 10.1016/j.jsbmb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Dai-Do D, Espinosa E, Liu G, Rabelink TJ, Julmy F, Yang Z, Mahler F, Luscher TF. 17 beta-estradiol inhibits proliferation and migration of human vascular smooth muscle cells: similar effects in cells from postmenopausal females and in males. Cardiovasc Res. 1996;32:980–985. [PubMed] [Google Scholar]
- 90.Suzuki A, Mizuno K, Ino Y, Okada M, Kikkawa F, Mizutani S, Tomoda Y. Effects of 17 beta-estradiol and progesterone on growth-factor-induced proliferation and migration in human female aortic smooth muscle cells in vitro. Cardiovasc Res. 1996;32:516–523. [PubMed] [Google Scholar]
- 91.Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “Age-Related Hypogonadism” –FDA Concerns. N Engl J Med. 2015;373:689–691. doi: 10.1056/NEJMp1506632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casquero AC, Berti JA, Salerno AG, Bighetti EJ, Cazita PM, Ketelhuth DF, Gidlund M, Oliveira HC. Atherosclerosis is enhanced by testosterone deficiency and attenuated by CETP expression in transgenic mice. J Lipid Res. 2006;47:1526–1534. doi: 10.1194/jlr.M600135-JLR200. [DOI] [PubMed] [Google Scholar]
- 93.von DG, von DO, Volker W, Langer C, Weinbauer GF, Behre HM, Nieschlag E, Assmann G, von EA. Atherosclerosis in apolipoprotein E-deficient mice is decreased by the suppression of endogenous sex hormones. Horm metab Res. 2001;33:110–114. doi: 10.1055/s-2001-12405. [DOI] [PubMed] [Google Scholar]
- 94.Henriques TA, Huang J, D’Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinol. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 95.Mukherjee TK, Dinh H, Chaudhuri G, Nathan L. Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:4055–4060. doi: 10.1073/pnas.052703199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiu Y, Yanase T, Hu H, Tanaka T, Nishi Y, Liu M, Sueishi K, Sawamura T, Nawata H. Dihydrotestosterone suppresses foam cell formation and attenuates atherosclerosis development. Endocrinol. 2010;151:3307–3316. doi: 10.1210/en.2009-1268. [DOI] [PubMed] [Google Scholar]
- 97.McRobb L, Handelsman DJ, Heather AK. Androgen-induced progression of arterial calcification in apolipoprotein E-null mice is uncoupled from plaque growth and lipid levels. Endocrinol. 2009;150:841–848. doi: 10.1210/en.2008-0760. [DOI] [PubMed] [Google Scholar]
- 98.Higgins CD, Swerdlow AJ, Schoemaker MJ, Wright AF, Jacobs PA. Mortality and cancer incidence in males with Y polysomy in Britain: a cohort study. Hum Genet. 2007;121:691–696. doi: 10.1007/s00439-007-0365-8. [DOI] [PubMed] [Google Scholar]
- 99.Charchar FJ, Bloomer LD, Barnes TA, et al. Inheritance of coronary artery disease in men: an analysis of the role of the Y chromosome. Lancet. 2012;379:915–922. doi: 10.1016/S0140-6736(11)61453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van PL, Bakalov VK, Bondy CA. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J Clin Endocrinol Metab. 2006;91:2867–2870. doi: 10.1210/jc.2006-0503. [DOI] [PubMed] [Google Scholar]
- 101.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51:147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 102.Hannawa KK, Eliason JL, Upchurch GR., Jr Gender differences in abdominal aortic aneurysms. Vascular. 2009;17(Suppl 1):S30–S39. doi: 10.2310/6670.2008.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Makrygiannis G, Courtois A, Drion P, Defraigne JO, Kuivaniemi H, Sakalihasan N. Sex differences in abdominal aortic aneurysm: the role of sex hormones. Ann Vasc Surg. 2014;28:1946–1958. doi: 10.1016/j.avsg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007:CD002945. doi: 10.1002/14651858.CD002945.pub2. [DOI] [PubMed] [Google Scholar]
- 105.Guirguis-Blake JM, Beil TL, Senger CA, Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:321–329. doi: 10.7326/M13-1844. [DOI] [PubMed] [Google Scholar]
- 106.Grootenboer N, Bosch JL, Hendriks JM, van Sambeek MR. Epidemiology, aetiology, risk of rupture and treatment of abdominal aortic aneurysms: does sex matter? Eur J Vasc Endovasc Surg. 2009;38:278–284. doi: 10.1016/j.ejvs.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 107.Skibba AA, Evans JR, Hopkins SP, Yoon HR, Katras T, Kalbfleisch JH, Rush DS. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429–1436. doi: 10.1016/j.jvs.2015.07.079. [DOI] [PubMed] [Google Scholar]
- 108.Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 109.Stuntz M. Modeling the Burden of Abdominal Aortic Aneurysm in the USA in 2013. Cardiology. 2016;135:127–131. doi: 10.1159/000446871. [DOI] [PubMed] [Google Scholar]
- 110.Laser A, Ghosh A, Roelofs K, Sadiq O, McEvoy B, DiMusto P, Eliason J, Upchurch GR., Jr Increased estrogen receptor alpha in experimental aortic aneurysms in females compared with males. J Surg Res. 2014;186:467–474. doi: 10.1016/j.jss.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnston WF, Salmon M, Su G, Lu G, Ailawadi G, Upchurch GR., Jr Aromatase is required for female abdominal aortic aneurysm protection. J Vasc Surg. 2015;61:1565–1574. doi: 10.1016/j.jvs.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin-McNulty B, Tham DM, Da CV, Ho JJ, Wilson DW, Rutledge JC, Deng GG, Vergona R, Sullivan ME, Wang YX. 17 Beta-estradiol attenuates development of angiotensin II-induced aortic abdominal aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1627–1632. doi: 10.1161/01.ATV.0000085842.20866.6A. [DOI] [PubMed] [Google Scholar]
- 113.Thatcher SE, Zhang X, Woody S, Wang Y, Alsiraj Y, Charnigo R, Daugherty A, Cassis LA. Exogenous 17-beta estradiol administration blunts progression of established angiotensin II-induced abdominal aortic aneurysms in female ovariectomized mice. Biol Sex Differ. 2015;6:12. doi: 10.1186/s13293-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1251–1256. doi: 10.1161/ATVBAHA.107.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res. 2012;110:e73–e85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, Thatcher S, Wu C, Daugherty A, Cassis LA. Castration of male mice prevents the progression of established angiotensin II-induced abdominal aortic aneurysms. J Vasc Surg. 2015;61:767–776. doi: 10.1016/j.jvs.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alsiraj Y, Thatcher SE, Charnigo R, Kuey C, Blalock E, Daugherty A, Cassis LA. Female Mice with an XY Sex Chromosome Complement Develop Severe Angiotensin II-Induced Abdominal Aortic Aneurysms. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 119.Parker WH, Jacoby V, Shoupe D, Rocca W. Effect of bilateral oophorectomy on women’s long-term health. Womens Health (Lond Engl) 2009;5:565–576. doi: 10.2217/whe.09.42. [DOI] [PubMed] [Google Scholar]
- 120.Song X, Li G, Vaage J, Valen G. Effects of sex, gonadectomy, and oestrogen substitution on ischaemic preconditioning and ischaemia-reperfusion injury in mice. Acta Physiol Scand. 2003;177:459–466. doi: 10.1046/j.1365-201X.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 121.Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I. Gender differences in cardiac ischemic injury and protection–experimental aspects. Exp Biol Med (Maywood) 2009;234:1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- 122.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, Bonaa KH. Lifelong Gender Gap in Risk of Incident Myocardial Infarction: The Tromso Study. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.5451. [DOI] [PubMed] [Google Scholar]
- 125.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parker WH. Ovarian conservation versus bilateral oophorectomy at the time of hysterectomy for benign disease. Menopause. 2014;21:192–194. doi: 10.1097/GME.0b013e31829be0a0. [DOI] [PubMed] [Google Scholar]
- 127.World Heart Federation. URL. 2016 Available at: URL: http://www.world-heart-federation.org/cardiovascular-health/cardiovascular-disease-risk-factors/hypertension/
- 128.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension. 2014;64:573–582. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pessoa BS, Slump DE, Ibrahimi K, Grefhorst A, van VR, Garrelds IM, Roks AJ, Kushner SA, Danser AH, van Esch JH. Angiotensin II type 2 receptor- and acetylcholine-mediated relaxation: essential contribution of female sex hormones and chromosomes. Hypertension. 2015;66:396–402. doi: 10.1161/HYPERTENSIONAHA.115.05303. [DOI] [PubMed] [Google Scholar]
- 133.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 134.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 135.Dantas AP, Franco MC, Silva-Antonialli MM, Tostes RC, Fortes ZB, Nigro D, Carvalho MH. Gender differences in superoxide generation in microvessels of hypertensive rats: role of NAD(P)H-oxidase. Cardiovasc Res. 2004;61:22–29. doi: 10.1016/j.cardiores.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 136.Arnold AP, Chen X, Link JC, Itoh Y, Reue K. Cell-autonomous sex determination outside of the gonad. Dev Dyn. 2013;242:371–379. doi: 10.1002/dvdy.23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murphy E, Steenbergen C. Sex, drugs, and trial design: sex influences the heart and drug responses. J Clin Invest. 2014;124:2375–2377. doi: 10.1172/JCI76262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda MD: National Heart, Lung, and Blood Institute; 2016. [Google Scholar]


