Abstract
Memory consolidation involves the process by which newly acquired information becomes stored in a long-lasting fashion. Evidence acquired over the past several decades, especially from studies using post-training drug administration, indicates that emotional arousal during the consolidation period influences and enhances the strength of the memory and that multiple different chemical signaling systems participate in this process. The mechanisms underlying the emotional influences on memory involve the release of stress hormones and activation of the basolateral amygdala, which work together to modulate memory consolidation. Moreover, work suggests that this amygdala-based memory modulation occurs with numerous types of learning and involves interactions with many different brain regions to alter consolidation. Additionally, studies suggest that emotional arousal and amygdala activity in particular influence synaptic plasticity and associated proteins in downstream brain regions. This review considers the historical understanding for memory modulation and cellular consolidation processes and examines several research areas currently using this foundational knowledge to develop therapeutic treatments.
I. Understanding the Systems and Cellular Processes of Memory Consolidation
Memory defines us as individuals; our personal preferences, skills, and wisdom are rooted in long-term memories. We draw on our memories to make sense of the present, and our memories help to direct our future behaviors. Although we all know much about memory from our personal experiences, investigating the neural processes underlying memory is not as simple as it may seem. We cannot directly observe memory; human memory as well as animal memory is inferred from experience-induced changes in behavior. And it is, of course, essential to distinguish the behavioral changes reflecting memory from those induced by the many other conditions that can directly influence behavior. Thus, experimental treatments that modulate memory, without directly influencing the behavior used to assess memory, are critical in understanding brain processes underlying memory.
From our personal perception, it may seem that information regarding an event is acquired and immediately stored in our brains. However, lasting memories are not formed instantly at the time of an experience. Furthermore, as we do not have lasting memories for every detail or even every event, there must be some process(es) by which information is selected for retention. By logical extension, the period of time between expression of a short- and long-term memory must be one in which the memory for that information is not in a permanent state but remains labile. The lability of memory processes occurring after learning permits postlearning neural influences to regulate the resulting strength of memory. Understanding the neural systems and processes involved in the endogenous modulation of memory consolidation can provide critical insights into the mechanisms and substrates for drug effects on memory.
Investigating emotionally influenced memories is especially advantageous for addressing these issues, as such memories are more likely to be stored for the long-term after a single experience compared with the memories for emotionally neutral events. This provides a temporal window of opportunity for investigation of the neural basis of memory. Furthermore, an understanding of the effects of emotional arousal on memory consolidation may provide critical insights for the development of treatments for memory-based disorders and problems. This review explores current understanding of the neural processes modulating and underlying the consolidation of long-lasting memories.
II. History of Memory Modulation and Consolidation
The findings of Müller and Pilzecker (1900) were the first to provide evidence supporting what was termed the perseveration consolidation hypothesis. But, the hypothesis, and the evidence supporting it, was largely neglected until the 1940s, when electroshock treatments were first used for treatments of mental disorders. In a classic study, Duncan (1949) reported that electroshock given to rats immediately after they were trained impaired the memory of the training. However, importantly, the degree of amnesia decreased as the interval between training and electroshock treatment was increased. These findings, which were extensively replicated and extended in many subsequent studies, clearly indicated that the storage of memories is time-dependent (McGaugh, 1966; McGaugh and Herz, 1972).
The evidence of experimentally induced retrograde amnesia had clear implications for understanding drug influences on memory. In an early study, Lashley (1917) reported that strychnine administered to rats before daily training enhanced their learning performance. Subsequent findings provided additional evidence of drug-induced learning enhancement (McGaugh and Petrinovich, 1959). These results suggested the possibility that strychnine influenced neural processes underlying learning. However, the findings of these studies, as well as those of all studies using drug administration prior to training, only indicate whether performance is influenced. The problem faced by such studies is that of determining whether the drug effect is due to enhanced learning or to some other influence on performance. For example, drug administration may alter pain sensation, thus producing effects on the acquisition of footshock-based learning, or may dilate pupils, leading to potential acquisition deficits for visual learning tasks. In each case, although retention tests would undoubtedly indicate differences between drug and control groups, it would be impossible to attribute those differences to effects on processes underlying memory.
The findings of experimentally induced retrograde amnesia (Duncan, 1949) suggested that, if strychnine enhances memory processes, it should be possible to enhance learning by administering the drug after training. Thus, as the animals would be drug-free during both training and subsequent testing, direct influences of the drug on performance could be excluded. Subsequent experiments using post-training administration of strychnine and other stimulant drugs provided extensive evidence supporting this implication (McGaugh, 1966; 1973; McGaugh and Herz, 1972). Studies using drug treatments administered prior to training or testing can, of course, provide important information about the actions of drugs. However, studies of drug influences on learning must dissociate learning effects from performance effects. Post-training drug administration enables the investigation of drug effects on memory consolidation without having to control for possible performance effects and has, thus, become the standard method for investigation of drug influences on learning and memory.
Concurrent with the development of the idea that memories are consolidated over time, other findings were beginning to provide an understanding of where memory consolidation occurs in the brain. Scoville and Milner (1957) reported the remarkable results obtained from patients whose medial temporal lobes were surgically removed in attempts to treat brain-based disorders. Following recovery, the patients demonstrated varying degrees of memory impairments. This study led, most famously, to decades-long research with one patient, Henry Molaison (referred to as H.M.). The findings indicated that medial temporal lobe damage caused significant anterograde amnesia, whereas memories from events that occurred significantly before the surgery remained largely intact. Importantly, the degree of the memory problems was related to the extent of bilateral damage to the medial temporal lobe, especially when the lesions extended into the hippocampus and hippocampal gyrus. Patients with less damage to the hippocampal complex or only unilateral damage showed less severe memory deficits or even no lasting deficit. These findings suggested that the formation and storage/retrieval of memories are distinct processes involving different regions of the brain, and that the medial temporal lobe, and especially the hippocampal formation, is of particular importance for the formation, but not long-term storage or retrieval, of memories.
During this time, a number of studies also investigated the role of the amygdala in brain functioning. Early work placed the amygdala in the limbic system, considered the major emotional processing circuit (MacLean, 1949; 1952). In a landmark study, Kluver and Bucy (1937) reported that lesions of the temporal lobe in monkeys produced large deficits in emotional processing, and Weiskrantz (1956) later demonstrated that these emotional deficits were due primarily to the loss of the amygdala. Following this work identifying the amygdala as part of an emotional processing system, Gold et al. (1975) proposed that the amygdala participated in the consolidation of emotional memories. Remarkably, they found that high-level electrical stimulation of the amygdala after training disrupted memory consolidation, whereas low-level stimulation enhanced memory. This finding suggested that the amygdala played a role in memory consolidation and that, in particular, alterations in amygdala activity after training could modulate (either enhance or impair) memory. The involvement of the amygdala in the modulation of memory consolidation (McGaugh and Gold, 1976) is addressed further below.
Concurrently, work in the cellular and molecular domains further advanced our understanding of the processes underlying memory consolidation. Hebb (1949) proposed the dual-trace theory of memory formation suggesting that short-term and long-term memories involve distinct processes. He suggested that the reverberatory activity of cells creates lasting memory by inducing structural changes in synapses. Subsequent studies suggested that such synaptic changes involve the synthesis of new proteins. In support of this implication, Flexner et al. (1963) found that intracerebral injections of the protein synthesis inhibitor puromycin impaired memory in mice, and Agranoff and Klinger (1964) reported that, in goldfish, intracranial injections of the puromycin immediately after training impaired retention. Puromycin injections administered immediately prior to the learning trials did not affect acquisition or short-term memory but impaired long-term retention (Agranoff et al., 1965). Injections administered after training also impaired retention, and the degree of impairment varied with the interval between training and subsequent puromycin injection (Agranoff et al., 1965). Together, these studies provided a critical set of findings suggesting that 1) the synthesis of new proteins is essential for creating long-term memory, 2) such synthesis occurs within a limited time window after learning, and 3) short-term memories do not depend on protein synthesis. The conclusions from these early studies were seminal in guiding subsequent investigation of the molecular bases of long-term memory formation (Davis and Squire, 1984).
However, protein synthesis inhibitors have many nonspecific effects that may interfere with memory. Flexner and Goodman (1975) suggested that memory impairments may be due to side effects of protein synthesis inhibitors on catecholamine activity. In support of this view, Canal and Gold (2007) reported an immediate and large increase in extracellular norepinephrine, dopamine, and serotonin near the site of an intra-amygdala infusion of the protein synthesis inhibitor anisomycin as well as an accompanying impairment in memory. The authors found that pretreatment with intra-amygdala infusions of the β-adrenergic receptor antagonist propranolol attenuated the memory-impairing effect of anisomycin, suggesting the alterations in catecholamine levels could be sufficient to produce memory impairments associated with protein synthesis inhibitors. Moreover, in addition to this noise produced by artificially provoked release of neurotransmitters (Gold, 2006), protein synthesis inhibitors may also influence memory processes by producing a superinduction, or rapid and superphysiological expression, of immediate early genes (Radulovic and Tronson, 2008). Therefore, caution may be warranted in assigning the mechanisms for the memory impairments observed with protein synthesis inhibitors.
III. Stress Hormones: Epinephrine and Glucocorticoids
Why should memory consolidation be susceptible to modulating influences, as this early work suggested? Most of our lives are full of mundane or trivial events, with significant occurrences interspersed throughout. Thus, a system that allows relatively selective remembrance of the more important events would be highly beneficial. Because significant events tend to be emotionally arousing, emotional arousal would appear to be a good candidate for driving such a system. And, as emotional arousal involves the release of adrenal stress hormones, stress hormones themselves would appear to be excellent candidate mechanisms for the endogenous modulation of memory consolidation.
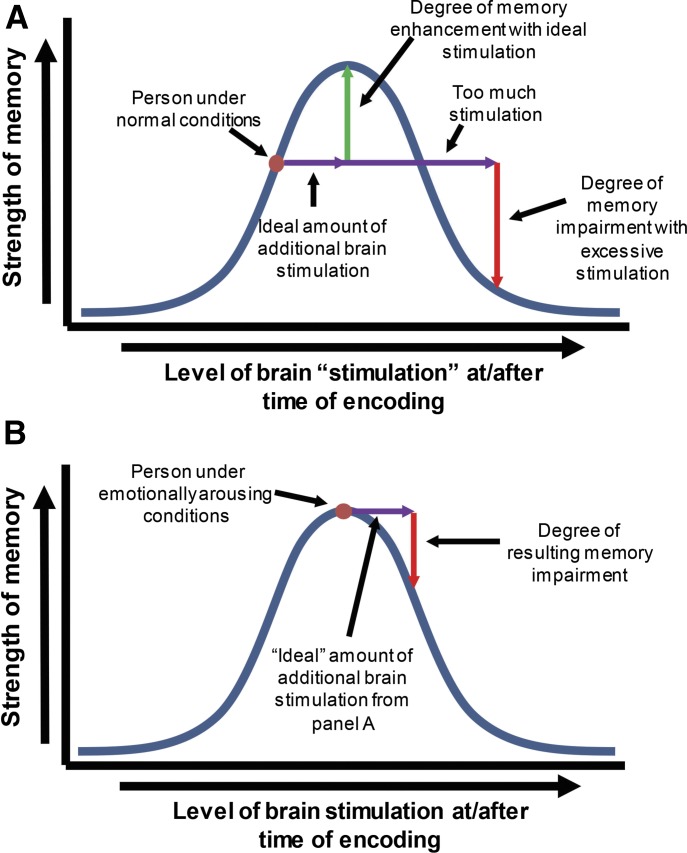
In the first studies to address this issue, Gold and van Buskirk (1975, 1976) found that systemic administration of epinephrine or adrenocorticotropic hormone (ACTH) enhanced memory when given to rats after training on an inhibitory avoidance task. In their study of ACTH (Gold and Van Buskirk, 1976), they found that ACTH enhanced memory when animals received training with low footshock intensity and impaired memory when training involved high footshock intensity. These findings suggested that memory modulation by ACTH follows an inverted-U curve, as illustrated in Fig. 1. Thus, administration of ACTH at high footshock levels, when endogenous ACTH is presumably high, appeared to increase the level beyond the peak of the dose–response curve and produce memory impairment. Subsequent studies have provided extensive evidence that post-training systemic administration of the stress hormones epinephrine or corticosterone modulates memory in a variety of learning and memory tasks (Gold and Van Buskirk, 1975; Flood et al., 1978; Sandi and Rose, 1994; Roozendaal and McGaugh, 1996). Of particular significance, these findings suggest that exogenous administration of agents like epinephrine and glucocorticoids (GCs) is effective because they work on an endogenous system that serves the purpose of enhancing important memories. However, they also highlight a complication in drug therapy for memory modulation: a given dose of a drug may enhance or impair memory, depending on the arousal state of the individual (see Fig. 1B for a depiction of this issue) (McGaugh and Roozendaal, 2009).
Fig. 1.
(A) Inverted “U” curve showing the relationship between the level of brain stimulation either at the time of or immediately after a learning event (encoding) and the long-term strength of the resulting memory. Stimulation refers to endogenous neural and hormonal activity (e.g., release of stress hormones) as well as any potential exogenous stimulation (e.g., drugs) intended to enhance memory consolidation. Under normal conditions, a person’s memory could be enhanced by providing the appropriate level of exogenous stimulation that would bring the memory’s strength to its peak. However, excessive stimulation runs a considerable risk of not only failing to enhance the strength of the resulting memory but even impairing retention. (B) Similar diagram as (A), but showing a person under highly emotionally arousing conditions. Evidence from animal studies indicates that, under periods of already-high emotional arousal, further exogenous administration of stress hormones or other non-natural methods of activating the memory-modulatory system provides no memory-enhancing benefit and can even impair memory. As a result, administration of exogenous agents that normally enhance memory carries the risk of impairing memory when the memory-modulatory system is already at its peak ability for strengthening memories.
IV. Amygdala Modulation of Memory
Much research has pointed to a critical role for the amygdala in mediating the effects of peripheral stress hormones on memory consolidation. Gold and van Buskirk’s (1978) finding that stressful footshock stimulation as well as epinephrine administration increases brain levels of norepinephrine suggested that the noradrenergic activation of the amygdala may be a key step in stress hormone effects on consolidation. In support of this possibility, Gallagher et al. (1977) reported that β-noradrenergic antagonists administered into the amygdala after training impair memory. Moreover, subsequent research found that the β-noradrenergic antagonist propranolol administered into the amygdala blocks the memory-enhancing effects of systemically administered epinephrine (Liang et al., 1986). Additionally, and importantly, many findings indicate that the basolateral amygdala (BLA) is a critical region involved in modulating memory consolidation (McIntyre et al., 2003). Norepinephrine infused selectively into the BLA after training enhances memory, and lesions of the BLA or infusions of propranolol into BLA after training impair memory (Hatfield and McGaugh, 1999; LaLumiere et al., 2003; Barsegyan et al., 2014). Figure 2 provides a schematic diagram, based on the evidence summarized to this point and below, for how the BLA modulates memory consolidation.
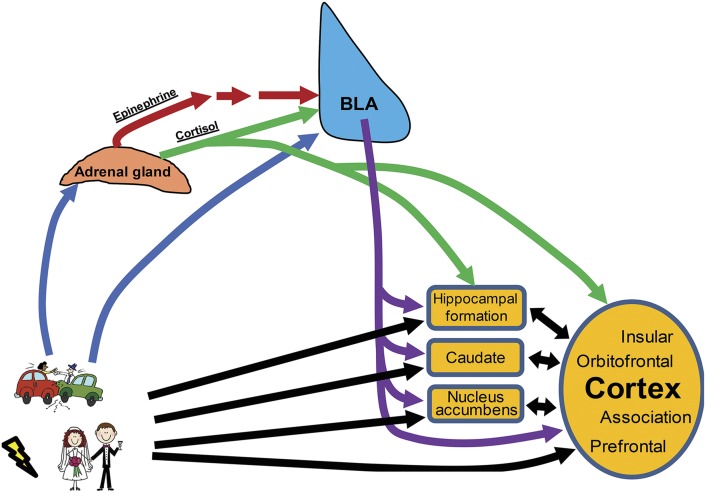
Fig. 2.
Schematic diagram illustrating how emotionally arousing events lead to enhanced memory consolidation. Events, regardless of their degree of emotional arousal, produce information (black arrows) that is processed in a number of different memory-related brain regions, depending on the type of learning, including the hippocampal formation, the caudate, the nucleus accumbens, and various cortical regions. However, emotional arousal also activates systems that influence how those memories are processed, specifically leading to enhanced memory consolidation compared with memories for emotionally neutral or mundane events. Such events, as shown in the lower-left corner, activate peripheral stress hormone systems, leading to a release of epinephrine and cortisol. Via indirect and direct routes, respectively, these hormones lead to activation of the BLA. In addition, emotionally arousing events may also activate the BLA through other means. The BLA, in turn, maintains widespread projections throughout the forebrain and, through these projections (purple arrows), modulates the memory processing in these other regions, enhancing the consolidation of such memories. In addition, as cortisol can cross the blood-brain barrier, it also directly influences memory consolidation in these other regions, although in a manner dependent on BLA activity.
Epinephrine does not freely cross the blood-brain barrier (Hardebo and Owman, 1980), so its effects on memory most likely involve peripheral mechanisms. Several findings suggest that the vagus nerve serves as a bridge between the peripheral stress response and amygdala norepinephrine activity. Peripheral epinephrine activates the vagus nerve (Miyashita and Williams, 2006), and stimulation of the vagus nerve enhances memory (Clark et al., 1995, 1998, 1999) and increases norepinephrine levels in the amygdala (Hassert et al., 2004). The vagus nerve projects to the nucleus of the solitary tract, which sends ascending projections to the central and medial nuclei of the amygdala both directly and through the bed nucleus of the stria terminalis (Ricardo and Koh, 1978). However, the noradrenergic response in the amygdala involves the afferent pathway from the nucleus of the solitary tract to the locus coeruleus via the nucleus paragigantocellularis (Ricardo and Koh, 1978; Ennis and Aston-Jones, 1988; Chiang and Aston-Jones, 1993; Garcia-Medina and Miranda, 2013). Like blockade of β-adrenergic receptors in the amygdala, inactivation of the nucleus of the solitary tract prevents the memory-enhancing effects of systemically administered epinephrine (Williams and McGaugh, 1993). Taken together, these results suggest that epinephrine influences memory consolidation by indirectly stimulating the release of norepinephrine in the BLA.
Unlike peripheral epinephrine, GCs (corticosterone in the rat; cortisol in the human) readily cross the blood-brain barrier and thus can directly influence many brain regions. Nonetheless, like epinephrine, GC effects on memory involve activation of the BLA. Selective lesions of the BLA or intra-BLA infusions of noradrenergic antagonists block the memory-enhancing effects of systemically administered GC agonists (Roozendaal and McGaugh, 1996; Roozendaal et al., 1996; Quirarte et al., 1997). Post-training BLA infusions of GC agonists and antagonists enhance and impair memory, respectively (Roozendaal and McGaugh, 1997b), and, as was found with epinephrine, concurrent BLA infusions of noradrenergic antagonists block GC agonist-induced enhancement (Roozendaal et al., 2002, 2006).
GC effects depend on cAMP signaling in the BLA. The finding that intra-BLA infusions of a synthetic cAMP analog reverse the memory-impairing effect of a GC antagonist indicates that, in the BLA, GCs influence memory through modulation of noradrenergic-cAMP interactions (Roozendaal et al., 2002). However, as GCs readily enter the brain, they influence activity in many brain regions. GCs enhance memory when administered directly into the hippocampus, dorsal striatum, or nucleus accumbens shortly after training (Roozendaal and McGaugh, 1997a; Quirarte et al., 2009; Wichmann et al., 2012), indicating that GCs can enhance memory through mechanisms other than a direct effect on noradrenergic-cAMP signaling within the BLA. It is important to note that the effects of administration in other brain regions are blocked by lesions of the BLA or noradrenergic antagonists infused into the BLA, suggesting that the BLA plays a permissive role and an interplay between the BLA and other memory systems is required for stress hormone–induced memory modulation.
Emotional modulation of consolidation involves other neurotransmitter systems in a noradrenergic receptor-dependent manner. Like norepinephrine, dopamine levels in the amygdala increase following stressors such as footshock and forced swimming (Coco et al., 1992; Bouchez et al., 2012). Post-training intra-BLA microinjections of dopamine enhance retention, whereas microinjections of either a D1 or D2 receptor antagonist impair retention (Lalumiere et al., 2004). Concurrent intra-BLA administration of β-adrenergic antagonists prevents the memory enhancement induced by dopamine infusions, consistent with the critical role of the β-adrenergic receptors in BLA modulation of memory consolidation. Evidence also suggests that the cholinergic system influences memory consolidation. Optical stimulation and inhibition of cholinergic terminals in the BLA, originating from the nucleus basalis magnocellularis, enhance and impair, respectively, retention of fear conditioning (Jiang et al., 2016). Blockade of muscarinic m1 and m2 cholinergic receptors impairs the consolidation of inhibitory avoidance learning (Power et al., 2003). Moreover, the ability of systemic dexamethasone administration to enhance memory consolidation depends on concurrent activation of muscarinic receptors in the BLA (Power et al., 2000). Other studies have demonstrated that opioid-peptide, GABAergic, and muscarinic cholinergic effects on memory depend on amygdala noradrenergic activation (McGaugh et al., 1988).
These findings provide compelling evidence that norepinephrine in the BLA has a central role in modulating memory consolidation (McIntyre et al., 2003). As stressors can produce a prolonged release of norepinephrine and dopamine in the BLA lasting over 2 hours (McIntyre et al., 2002; Bouchez et al., 2012), these catecholamine neurotransmitters most likely play a critical role in maintaining increased BLA activity and, particularly, increased activity in the BLA projection neurons (Zhang et al., 2013) following an emotionally arousing event that enables the modulation of memory consolidation. In support of this conclusion, in vivo microdialysis studies indicate that the amount of norepinephrine released following inhibitory avoidance training correlates strongly with the rat’s degree of memory retention 2 days later (McIntyre et al., 2002).
Recent work has examined how patterns of BLA activity influence memory consolidation. Findings of electrophysiological recording studies indicate that the coherence of BLA activity with other memory-related structures in the γ frequency range (35–45 Hz) increases across learning trials (Bauer et al., 2007; Popescu et al., 2009), although such studies have not determined whether the activity is causally involved in memory processes. Recent results suggest that optogenetic stimulation of BLA projection neurons, using bursts of 40 Hz light pulses given immediately after training, enhances retention for inhibitory avoidance learning (Huff et al., 2013). Similar stimulation using bursts of 20 Hz light pulses, however, does not alter retention, suggesting the possible importance of BLA activity in the frequency range at approximately 40 Hz following a learning event.
Further work addressing whether stimulating the BLA with bursts of activity in that frequency range drives or enhances the coherence of γ oscillatory activity between the amygdala and other structures will be necessary to demonstrate the function of such activity in the ability of the BLA to modulate consolidation through its effects on other regions. However, optogenetic approaches will undoubtedly advance our understanding of the amygdala modulation of memory process, as such approaches can provide temporally precise control of activity, spatially precise control of particular pathways, and genetically precise control of specific neuronal subpopulations within a heterogeneous structure. For example, recent optogenetic and electrophysiological recording studies indicate that reward-predictive versus aversion-predictive cues increase activity in BLA neurons that project to the nucleus accumbens versus the central amygdala, respectively (Beyeler et al., 2016). Thus, heterogeneity within the BLA, even simply differentiated by the projection targets of the neurons, most likely plays a critical role in how the BLA modulates memory consolidation.
Of particular interest in understanding how the BLA modulates memory consolidation is the question of how general the effects of such modulation are in terms of the types of memory. Although much of the research on BLA memory modulation has used inhibitory avoidance, a footshock-based contextual learning task, evidence suggests that the BLA influences the consolidation of long-term memories for many different types of learning. This includes the following: closely related types such as contextual fear conditioning (LaLumiere et al., 2003); other forms of memory that most likely involve significant emotional arousal, including spatial and cued water-maze learning (Packard et al., 1994); learning based on emotionally arousing interoceptive events such as conditioned taste aversion (Miranda et al., 2003; Guzman-Ramos and Bermudez-Rattoni, 2012); reward-based learning such as associations between stimuli and cocaine (Fuchs et al., 2006); and novel object and context recognition memory (Malin and McGaugh, 2006; Barsegyan et al., 2014; Beldjoud et al., 2015). Indeed, although much of the evidence on the amygdala in previous research has originated from studies using negatively valenced learning tasks, recent findings make it clear that the amygdala influences reward-based/appetitive processes, most likely through divergent projections. For example, optical stimulation of the BLA inputs to the nucleus accumbens reinforces instrumental behaviors (Stuber et al., 2011). Similarly, recent recording work suggests that distinct neuronal populations within the BLA code for reward-associated versus aversion-associated stimuli (Beyeler et al., 2016). Thus, manipulations, such as drug infusions into the BLA, would be expected to alter neural processing for both aversive and appetitive stimuli.
The final examples in the list above (i.e., novel object/context recognition) raise an important question of whether emotional arousal is necessary for the BLA to modulate memory consolidation, as novel object and context recognition would seem to lack any obvious emotional arousal. However, post-training systemic administration of corticosterone enhances consolidation only in rats that were not previously habituated to the environment (Roozendaal et al., 2006), suggesting that novelty itself may provide sufficient activation of emotional arousal systems for memory modulation to occur. Systemic administration of yohimbine, which stimulates norepinephrine release, restores the ability of corticosterone to enhance consolidation in rats previously habituated to the environment. Together, these results suggest that mimicking the effects of emotional arousal, through both noradrenergic and glucocorticoid activation, can modulate memory consolidation even for those types of learning in which emotional arousal has been minimized. Nonetheless, the difficulty in determining whether a learning task involves no emotional arousal makes it virtually impossible to definitively state whether the BLA influences memory consolidation for nonemotionally arousing learning.
V. Amygdala Interactions with Other Brain Regions
The BLA maintains major connections with a vast array of forebrain structures, including projections to memory-processing regions such as the hippocampal formation, striatum, and prefrontal cortex. Considerable evidence suggests that the BLA modulates memory consolidation through interactions with these other brain regions (McGaugh et al., 2002), although it should be noted that, with a few exceptions, most of these studies have relied on tasks with clear emotional components. In an early study examining recognition memory in monkeys, combined lesions of the hippocampus and the amygdala, but not lesions of either separately, produced profound deficits, suggesting that the two regions interacted during memory processing (Mishkin, 1978). The BLA receives inputs from the entorhinal cortex and ventral hippocampus and, similarly, projects to these subregions of the hippocampal formation (Pitkanen et al., 2000; Petrovich et al., 2001; Witter and Amaral, 2004). As the entorhinal cortex is a major processing center for information going to and coming from the dorsal hippocampus (Hargreaves et al., 2005; Knierim et al., 2006), the BLA is able to interact with all regions of the hippocampal formation, either directly or indirectly.
BLA lesions and β-adrenergic receptor blockade in the BLA prevent the memory-enhancing effects of microinjections of GC receptor agonists into the dorsal hippocampus (Roozendaal and McGaugh, 1997a; Roozendaal et al., 1999). Similarly, BLA inactivation prevents the enhancement of memory consolidation produced by histone deacetylase inhibition in the dorsal hippocampus (Blank et al., 2014). As noted earlier, recording studies suggest that BLA activity becomes increasingly coupled with activity in downstream brain regions, including the entorhinal cortex, during training, and, moreover, that the increased coupling is related to the degree of learning (Bauer et al., 2007; Popescu et al., 2009). As findings indicate that BLA inactivation prevents learning-induced changes in ventral hippocampal plasticity (Farmer and Thompson, 2012), the interactions between the BLA and the hippocampus that influence memory consolidation most likely involve BLA influences on plasticity within the hippocampus. This issue is discussed further below.
Other studies have investigated interactions between the BLA and different regions of the striatum during memory consolidation. Contralateral unilateral lesions of the BLA and nucleus accumbens block the memory enhancement produced by systemic GC administration (Setlow et al., 2000). Similarly, accumbens lesions prevent the memory enhancement produced by intra-BLA or intrahippocampal infusions of a GC receptor agonist (Roozendaal et al., 2001). Other work suggests that the modulation of memory consolidation by the BLA depends specifically on activation of dopamine receptors in the accumbens shell, as blockade of such receptors in the shell, but not the core, prevents the memory enhancement induced by intra-BLA microinjections of dopamine (LaLumiere et al., 2005).
Findings of several studies indicate that the amygdala interacts with the dorsal regions of the striatum, specifically the caudate nucleus, during memory consolidation. Amphetamine administration into the amygdala or the caudate enhances the consolidation of cued water-maze learning, whereas similar administration into the hippocampus has no effect on such cued learning, indicating an important circuit involving the amygdala and the caudate during the consolidation of this type of learning (Packard et al., 1994). Importantly, for the cued water-maze task, amygdala inactivation before the retention test does not prevent the enhancement produced by post-training amphetamine administration in the amygdala (Packard et al., 1994). This finding indicates that the amygdala is not the storage site for the memory, but, rather, that the amygdala is selectively involved in modulating memory consolidation. Other findings indicate that caudate inactivation prevents the memory-enhancing effects of post-training intra-amygdala microinjections of amphetamine for learning in the cued, but not the spatial, version of the water-maze task (Packard and Teather, 1998), providing key evidence that the amygdala modulates memory processing in multiple memory systems.
As highlighted in the findings of caudate-amygdala studies, considerable evidence suggests that the amygdala’s interactions with other brain regions are most likely dependent on the type of learning/information being processed. Indeed, the caudate results discussed above were based on a larger set of studies indicating that the amygdala modulates the consolidation for both hippocampus-dependent spatial learning and caudate-dependent cued or response learning. The amygdala’s ability to modulate memory consolidation appears to be highly promiscuous, as studies have demonstrated its role in doing so across a wide variety of learning tasks (Packard et al., 1994; Hatfield and McGaugh, 1999; LaLumiere et al., 2003; McGaugh, 2004; Barsegyan et al., 2014). This modulation, however, most likely involves the regulation of activity and plasticity in many different downstream brain regions playing more selective roles during memory consolidation (McGaugh, 2002).
Several studies have investigated this issue by using a modified form of inhibitory avoidance or contextual fear conditioning in which learning regarding the context is separated from the learning involving the footshock (Liang, 1999). Intra-BLA administration of the muscarinic cholinergic agonist oxotremorine enhances retention when given after either the context learning or the footshock learning (Malin and McGaugh, 2006), suggesting that the BLA modulates the consolidation for each component of learning. Moreover, unlike many of the tasks described above, the context learning component does not involve an obvious emotional component, although novelty itself may be emotionally arousing (Roozendaal et al., 2006). In contrast, oxotremorine administration into the dorsal hippocampus after context training, but not footshock training, enhances retention, whereas similar administration into the anterior cingulate cortex enhances retention when given after footshock, but not context, training (Malin and McGaugh, 2006). These findings provide evidence of the promiscuity of BLA in memory modulation and the more selective roles of other structures. Recent findings using optogenetic approaches suggest that this is likely due to specific populations of BLA neurons that project to distinct structures. Using the same modified contextual fear conditioning task noted above, Huff et al. (2016) found that postfootshock, but not postcontext, stimulation of the BLA inputs to the ventral hippocampus enhanced retention, again most effectively with bursts of 40 Hz stimulation. Thus, whereas the BLA as a whole modulates many different kinds of memories, separate subpopulations, as differentiated by their projection targets, most likely influence memory consolidation in more selective manners.
VI. Synaptic Plasticity
Pharmacological and systems neuroscience studies, including those described above, have informed our understanding of the brain regions, hormones, and neurotransmitters that participate in the consolidation of long-term memories. However, the substrate of memory, that is, the physical trace that persists as long as the memory itself, continues to elude the field. Since 1950, when Lashley (1950) reported his decades of work that failed to disrupt specific memories by making lesions in the rat cortex, generations of memory researchers have continued Lashley’s search for the engram. Some searches have been guided by specific molecules (Sacktor et al., 1993; Josselyn, 2010), whereas others have focused on processes (Song et al., 2000; Routtenberg, 2008). Most agree, though, that changes in synaptic strength play a role in the conversion of percepts to memories.
Bliss and Lomo (1973) discovered lasting changes in the postsynaptic response in the hippocampus following high-frequency stimulation of the perforant path. This long-term potentiation (LTP) of synaptic strength shares many characteristics with memory consolidation. For example, LTP induction does not require new protein synthesis, yet maintenance of the altered synaptic state does (Krug et al., 1984; Frey et al., 1988). Although LTP and memory share these and other qualities, definitive proof that learning induces LTP or that LTP is a basis of learning and memory is difficult to acquire.
Whitlock et al. (2006) reported results from a series of experiments designed to test the hypothesis that memory consolidation involves LTP-like mechanisms in the hippocampus. They trained rats on a single-trial inhibitory avoidance task, to limit the timeframe of synaptic consolidation, and targeted the dorsal hippocampus due to its established role in consolidation of the memory involved in this task. Using a multi-electrode recording array, the authors found small changes in field potentials after training. Additionally, they identified increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in synaptic fractions from the dorsal hippocampus in rats trained on the inhibitory avoidance task. As LTP induction alters AMPA receptor number and phosphorylation state, the increase in hippocampal AMPA receptors following inhibitory avoidance training provided indirect evidence that learning induced LTP. They also reported increased phosphorylation at the ser831 site of AMPA receptors following training, an indicator of LTP in those synapses. Moreover, they were unable to induce LTP in the synapses that were potentiated following inhibitory avoidance training. Occlusion of further LTP at these synapses suggests that the learning-induced potentiation saturated the mechanisms involved in electrically induced LTP.
These findings suggest that synaptic strength changes following experience and that this change in synapse function is involved in memory storage, yet plasticity is typically observed only in select synapses. Thus, considerable research has focused on identifying mechanisms underlying synapse-specific alterations. Steward and Levy (1982) discovered polyribosomes in dendritic spines in the dentate gyrus, suggesting the possibility of local translation in the dendrites, and Steward et al. (1998) later identified the mRNA for the immediate early gene Arc near postsynaptic densities. These findings suggested that plasticity-associated proteins could be translated near the synapse. Control of local translation would provide an elegant mechanism for addressing how synaptic alterations can be made in a timely fashion because it can be more rapid and efficient than somatic protein synthesis, which requires transport of a signal from the synapse to the nucleus and then transport of proteins from the soma back to the synapse. Moreover, local protein translation provides a straightforward mechanism for ensuring synapse-specific modifications. In confirmation of this hypothesis, Tang et al. (2002) found that mechanistic target of rapamycin (mTOR) kinase, which plays a critical role in the late phase of hippocampal LTP, regulates local translation of plasticity-related proteins.
Alternatively, synapse-specific changes can occur through a synaptic tagging process. In the synaptic tag-and-capture mechanism of synapse-specific plasticity, first proposed by Frey and Morris (1997), a synaptic tag, temporarily present in a weakly stimulated postsynaptic site, links that synapse to the plasticity-inducing proteins that are translated at a strongly stimulated synapse. This hypothesis provides an explanation for why weak stimulation of a synapse does not induce protein synthesis, whereas strong stimulation of a neighboring synapse is sufficient to produce new proteins that appear selectively in the two stimulated synapses, but not in nearby synapses that received no stimulation at all. Evidence for this mechanism comes from the finding that induction of short-lasting LTP in one pathway with a weak stimulus results in long-lasting LTP when it is paired with strong stimulation of a different synapse on the same neuron (Frey and Morris, 1997, 1998).
VII. Amygdala Modulation of Cellular Consolidation
These discoveries made in studies of synaptic plasticity have informed hypotheses about how the amygdala modulates memory storage through interactions with downstream brain regions. As noted earlier, considerable research has focused on the hippocampus for its interactions with the amygdala during memory processing. Early studies of the influence of the amygdala on cellular processing in the hippocampus revealed that BLA stimulation and lesions enhance and impair, respectively, perforant path stimulation-induced LTP in the dentate gyrus (Ikegaya et al., 1994; 1995). Stimulation of the lateral perforant path together with the medial perforant path produces a similar enhancement, suggesting that BLA influences on dentate gyrus plasticity involve the BLA–entorhinal cortex pathway (Nakao et al., 2004). Soon after the discovery that the BLA modulates the strength of perforant path–dentate gyrus synapses, Frey and colleagues (2001) reported that BLA stimulation converts the early phase of LTP to late-phase LTP and that late-phase LTP depends on protein kinase A activation (Huang et al., 1995; Abel et al., 1997), indicating a role for catecholamines at the site of cellular consolidation.
A. Basolateral Amygdala Influence on Expression of Plasticity-Related Proteins in the Hippocampus
The evidence that the BLA modulates hippocampal synaptic plasticity, as well as memories of various kinds, suggests that sensory input determines the specific synapses targeted for plasticity and that BLA actions influence consolidation of functional changes at those synapses (McIntyre et al., 2005; McReynolds and McIntyre, 2012). This hypothesis shares some characteristics with the emotional tagging hypothesis described by Richter-Levin and Akirav (2003). Both attempt to explain why most memories are lost almost instantly, whereas emotionally arousing memories frequently persist. According to the emotional tagging hypothesis, hippocampal activation by experience sets a local synaptic tag that emotional arousal reinforces, thus promoting cellular consolidation that supports hippocampus-dependent long-term memory. Research over the past 18 years provides evidence that the BLA influences the expression of plasticity-related proteins that promote synaptic consolidation in the hippocampus and in other areas of the brain implicated in memory processing.
The mRNA for the immediate early gene Arc is found specifically in dendritic regions that are near stimulated synapses (Steward et al., 1998; Dynes and Steward, 2012), and it can be translated to protein in synapses that are isolated from the soma (Yin et al., 2002; Waung et al., 2008). Administration of Arc antisense oligodeoxynucleotides (ODNs) into the dorsal hippocampus interferes with translation of Arc mRNA to protein and impairs memory and plasticity. Guzowski et al. (2000) found that intradorsal hippocampus infusions of Arc antisense ODNs impair the maintenance of LTP, without affecting induction, and impair long-term memory for a spatial water-maze task. Such infusions did not affect initial learning. These findings suggest that ARC protein is necessary for hippocampal synaptic plasticity that contributes to memory consolidation.
Based on this evidence, several studies have examined the influence of the BLA on hippocampal expression of ARC. Memory-enhancing infusions of the β-adrenergic receptor agonist clenbuterol into the BLA immediately after inhibitory avoidance training increase expression of ARC protein in the dorsal hippocampus, whereas post-training infusions of the sodium channel blocker lidocaine impair long-term memory and reduce ARC protein levels in the dorsal hippocampus (McIntyre et al., 2005). Infusions of ARC antisense ODNs into the dorsal hippocampus impair long-term memory (McIntyre et al., 2005), indicating a critical role for hippocampal ARC protein. The study found that changes in protein do not correlate with changes in mRNA levels. Taken together, these results suggest that the BLA modulates memory consolidation through actions on the synaptic plasticity-related protein ARC in the hippocampus. Moreover, the results indicated that inhibitory avoidance training increases hippocampal Arc mRNA, but that intra-BLA infusions of clenbuterol do not further increase Arc mRNA (McIntyre et al., 2005). Similarly, Ren et al. (2008) found that the anesthesia propofol decreases ARC expression in the hippocampus and that intra-BLA muscimol reverses the propofol effect on hippocampal ARC protein, but not mRNA, suggesting that the BLA exerts a post-transcriptional influence on hippocampal ARC expression. However, Huff et al. (2006) reported evidence indicating that the BLA also modulates Arc mRNA in the hippocampus. Nonetheless, detection methods for mRNA are generally more sensitive than those for protein, suggesting that reports of protein differences without accompanying mRNA differences indicate that the BLA influence on hippocampal ARC expression occurs, at least at times, via a post-transcriptional mechanism.
In view of the evidence that Arc mRNA is transported to stimulated regions of the dendrite where it can be locally translated and that the BLA can modulate ARC protein expression without affecting Arc mRNA, it is likely that Arc mRNA is transcribed following novel context-associated sensory input and driven specifically to postsynaptic regions of synapses that are active in response to the sensory input. Arc translation is necessary for consolidation of hippocampus-dependent memories, but not all novel contexts become long-lasting memories. According to this model, Arc mRNA is translated to protein when BLA actions reinforce the synapse. By extension, novel experiences prime hippocampal synapses for consolidation, but those experiences (and related synapses) are rapidly consolidated only if they are emotionally arousing. In support of this model, post-training administration of the stress hormone corticosterone enhances consolidation of inhibitory avoidance and increases ARC protein expression in synaptoneurosomes (synapse-enriched samples) taken from the dorsal hippocampus. Blockade of BLA β-adrenergic receptors attenuates the corticosterone effects on memory and hippocampal ARC expression (McReynolds et al., 2010). These findings suggest that dendritically localized synaptic plasticity-related mRNAs like Arc are markers of where synaptic plasticity is occurring and that BLA activation is a regulator of when it occurs. Na et al. (2016) recently found that glutamate induced rapid translation of ARC in hippocampal dendrites and that reactivation of stalled polyribosomes mediated this effect. Figure 3 provides a schematic diagram for how the BLA influences ARC expression in regions, such as the hippocampus, important for processing declarative memory information.
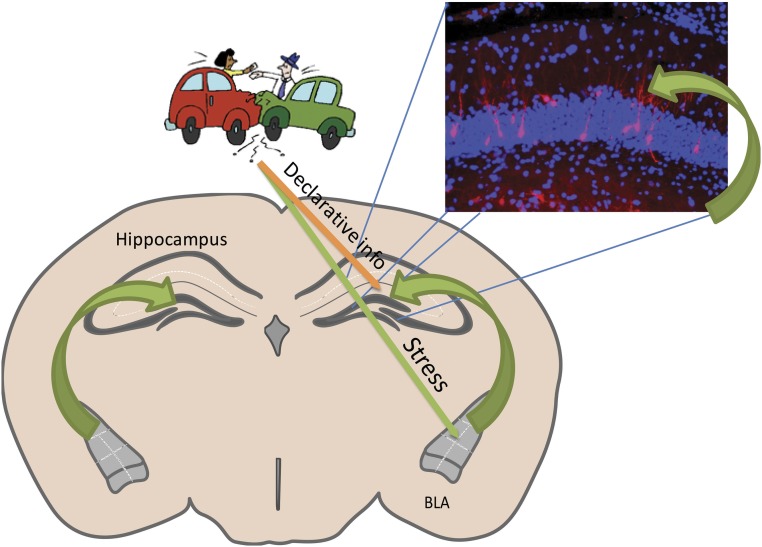
Fig. 3.
Schematic diagram illustrating a theoretical model of amygdala modulation of memory and synaptic plasticity in the hippocampus. Sensory and contextual stimuli activate specific synapses of the hippocampus (orange arrow), leading to the encoding of declarative or declarative-like memories. The activity-dependent immediate early gene Arc is transcribed and delivered to activated synapses, where it is degraded. In an event that is sufficiently emotionally arousing to be rapidly stored as a long-term memory, the coincident amygdala response to stress hormones directly or indirectly (green arrow) influences translation of Arc mRNA to protein in the recently activated synapse. Fluorescent immunohistochemistry image shows nuclei stained with 4′,6′-diamidino-2-phenylindole (blue) and ARC protein in neuronal cell bodies and dendrites (red) in hippocampal tissue.
B. Basolateral Amygdala Influence on Expression of Plasticity-Related Proteins in Other Brain Regions
Following these results, other studies examined whether the BLA exerts a similar influence on ARC expression in other regions involved in memory consolidation, including the prelimbic region of the medial prefrontal cortex (Roozendaal et al., 2009) and the rostral anterior cingulate (Malin et al., 2007). Post-training β-adrenergic receptor activation in the BLA increases synaptic expression of ARC protein in the medial prefrontal cortex (McReynolds et al., 2014) and the rostral anterior cingulate (Holloway-Erickson et al., 2012), and blockade of ARC protein expression in either area impairs memory consolidation (Holloway and McIntyre, 2011; McReynolds et al., 2014). Blockade of β-adrenergic receptors in the BLA attenuates the memory enhancement produced by post-training administration of corticosterone, but, in contrast to the BLA-mediated reduction in hippocampal ARC expression, such blockade in the BLA increases ARC expression in the prelimbic region of the medial prefrontal cortex (McReynolds et al., 2014). These findings suggest that the BLA is a regulator of ARC expression elsewhere in the brain. Although ARC expression in the medial prefrontal cortex is necessary for normal memory consolidation of inhibitory avoidance, additional expression does not benefit memory consolidation.
C. Mechanisms of Action of ARC
Although many reports have established a critical role for ARC in memory and synaptic plasticity (Guzowski et al., 2000; Plath et al., 2006; Shepherd et al., 2006; Bramham et al., 2008; Ploski et al., 2008; Korb and Finkbeiner, 2011; Shepherd and Bear, 2011), the precise function of ARC at the synapse that contributes to memory consolidation remains unknown (Steward et al., 2015). ARC contributes to actin polymerization (Messaoudi et al., 2007), suggesting a role in strengthening synapses through influences on cytoarchitecture. ARC is necessary for the maintenance of LTP (Guzowski et al., 2000), but ARC also promotes long-term depression by stimulating the endocytosis of AMPA receptors (Waung et al., 2008; Jakkamsetti et al., 2013). Thus, rather than playing a direct role in the strengthening of stimulated synapses, it is possible that ARC participates in enhancing the signal-to-noise ratio at the synapse (Okuno et al., 2012) or contributes to synaptic homeostasis (Turrigiano, 2007), allowing synapses to compensate for changes in neuronal excitability (Beique et al., 2011).
D. Going beyond ARC
It is unlikely that ARC is the only synaptic plasticity-associated protein that emotional arousal or amygdala activity influences. For example, memory-enhancing post-training intra-BLA infusions of clenbuterol increase CaMKIIα expression in the anterior cingulate (Holloway-Erickson et al., 2012). As with ARC, CaMKIIα mRNA is transported to dendrites, where it can be translated to protein (Mori et al., 2000; Lee et al., 2009), and dendritic targeting of CaMKIIα is necessary for normal hippocampal plasticity as well as spatial memory, fear conditioning, and object recognition memory (Miller et al., 2002). In contrast to ARC and CaMKIIα, memory-enhancing intra-BLA infusions of clenbuterol do not affect expression of the protein product of the immediate early gene c-Fos in the anterior cingulate or dorsal hippocampus (McIntyre et al., 2005; Holloway-Erickson et al., 2012). These findings indicate that the two immediate early genes (Arc and c-Fos) do not respond in the same manner to memory-enhancing BLA manipulations, but two mRNAs that are locally translated (Arc and CaMKIIα) do, providing additional evidence that the BLA influences the local translation of synaptic plasticity-related proteins. However, it is important to consider the possibility that these proteins may be regulated at the level of transport to the synapses, translation, or degradation (see McReynolds and McIntyre, 2012 for review).
Finally, although beyond the scope of the present review, it is important to note that strictly neuronal accounts of memory consolidation are incomplete, as considerable evidence in recent years has pointed to the influence of non-neuronal cells, especially glia-based systems, in mediating the effects of neurotransmitters and other chemicals on plasticity (Pearson-Leary et al., 2016). For example, glucose, which modulates memory consolidation, most likely does so at least in part via effects on astrocytic release of lactate, which then influences neuronal functioning (Gold, 2014). Recent work has also found that astrocytic β2-adrenergic receptors in the hippocampus are involved in the consolidation of contextual learning in a fear-conditioning task (Gao et al., 2016), suggesting the possibility that glia mediate some noradrenergic influences on memory consolidation.
VIII. Memory Modulation—Evidence from Human Studies
The evidence discussed above concerning the role of the amygdala and hormone systems in influencing memory consolidation was largely based on nonhuman animal studies. However, the potential for translational implications of these findings depends on whether these systems operate similarly in humans. Stimulation of the vagus nerve enhances memory in humans (Clark et al., 1999), providing clinical confirmation of this important component of the memory-modulatory system. Other studies, using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have provided considerable evidence indicating that amygdala activation influences the strength of memories of human subjects in a manner similar to that observed with nonhuman animals. In an early study, subjects viewed two different videos, consisting, respectively, of emotionally arousing film clips or emotionally neutral clips while undergoing PET imaging for glucose metabolism (Cahill et al., 1996) and were then tested for memory of the films 3 weeks later. The findings indicated that amygdala activity at the time of encoding significantly correlated with memory of the emotionally arousing clips. Additional evidence of a relationship between amygdala activation induced by exposure to visual stimuli and subsequent memory was obtained in studies using fMRI (Hamann et al., 1999; Canli et al., 2000).
Moreover, work using PET and fMRI approaches has reported that, during emotionally influenced learning, amygdala activity not only correlates with enhanced memory but also with activity in hippocampal regions (Kilpatrick and Cahill, 2003; Dolcos et al., 2004, 2005). Analyses of fMRI studies have revealed the existence of large-scale networks, in which distinct brain regions appear to show correlational changes in activity that may reveal such amygdala interactions with other regions with regard to mnemonic processes. Of particular interest, a network identified as the default mode network includes a variety of prefrontal regions that demonstrate functional connectivity with medial temporal lobe structures, including both the hippocampus and amygdala (Raichle et al., 2001). Coordinated activity among these structures during rest may be an important mechanism by which the amygdala influences the activity and plasticity in these other brain regions, thereby modulating the strength of memories (Hermans et al., 2014).
Considering the widespread reports suggesting sex differences in a variety of disorders that involve amygdala functioning (Kessler et al., 2003; Bangasser and Valentino, 2014; Hyde, 2014), it is worth considering evidence for sex differences in amygdala functioning in memory processes. Indeed, whereas amygdala activity appears to play the same role in both males and females in terms of influencing memory consolidation, imaging studies have suggested an interaction between sex and lateralization, with females having stronger activation of the left amygdala and males having stronger activation of the right amygdala (Canli et al., 2002; Cahill et al., 2004; Mackiewicz et al., 2006). In attempting to understand some of the sex differences with regard to amygdala, early research suggested that males have larger amygdala volumes than females, even corrected for overall brain size (Giedd et al., 1996; Brierley et al., 2002). However, a recent meta-analysis suggests that corrected amygdala volumes are the same for both sexes (Marwha et al., 2017). Nonetheless, even if there are no basic anatomic differences in the amygdala between men and women, sex differences in amygdala-based memory tasks may arise from differences in connectivity between the amygdala and other brain regions or, alternatively, from sex steroid–hormone interactions.
IX. Pharmacological Implications for Brain-Based Disorders
For most of us, it is difficult to remember information such as the state capitals without committing a great deal of time and attention to practice, whereas memories of personal tragedies seem to stay with us indefinitely, a paradox explained by the findings and conclusions described in the previous sections. An unfortunate outcome of this, however, is that it frequently requires effort to retain the memories we wish to keep, yet we cannot forget the memories of life’s most troubling moments even when such memories create significant dysfunction in our lives. Therefore, efforts have attempted to tap into the processes and mechanisms underlying memory consolidation to artificially drive and inhibit memory and synaptic plasticity. This work has led to potential pharmacotherapies for the treatment of anxiety disorders and post-traumatic stress disorder (PTSD), as well as new approaches to enhance memory and facilitate rehabilitation from stroke and traumatic brain injury.
A. Extinction and Exposure Therapy
Although antidepressant and anxiolytic drugs show some beneficial outcomes for the treatment of anxiety and PTSD, they treat the symptoms and not the cause(s) of these problems (Lee et al., 2016). Whether the core feature of the disorder is a troubling memory or an irrational belief, therapies can use new learning to overcome maladaptive thoughts and behaviors. Exposure-based therapies, in which patients are exposed to the fear- or anxiety-inducing stimuli, are considered the gold standard of treatment of PTSD and anxiety disorders because patients’ responses to the stimuli extinguish and/or the patients learn new, more adaptive associations or responses to triggers (Rauch et al., 2012). Unfortunately, and especially in the most clinically significant manifestations of these disorders, such treatments do not always provide long-lasting benefit, as strong traumatic memories often overcome the new learning as time passes or reminders arise (Boschen et al., 2009; Vervliet et al., 2013).
B. Reconsolidation
Ideally, therapies would target problematic memories for permanent erasure or alteration, and, in fact, some research has attempted to use mechanisms of memory consolidation to interfere with specific traumatic memories. These attempts largely stem from findings in rodents indicating that post-training intra-BLA infusions of the protein synthesis inhibitor anisomycin impair consolidation of a conditioned fear memory (Schafe and LeDoux, 2000) and that similar infusions given after a fear-conditioning retrieval session (i.e., following a brief exposure to the conditioned stimulus) in the absence of reinforcement also appear to impair the original fear-conditioning memory, even when tested 1 or 14 days later (Nader et al., 2000). The authors proposed that retrieval makes memories (and their neural substrates) labile and susceptible to disruption. Just as the initial consolidation of long-term memory requires protein synthesis, the reconsolidation of memories requires protein synthesis when memories are retrieved (Nader et al., 2000).
This finding led to a great deal of enthusiasm because it suggested that a disabling memory could be weakened or even eliminated altogether with a simple retrieval followed by a memory-impairing treatment. Because humans would have physical difficulty tolerating the systemic administration of an anisomycin dose sufficient to block protein synthesis in the amygdala, research has focused on developing other approaches. One procedure involves a combination of a reactivation session with extinction training. Specifically, the person undergoes a brief retrieval trial followed by extinction training. Using this procedure with fear conditioning, previous work has found an attenuated fear response in rats (Monfils et al., 2009) and humans (Schiller et al., 2010), and the fear response did not return in humans 1 year later. Although potentially promising, these findings came from healthy humans who were not susceptible to the relapse of fear that is seen in pathologic conditions such as PTSD, and there may be other potential limitations to the application of reconsolidation findings, noted below. Moreover, from a mechanistic level, it is curious that a combination of extinction and reconsolidation procedures, which would normally be expected to produce opposing effects on memory, could work well together to produce a beneficial effect.
C. Norepinephrine
Evidence described in earlier sections suggests that β-adrenergic receptor activation after a learning event is a critical component for the emotional enhancement of such memories. Based on this idea and in an attempt to prevent the development of PTSD, Pitman et al. (2002) identified individuals who entered the emergency room after suffering acute psychologic traumas and were thus at risk for developing PTSD and administered the β-adrenergic antagonist propranolol to the patients. One month later, clinician-administered PTSD scale scores were lower in propranolol-treated patients than in placebo-treated control subjects. Furthermore, 6 of 14 placebo control subjects showed physiologic responses to imagery of the traumatic event, whereas none of the propranolol-treated group responded (Pitman et al., 2002). These findings suggest that interfering with noradrenergic signaling immediately following a traumatic event reduces or even prevents the development of PTSD.
However, it is not always possible to intervene in the time between the trauma and consolidation of the memory. Furthermore, only a small portion of trauma sufferers develops PTSD, and it would, from a treatment perspective, be better to treat only those who go on to develop PTSD. Therefore, Pitman et al. (2002) designed a study based on reconsolidation findings indicating that the same systems that are involved in initial consolidation are required for the reconsolidation of the memory. In a study of patients diagnosed with PTSD, they found that propranolol administration after retrieval of the traumatic event reduced physiologic responses to imagery related to the traumatic event 1 week later (Brunet et al., 2008). However, in a later study, the same group repeated the experiment, adding a control group that was given propranolol without reactivation of the memory. In this follow-up study, the group failed to replicate their earlier findings (Wood et al., 2015). Reactivity to traumatic mental imagery was slightly higher in the reactivation plus propranolol-treated group in the follow-up study, which included only men, whereas the first study was carried out in men and women. Another difference was that propranolol was administered postreactivation in the first study and before memory reactivation in the follow-up study. Furthermore, reactivity fell below PTSD levels in the propranolol-alone control group, suggesting that propranolol may have had a lasting, nonspecific effect on physiologic responding. The authors concluded that clinical applications based on the animal reconsolidation literature are not likely to be straightforward.
Another potential approach based on the importance of noradrenergic signaling in consolidation has been combining extinction-based therapy with drugs targeting the noradrenergic system. Just as post-training administration of norepinephrine enhances consolidation of conditioned fear, intra-BLA infusions of norepinephrine immediately after extinction training enhance the consolidation of extinction (Berlau and McGaugh, 2006). Extinction procedures in rodents have provided the basis for exposure therapy in patients, as described earlier. As a result, clinicians have examined the effect of the α-adrenergic antagonist yohimbine on extinction in humans with social anxiety disorder and claustrophobia (Powers et al., 2009; Smits et al., 2014). Yohimbine acts presynaptically to stimulate norepinephrine release and, therefore, would be expected to have memory-strengthening effects for any recent learning, such as extinction learning. Consistent with animal literature, yohimbine improves exposure therapy outcomes (Powers et al., 2009; Smits et al., 2014). The extinction-enhancing benefits of noradrenergic receptor activation extend to cocaine-seeking behavior, as activation and blockade of such receptors in the ventral medial prefrontal cortex enhance and impair, respectively, the extinction of cocaine seeking in rats (LaLumiere et al., 2010), suggesting the potentially broad-ranging utility of targeting the noradrenergic system to alter memories for therapeutic benefit.
One challenge in translating findings from animal research to mental health benefits in humans is the large difference in conditions between controlled laboratory studies in animals versus clinical treatment of human psychopathologies. In particular, it should be noted that the effects of manipulations on reconsolidation in the laboratory, and especially in rodent studies, are typically observed under specific, highly controlled conditions that may be impossible to control in human patients. Indeed, even in the laboratory, there appear to be boundary conditions that determine whether manipulations can alter reconsolidation, including the age of the memory, the predictability of the reactivation stimulus, training intensity, and whether a memory is reactivated directly or indirectly (Nader and Einarsson, 2010). Older memories of intense and complicated traumas may not be as susceptible to reconsolidation blockade as are the simple, recent, and well-controlled memories made in the laboratory.
Additionally, because much of the clinical research is, for good reason, targeted at patients with PTSD and similar disorders, it is important to consider that the prior stressors these patients have endured have most likely altered their neurobiology in fundamental ways (Aubry et al., 2016). Indeed, evidence from the animal literature suggests that chronic stress sensitizes noradrenergic responses to later acute stress and alters hypothalamic-pituitary-adrenal axis functioning (Nisenbaum et al., 1991; Herman, 2013). Similarly, previous findings indicate that prior chronic stress alters the consolidation of long-term memories for later learning as well as alters plasticity mechanisms in relevant brain regions, including the BLA (Vyas et al., 2002; Monsey et al., 2014; Suvrathan et al., 2013). Thus, differences between animal and human clinical findings may also result from such issues, and assumptions that patients with PTSD will respond to treatment paradigms developed in animal models lacking the chronic stress component should be treated cautiously.
D. Glucocorticoids
As discussed earlier, emotionally arousing situations induce release of GCs that both help to mobilize energy resources and modulate the consolidation of memories for the situation that may be important for survival. However, administration of a dose of corticosterone that enhances memory consolidation impairs the retrieval of a spatial memory in rats (de Quervain et al., 1998). Similarly, acute administration of cortisone in humans impairs retrieval of a long-term declarative memory (de Quervain et al., 2000). These findings offer interesting mechanistic repercussions for understanding the relationship between consolidation and retrieval processes, and they also have potential clinical implications, including the possibility that GCs could provide benefits to patients suffering from anxiety disorders by impairing the retrieval of aversive memories while simultaneously enhancing consolidation of the extinction memory (de Quervain et al., 2009). In support of this hypothesis, patients given hydrocortisone after cardiac surgery, an event known to produce PTSD in some patients, demonstrate resistance to the development of PTSD symptoms (Schelling et al., 2004; Weis et al., 2006). Similarly, cortisol administration to patients during exposure to reminders of phobic fears, such as photos of spiders, enhances extinction of the conditioned fear (Soravia et al., 2006).
Individual differences in endogenous cortisol signaling are associated with PTSD risk. For example, the cortisol response to dexamethasone is suppressed in PTSD patients (Yehuda et al., 2004), and results of a recent epigenetic study indicate that methylation of the GC receptor gene NR3C1 is associated with reduced risk of PTSD in survivors of the Rwandan genocide (Vukojevic et al., 2014). In addition to enhancing the consolidation of the new learning during exposure therapy while suppressing retrieval of the traumatic memory, GCs signal the hypothalamus-pituitary-adrenal axis to terminate the stress response (Yehuda and LeDoux, 2007). Taken together, these results suggest that short-term administration of GCs, specifically during exposure therapy, may be a relatively safe and effective approach to the treatment of PTSD.
E. Endocannabinoids
Preclinical findings indicate that endocannabinoid signaling in the BLA, medial prefrontal cortex, and hippocampus is critically involved in the consolidation of emotionally arousing memories (Campolongo et al., 2009; Morena et al., 2014). Further evidence suggests that endocannabinoids are involved in the consolidation of the extinction of conditioned fear (Marsicano et al., 2002). Studies of human patients further indicate that endocannabinoids play a role in PTSD pathology. A single-nucleotide polymorphism of fatty acid amide hydrolase (FAAH), an enzyme that breaks down endocannabinoids, is significantly associated with PTSD diagnosis in Vietnam war veterans (Pardini et al., 2012), and another common human mutation in the FAAH gene increases endocannabinoid levels and enhances extinction learning in mice (Dincheva et al., 2015), suggesting that pharmacological inhibitors of FAAH could be used to enhance the effects of exposure therapy. Likewise, administration of a synthetic cannabinoid to human volunteers enhances the extinction of conditioned fear (Rabinak et al., 2013). In a study conducted 4–6 years after the attacks on the World Trade Center, circulating levels of endocannabinoids were significantly reduced in individuals who developed PTSD versus individuals who never met the criteria for PTSD (Hill et al., 2013). A recent study found that acute stress led to GC-mediated retrograde endocannabinoid release in the amygdala, and this endocannabinoid action regulates GABA release in the BLA (Di et al., 2016), suggesting a mechanism by which endocannabinoids produce memory-modulating effects. Although research demonstrating a role for endocannabinoids in the extinction of fear and PTSD is relatively new, a great deal of work is now dedicated to exploring the neural mechanisms of endocannabinoids as modulators of traumatic memories.
F. N-Methyl-D-Aspartate and α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptors
Research investigating the use of the partial N-methyl-D-aspartate (NMDA) receptor agonist D-cycloserine (DCS) as an adjunct to exposure therapy has played a major role in translation of psychopharmacology studies in rats to clinical treatments for anxiety in humans. Glutamate actions at the NMDA receptor are critical for most forms of plasticity and memory, and, indeed, NMDA antagonists impair extinction of conditioned fear (Falls et al., 1992; Burgos-Robles et al., 2007). However, increased NMDA activity can produce cellular excitotoxicity, making the NMDA receptor a target for modulation, rather than direct and persistent activation. In the laboratory of Michael Davis, Walker et al. (2002) found that administration of DCS, a drug that is effective in treatment of tuberculosis, enhanced the consolidation of extinction of fear-potentiated startle in rats. In addition to its antibiotic effects, DCS positively modulates the NMDA receptor through actions at the L-glycine site on the GluN1 subunit. Soon after the discovery of extinction enhancement in rats, Ressler et al. (2004) reported significantly improved outcomes in a study of acrophobia symptoms following DCS pairing with exposure therapy. The proposal that DCS and other cognitive enhancers could be used to improve extinction of fear in humans who suffer from anxiety disorders has profoundly influenced learning and memory research (Singewald et al., 2015; Maren and Holmes, 2016). Moreover, several other groups found that DCS administration enhances the effectiveness of exposure therapy in patients with social anxiety disorder (Hofmann et al., 2006), obsessive-compulsive disorder (Kushner et al., 2007), and panic disorder (Otto et al., 2010).
However, studies of DCS effects on exposure therapy outcomes in the treatment of PTSD have provided mixed results (Litz et al., 2012; Rothbaum et al., 2014; Bowers and Ressler, 2015). One possible explanation for the failure of DCS is that its cognitive-enhancing properties may serve to reinforce the negative association of reminders of the trauma with the fear response during unsuccessful exposure therapy sessions. Because the effectiveness of DCS in treatment of anxiety disorders appears to be related to the acute effectiveness of the exposure session prior to which the DCS was administered (Smits et al., 2013b), current studies are examining the specific variables associated with DCS and therapy that will be predictive of successful outcomes (Smits et al., 2013a; de Kleine et al., 2014). Post-training DCS following only successful sessions may be the optimal approach to using DCS to augment the effects of exposure therapy. Another limitation of DCS is that it cannot be given chronically due to rapid tolerance (Quartermain et al., 1994; Singewald et al., 2015). Difficult-to-treat disorders such as PTSD may not be well suited for DCS augmentation of exposure therapy due to the requirement of repeated exposure therapy sessions. Despite the limitations of DCS as an adjunct to exposure therapy in the treatment of PTSD, research investigating cognitive enhancers as adjuncts to exposure therapy owes its start to the discovery and translation of DCS enhancement of extinction of conditioned fear in rats.
AMPA receptors are responsible for much of the postsynaptic current associated with fast excitatory transmission in the brain, making them a natural target for pharmacotherapies and cognitive enhancement. As with NMDA receptors, direct activation of the AMPA receptors for glutamate carries significant risk of excitoxicity, and, consequently, fewer translational studies have focused on these receptors with regard to memory. In recent years, however, the development of a drug class, often referred to as ampakines, that allosterically modulates activity at these receptors has provided a potential route for targeting these receptors. They may provide benefits through two mechanisms. First, by positively modulating AMPA receptor activity, these compounds may act as acute cognitive enhancers (Lynch et al., 2011). Second, by increasing the postsynaptic response, these compounds can enhance postsynaptic plasticity mechanisms and thus enhance memory-based mechanisms for long-lasting therapeutic improvement (Lynch et al., 2008).
For example, research using the allosteric AMPA receptor potentiator 4-[2-(phenylsulphonylamino)ethylthio]-2,6-difluorophenoxyacetamide (PEPA) has found that acute administration of this drug enhances performance during a water-maze task in rats with ischemia-induced memory deficits and selectively enhances the extinction of contextual fear conditioning without affecting the initial acquisition of the fear conditioning (Sekiguchi et al., 1997; Zushida et al., 2007). The latter finding appears to be due to a preferential effect of PEPA on the medial prefrontal cortex, known to be involved in extinction learning for fear conditioning, rather than the BLA or hippocampus. Indeed, PEPA administration into the ventral medial prefrontal cortex enhances the consolidation of extinction learning for cocaine-seeking behavior as well and acutely inhibits cocaine seeking in rats (LaLumiere et al., 2010, 2012), suggesting the potential for its use in drug addiction. Although ampakines carry potential, the clinical research on ampakine compounds has reported mixed effects (Ingvar et al., 1997; Wezenberg et al., 2007; Fond et al., 2015), and the amount of attention devoted to this class of drugs has been relatively low. Moreover, clinical research has frequently used acute drug administration to look at the drugs’ immediate effects on performance rather than to consider how they may be used to enhance the long-term consolidation of memories in a therapeutic manner.
G. Vagus Nerve Stimulation
Although epinephrine does not cross the blood-brain barrier, post-training administration of epinephrine enhances memory consolidation in both rats (Gold and Van Buskirk, 1975) and humans (Cahill and Alkire, 2003), an effect most likely mediated by the vagus nerve. Indeed, the vagus nerve is responsive to exogenously administered epinephrine (Miyashita and Williams, 2006), and electrical stimulation of the vagus nerve (VNS) increases norepinephrine levels in the amygdala (Hassert et al., 2004) and enhances memory in rats (Clark et al., 1995, 1998) and humans (Clark et al., 1999). Because surgeons can, with relative ease, access the vagus nerve, these findings suggest a possible therapeutic use for controlling the vagus nerve in the alteration of memory consolidation.
In 1997, the Food and Drug Administration approved the use of chronic VNS as a seizure prevention therapy in humans, enabling investigation of other uses, including influencing memory-related processes and plasticity. Recent research has worked to use control over the vagus nerve to promote brain plasticity by precisely timing VNS with training and rehabilitation (Hays, 2016). Work in rats suggests that pairing VNS with auditory tones remodels the auditory cortex and eliminates the behavioral correlate of chronic tinnitus (Engineer et al., 2011), and that pairing VNS with exposure to conditioned cues enhances the extinction of the conditioned fear response (Peña et al., 2013). Thus, by tapping into the system that evolved to signal the brain to store memories for emotionally arousing events, clinical treatments using VNS may ameliorate neurologic symptoms and problems resulting from stroke, brain injury, and even PTSD. Indeed, evidence indicates that precisely pairing VNS with skilled reaching and strength training during recovery from stroke or traumatic brain injury significantly improves performance on these skills (Khodaparast et al., 2013; Pruitt et al., 2016). Together, the animal and human research findings are the basis for clinical trials currently in progress investigating the efficacy of VNS pairing with rehabilitation in stroke and tinnitus patients.
X. Conclusion
The present review has brought together several areas of research, just as those lines of research have themselves come together in recent years. In particular, the early studies suggesting that physiological processes modulate memory consolidation, combined with those studies focusing on activity-dependent synaptic plasticity, led to current work attempting to understand the role of the amygdala in modulating synaptic plasticity and to the identification of treatment targets for a variety of disorders. Overall, the findings discussed in this work indicate that a major mechanism influencing memory consolidation is emotional arousal, which activates the BLA, leading to sustained influences on activity and plasticity in a variety of brain regions downstream of the BLA. In particular, the evidence presented in this review strongly points to the critical role of the noradrenergic system in the BLA and other regions for influencing memory consolidation and plasticity. Current and future work will undoubtedly focus on the synaptic mechanisms altered by emotional arousal and BLA activity. Moreover, the emergence of new techniques for addressing circuit-level questions will provide additional and better methods to investigate the influences of the BLA on synaptic plasticity in specific downstream regions.
These decades of work as well as the ongoing work in the field have made enormous strides in our understanding of these memory systems, enabling the development of potential therapies for memory- and plasticity-based disorders. Each of the treatment approaches discussed in this work has taken advantage of different advances and aspects of the research, leading to multiple promising therapeutic avenues. In particular, these treatment approaches use classic pharmacotherapy with innovative behavioral paradigms, in which the treatment is given at a specific time within the therapy and under specific limited conditions. This approach may be a necessary and critical component to successful treatment, as nonspecific exogenous administration of different drugs or compounds has the potential to produce a variety of both nonspecific drug effects and unwanted memory effects (see Fig. 1B). Therefore, identifying the appropriate cognitive-behavioral treatment paradigm will be just as important as identifying the appropriate pharmacological treatments in developing effective combinations in the successful efforts to ameliorate a variety of disorders.
Abbreviations
- ACTH
adrenocorticotrophic hormone
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BLA
basolateral amygdala
- DCS
D-cycloserine
- FAAH
fatty acid amide hydrolase
- fMRI
functional magnetic resonance imaging
- GC
glucocorticoid
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- ODN
oligodeoxynucleotide
- PEPA
4-[2-(phenylsulphonylamino)ethylthio]-2,6-difluorophenoxyacetamide
- PET
positron emission tomography
- PTSD
post-traumatic stress disorder
- VNS
vagus nerve stimulation
Authorship Contributions
Wrote or contributed to the writing of the manuscript: LaLumiere, McGaugh, McIntyre.
Footnotes
This work was supported by the National Institutes of Health [Grants MH104384 (to R.T.L. and C.K.M.), DA034684 (to R.T.L.), DA037216 (to R.T.L.), MH105014 (to C.K.M.), and MH099655 (to C.K.M.)].
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. (1997) Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88:615–626. [DOI] [PubMed] [Google Scholar]
- Agranoff BW, Davis RE, Brink JJ. (1965) Memory fixation in the goldfish. Proc Natl Acad Sci USA 54:788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranoff BW, Klinger PD. (1964) Puromycin effect on memory fixation in the goldfish. Science 146:952–953. [DOI] [PubMed] [Google Scholar]
- Aubry AV, Serrano PA, Burghardt NS. (2016) Molecular mechanisms of stress-induced increases in fear memory consolidation within the amygdala. Front Behav Neurosci 10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. (2014) Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35:303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, McGaugh JL, Roozendaal B. (2014) Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Front Behav Neurosci 8:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Paz R, Paré D. (2007) Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci 27:9369–9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, Na Y, Kuhl D, Worley PF, Huganir RL. (2011) Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci USA 108:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldjoud H, Barsegyan A, Roozendaal B. (2015) Noradrenergic activation of the basolateral amygdala enhances object recognition memory and induces chromatin remodeling in the insular cortex. Front Behav Neurosci 9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. (2006) Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 86:123–132. [DOI] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, Tye KM. (2016) Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron 90:348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Dornelles AS, Werenicz A, Velho LA, Pinto DF, Fedi AC, Schröder N, Roesler R. (2014) Basolateral amygdala activity is required for enhancement of memory consolidation produced by histone deacetylase inhibition in the hippocampus. Neurobiol Learn Mem 111:1–8. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen MJ, Neumann DL, Waters AM. (2009) Relapse of successfully treated anxiety and fear: theoretical issues and recommendations for clinical practice. Aust N Z J Psychiatry 43:89–100. [DOI] [PubMed] [Google Scholar]
- Bouchez G, Millan MJ, Rivet JM, Billiras R, Boulanger R, Gobert A. (2012) Quantification of extracellular levels of corticosterone in the basolateral amygdaloid complex of freely-moving rats: a dialysis study of circadian variation and stress-induced modulation. Brain Res 1452:47–60. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. (2015) An overview of translationally informed treatments for posttraumatic stress disorder: animal models of Pavlovian fear conditioning to human clinical trials. Biol Psychiatry 78:E15–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. (2008) The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci 28:11760–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley B, Shaw P, David AS. (2002) The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev 39:84–105. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. (2008) Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 42:503–506. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. (2007) Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53:871–880. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. (2003) Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem 79:194–198. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. (1996) Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA 93:8016–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. (2004) Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn Mem 11:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. (2009) Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA 106:4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Gold PE. (2007) Different temporal profiles of amnesia after intra-hippocampus and intra-amygdala infusions of anisomycin. Behav Neurosci 121:732–741. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. (2002) Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA 99:10789–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. (2000) Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20:RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G. (1993) Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the rostral ventral medulla. Neuroscience 53:705–715. [DOI] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA. (1995) Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem 63:213–216. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. (1999) Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2:94–98. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. (1998) Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem 70:364–373. [DOI] [PubMed] [Google Scholar]
- Coco ML, Kuhn CM, Ely TD, Kilts CD. (1992) Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: attenuation by diazepam. Brain Res 590:39–47. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. (1984) Protein synthesis and memory: a review. Psychol Bull 96:518–559. [PubMed] [Google Scholar]
- de Kleine RA, Hendriks GJ, Smits JA, Broekman TG, van Minnen A. (2014) Prescriptive variables for d-cycloserine augmentation of exposure therapy for posttraumatic stress disorder. J Psychiatr Res 48:40–46. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. (2009) Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol 30:358–370. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. (1998) Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394:787–790. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. (2000) Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci 3:313–314. [DOI] [PubMed] [Google Scholar]
- Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW, Tasker JG. (2016) Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J Neurosci 36:8461–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, et al. (2015) FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 6:6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. (2004) Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42:855–863. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. (2005) Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA 102:2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CP. (1949) The retroactive effect of electroshock on learning. J Comp Physiol Psychol 42:32–44. [DOI] [PubMed] [Google Scholar]
- Dynes JL, Steward O. (2012) Arc mRNA docks precisely at the base of individual dendritic spines indicating the existence of a specialized microdomain for synapse-specific mRNA translation. J Comp Neurol 520:3105–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. (2011) Reversing pathological neural activity using targeted plasticity. Nature 470:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. (1988) Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci 8:3644–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. (1992) Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer GE, Thompson LT. (2012) Learning-dependent plasticity of hippocampal CA1 pyramidal neuron postburst afterhyperpolarizations and increased excitability after inhibitory avoidance learning depend upon basolateral amygdala inputs. Hippocampus 22:1703–1719. [DOI] [PubMed] [Google Scholar]
- Flexner JB, Flexner LB, Stellar E. (1963) Memory in mice as affected by intracerebral puromycin. Science 141:57–59. [DOI] [PubMed] [Google Scholar]
- Flexner LB, Goodman RH. (1975) Studies on memory: inhibitors of protein synthesis also inhibit catecholamine synthesis. Proc Natl Acad Sci USA 72:4660–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Vidal D, Bennett EL, Orme AE, Vasquez S, Jarvik ME. (1978) Memory facilitating and anti-amnesic effects of corticosteroids. Pharmacol Biochem Behav 8:81–87. [DOI] [PubMed] [Google Scholar]
- Fond G, Micoulaud-Franchi JA, Brunel L, Macgregor A, Miot S, Lopez R, Richieri R, Abbar M, Lancon C, Repantis D. (2015) Innovative mechanisms of action for pharmaceutical cognitive enhancement: a systematic review. Psychiatry Res 229:12–20. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. (2001) Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci 21:3697–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. (1988) Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res 452:57–65. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. (1997) Synaptic tagging and long-term potentiation. Nature 385:533–536. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. (1998) Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci 21:181–188. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. (2006) The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci 23:2809–2813. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. (1977) Memory formation: evidence for a specific neurochemical system in the amygdala. Science 198:423–425. [DOI] [PubMed] [Google Scholar]
- Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM. (2016) Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc Natl Acad Sci USA 113:8526–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Medina NE, Miranda MI. (2013) Nucleus of the solitary tract chemical stimulation induces extracellular norepinephrine release in the lateral and basolateral amygdala. Brain Stimulat 6:198–201. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. (1996) Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol 366:223–230. [DOI] [PubMed] [Google Scholar]
- Gold PE. (2006) The many faces of amnesia. Learn Mem 13:506–514. [DOI] [PubMed] [Google Scholar]
- Gold PE. (2014) Regulation of memory: from the adrenal medulla to liver to astrocytes to neurons. Brain Res Bull 105:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. (1975) Memory interference and facilitation with posttrial amygdala stimulation: effect on memory varies with footshock level. Brain Res 86:509–513. [DOI] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk R. (1976) Enhancement and impairment of memory processes with post-trial injections of adrenocorticotrophic hormone. Behav Biol 16:387–400. [DOI] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk R. (1978) Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav Biol 23:509–520. [DOI] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB. (1975) Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13:145–153. [DOI] [PubMed] [Google Scholar]
- Guzman-Ramos K, Bermudez-Rattoni F. (2012) Interplay of amygdala and insular cortex during and after associative taste aversion memory formation. Rev Neurosci 23:463–471. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. (2000) Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20:3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. (1999) Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci 2:289–293. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Owman C. (1980) Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann Neurol 8:1–31. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. (2005) Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308:1792–1794. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. (2004) The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci 118:79–88. [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. (1999) Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem 71:232–239. [DOI] [PubMed] [Google Scholar]
- Hays SA. (2016) Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics 13:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. (1949) The Organization of Behavior: a Neuropsychological Theory, Wiley, New York. [Google Scholar]
- Herman JP. (2013) Neural control of chronic stress adaptation. Front Behav Neurosci 7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Battaglia FP, Atsak P, de Voogd LD, Fernández G, Roozendaal B. (2014) How the amygdala affects emotional memory by altering brain network properties. Neurobiol Learn Mem 112:2–16. [DOI] [PubMed] [Google Scholar]
- Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, Hillard CJ, Yehuda R. (2013) Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38:2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. (2006) Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 63:298–304. [DOI] [PubMed] [Google Scholar]
- Holloway CM, McIntyre CK. (2011) Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiol Learn Mem 95:425–432. [DOI] [PubMed] [Google Scholar]
- Holloway-Erickson CM, McReynolds JR, McIntyre CK. (2012) Memory-enhancing intra-basolateral amygdala infusions of clenbuterol increase Arc and CaMKIIα protein expression in the rostral anterior cingulate cortex. Front Behav Neurosci 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R. (1995) A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell 83:1211–1222. [DOI] [PubMed] [Google Scholar]
- Huff ML, Emmons EB, Narayanan NS, LaLumiere RT. (2016) Basolateral amygdala projections to ventral hippocampus modulate the consolidation of footshock, but not contextual, learning in rats. Learn Mem 23:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Miller RL, Deisseroth K, Moorman DE, and Lalumiere RT (2013) Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc Natl Acad Sci USA 110:3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. (2006) Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci 26:1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS. (2014) Gender similarities and differences. Annu Rev Psychol 65:373–398. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. (1994) Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Res 656:157–164. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. (1995) Requirement of basolateral amygdala neuron activity for the induction of long-term potentiation in the dentate gyrus in vivo. Brain Res 671:351–354. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. (1997) Enhancement by an ampakine of memory encoding in humans. Exp Neurol 146:553–559. [DOI] [PubMed] [Google Scholar]
- Jakkamsetti V, Tsai NP, Gross C, Molinaro G, Collins KA, Nicoletti F, Wang KH, Osten P, Bassell GJ, Gibson JR, et al. (2013) Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron 80:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, López-Hernández GY, Ballinger EC, Wang S, Talmage DA, Role LW. (2016) Cholinergic signaling controls conditioned fear behaviors and enhances plasticity of cortical-amygdala circuits. Neuron 90:1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA. (2010) Continuing the search for the engram: examining the mechanism of fear memories. J Psychiatry Neurosci 35:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey Replication (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL, 2nd, Kilgard MP. (2013) Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 60:80–88. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. (2003) Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage 20:2091–2099. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. (1937) “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. Am J Physiol 119:352–353. [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. (2006) Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus 16:755–764. [DOI] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S. (2011) Arc in synaptic plasticity: from gene to behavior. Trends Neurosci 34:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M, Lössner B, Ott T. (1984) Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull 13:39–42. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. (2007) D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 62:835–838. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. (2003) Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci 23:6754–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Nawar EM, McGaugh JL. (2005) Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions. Learn Mem 12:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Nguyen LT, McGaugh JL. (2004) Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. Eur J Neurosci 20:2804–2810. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. (2010) The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem 17:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. (2012) Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci 35:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS. (1917) The effects of strychnine and caffeine upon the rate of learning. Psychobiology 1:141–169. [Google Scholar]
- Lashley KS. (1950) In search of the engram. Brain Physiology and Psychology 4:454–482. [Google Scholar]
- Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. (2016) Psychotherapy versus pharmacotherapy for posttraumatic stress cisorder: systemic review and meta-analyses to determine first-line treatments. Depress Anxiety 33:792–806. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. (2009) Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC. (1999) Pre- or post-training injection of buspirone impaired retention in the inhibitory avoidance task: involvement of amygdala 5-HT1A receptors. Eur J Neurosci 11:1491–1500. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. (1986) Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res 368:125–133. [DOI] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. (2012) A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res 46:1184–1190. [DOI] [PubMed] [Google Scholar]
- Lynch G, Palmer LC, Gall CM. (2011) The likelihood of cognitive enhancement. Pharmacol Biochem Behav 99:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. (2008) The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol 585:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. (2006) The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci USA 103:14200–14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. (1949) Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med 11:338–353. [DOI] [PubMed] [Google Scholar]
- MacLean PD. (1952) Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalogr Clin Neurophysiol 4:407–418. [DOI] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. (2007) Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem 87:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. (2006) Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci USA 103:1959–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A. (2016) Stress and fear extinction. Neuropsychopharmacology 41:58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, et al. (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534. [DOI] [PubMed] [Google Scholar]
- Marwha D, Halari M, Eliot L. (2017) Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume. Neuroimage 147:282–294. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (1966) Time-dependent processes in memory storage. Science 153:1351–1358. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (1973) Drug facilitation of learning and memory. Annu Rev Pharmacol 13:229–241. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2002) Memory consolidation and the amygdala: a systems perspective. Trends Neurosci 25:456. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Gold PE. (1976) Modulation of memory by electrical stimulation of the brain, in Neural Mechanisms of Learning and Memory (Rosenzweig MR, Bennett EL. eds) pp 549–560, MIT Press, Cambridge, MA. [Google Scholar]
- McGaugh JL, Herz MJ. (1972) Memory Consolidation, Albion Publishing Company, San Francisco, CA. [Google Scholar]
- McGaugh JL, Introini-Collison IB, Nagahara AH. (1988) Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Res 446:37–49. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. (2002) Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem 78:539–552. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Petrinovich L. (1959) The effects of strychnine sulphate on maze learning. Am J Psychol 72:99–102. [Google Scholar]
- McGaugh JL, Roozendaal B. (2009) Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 202:3–14. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. (2002) Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci 16:1223–1226. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. (2005) Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci USA 102:10718–10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Power AE, Roozendaal B, McGaugh JL. (2003) Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci 985:273–293. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. (2010) Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem 93:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Holloway-Erickson CM, Parmar TU, McIntyre CK. (2014) Corticosterone-induced enhancement of memory and synaptic Arc protein in the medial prefrontal cortex. Neurobiol Learn Mem 112:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, McIntyre CK. (2012) Emotional modulation of the synapse. Rev Neurosci 23:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. (2007) Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci 27:10445–10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. (2002) Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36:507–519. [DOI] [PubMed] [Google Scholar]
- Miranda MI, LaLumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. (2003) Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur J Neurosci 18:2605–2610. [DOI] [PubMed] [Google Scholar]
- Mishkin M. (1978) Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 273:297–298. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. (2006) Epinephrine administration increases neural impulses propagated along the vagus nerve: role of peripheral beta-adrenergic receptors. Neurobiol Learn Mem 85:116–124. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsey MS, Boyle LM, Zhang ML, Nguyen CP, Kronman HG, Ota KT, Duman RS, Taylor JR, Schafe GE. (2014) Chronic corticosterone exposure persistently elevates the expression of memory-related genes in the lateral amygdala and enhances the consolidation of a Pavlovian fear memory. PLoS One 9:e91530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Roozendaal B, Trezza V, Ratano P, Peloso A, Hauer D, Atsak P, Trabace L, Cuomo V, McGaugh JL, et al. (2014) Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc Natl Acad Sci USA 111:18333–18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. (2000) Two cis-acting elements in the 3′ untranslated region of alpha-CaMKII regulate its dendritic targeting. Nat Neurosci 3:1079–1084. [DOI] [PubMed] [Google Scholar]
- Müller GE, Pilzecker A. (1900) Experimentelle Beiträge zur Lehre vom Gedächtnis. Z Psychol Ergänzungsband 1:1–300. [Google Scholar]
- Na Y, Park S, Lee C, Kim DK, Park JM, Sockanathan S, Huganir RL, Worley PF. (2016) Real-time imaging reveals properties of glutamate-induced Arc/Arg 3.1 translation in neuronal dendrites. Neuron 91:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. (2010) Memory reconsolidation: an update. Ann N Y Acad Sci 1191:27–41. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. (2000) The labile nature of consolidation theory. Nat Rev Neurosci 1:216–219. [DOI] [PubMed] [Google Scholar]
- Nakao K, Matsuyama K, Matsuki N, Ikegaya Y. (2004) Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci USA 101:14270–14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. (1991) Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci 11:1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, et al. (2012) Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell 149:886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. (2010) Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry 67:365–370. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. (1994) Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA 91:8477–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Teather LA. (1998) Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol Learn Mem 69:163–203. [DOI] [PubMed] [Google Scholar]
- Pardini M, Krueger F, Koenigs M, Raymont V, Hodgkinson C, Zoubak S, Goldman D, Grafman J. (2012) Fatty-acid amide hydrolase polymorphisms and post-traumatic stress disorder after penetrating brain injury. Transl Psychiatry 2:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Leary J, Osborne DM, McNay EC. (2016) Role of glia in stress-induced enhancement and impairment of memory. Front Integr Nuerosci 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña DF, Engineer ND, McIntyre CK. (2013) Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry 73:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. (2001) Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 38:247–289. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. (2000) Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: a review. Ann N Y Acad Sci 911:369–391. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. (2002) Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 51:189–192. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. (2006) Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52:437–444. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. (2008) The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of Pavlovian fear conditioning in the lateral amygdala. J Neurosci 28:12383–12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Paré D. (2009) Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci 12:801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, McIntyre CK, Litmanovich A, McGaugh JL. (2003) Cholinergic modulation of memory in the basolateral amygdala involves activation of both m1 and m2 receptors. Behav Pharmacol 14:207–213. [DOI] [PubMed] [Google Scholar]
- Power AE, Roozendaal B, McGaugh JL. (2000) Glucocorticoid enhancement of memory consolidation in the rat is blocked by muscarinic receptor antagonism in the basolateral amygdala. Eur J Neurosci 12:3481–3487. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JA, Otto MW, Sanders C, Emmelkamp PM. (2009) Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. J Anxiety Disord 23:350–356. [DOI] [PubMed] [Google Scholar]
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL. (2016) Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma 33:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. (1994) Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol 257:7–12. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, de la Teja IS, Casillas M, Serafín N, Prado-Alcalá RA, Roozendaal B. (2009) Corticosterone infused into the dorsal striatum selectively enhances memory consolidation of cued water-maze training. Learn Mem 16:586–589. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. (1997) Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA 94:14048–14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. (2013) Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 64:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. (2008) Protein synthesis inhibitors, gene superinduction and memory: too little or too much protein? Neurobiol Learn Mem 89:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, Eftekhari A, Ruzek JI. (2012) Review of exposure therapy: a gold standard for PTSD treatment. J Rehabil Res Dev 49:679–687. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhang FJ, Xue QS, Zhao X, Yu BW. (2008) Bilateral inhibition of gamma-aminobutyric acid type A receptor function within the basolateral amygdala blocked propofol-induced amnesia and activity-regulated cytoskeletal protein expression inhibition in the hippocampus. Anesthesiology 109:775–781. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. (2004) Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. (1978) Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153:1–26. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. (2003) Emotional tagging of memory formation: in the search for neural mechanisms. Brain Res Brain Res Rev 43:247–256. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJ, Ferry B, Setlow B, McGaugh JL. (2001) Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J Neurosci 21:2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. (1996) Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem 65:1–8. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. (1997a) Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci 9:76–83. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. (1997b) Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol Learn Mem 67:176–179. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. (2009) Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci 29:14299–14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. (1999) Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci USA 96:11642–11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. (2006) Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA 103:6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. (1996) Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav Neurosci 110:1074–1083. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. (2002) Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation. Eur J Neurosci 15:553–560. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Duncan EJ, Rizzo A, et al. (2014) A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry 171:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A. (2008) The substrate for long-lasting memory: if not protein synthesis, then what? Neurobiol Learn Mem 89:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. (1993) Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA 90:8342–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Rose SP. (1994) Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Res 647:106–112. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. (2000) Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 20:RC96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling G, Kilger E, Roozendaal B, de Quervain DJ, Briegel J, Dagge A, Rothenhäusler HB, Krauseneck T, Nollert G, Kapfhammer HP. (2004) Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry 55:627–633. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. (2010) Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. (1997) A novel allosteric potentiator of AMPA receptors: 4--2-(phenylsulfonylamino)ethylthio--2,6-difluoro-phenoxyaceta mide. J Neurosci 17:5760–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. (2000) Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci 12:367–375. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. (2011) New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci 14:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. (2006) Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. (2015) Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 149:150–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Hofmann SG, Rosenfield D, DeBoer LB, Costa PT, Simon NM, O’Cleirigh C, Meuret AE, Marques L, Otto MW, et al. (2013a) D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: prognostic and prescriptive variables. J Consult Clin Psychol 81:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, Tuerk P, Shiekh M, Rosenfield B, Hofmann SG, et al. (2014) Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol Psychiatry 75:840–846. [DOI] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, Simon NM, Pollack MH, Hofmann SG. (2013b) D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res 47:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Miller KD, Abbott LF. (2000) Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci 3:919–926. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. (2006) Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci USA 103:5585–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Farris S, Pirbhoy PS, Darnell J, Driesche SJ. (2015) Localization and local translation of Arc/Arg3.1 mRNA at synapses: some observations and paradoxes. Front Mol Neurosci 7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Levy WB. (1982) Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci 2:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. (1998) Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21:741–751. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S. (2013) Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci 369:20130151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 99:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. (2007) Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol 17:318–324. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. (2013) Fear extinction and relapse: state of the art. Annu Rev Clin Psychol 9:215–248. [DOI] [PubMed] [Google Scholar]
- Vukojevic V, Kolassa IT, Fastenrath M, Gschwind L, Spalek K, Milnik A, Heck A, Vogler C, Wilker S, Demougin P, et al. (2014) Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. J Neurosci 34:10274–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. (2002) Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci 22:2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. (2008) Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis F, Kilger E, Roozendaal B, de Quervain DJ, Lamm P, Schmidt M, Schmölz M, Briegel J, Schelling G. (2006) Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg 131:277–282. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. (1956) Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol 49:381–391. [DOI] [PubMed] [Google Scholar]
- Wezenberg E, Verkes RJ, Ruigt GS, Hulstijn W, Sabbe BG. (2007) Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology 32:1272–1283. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. (2006) Learning induces long-term potentiation in the hippocampus. Science 313:1093–1097. [DOI] [PubMed] [Google Scholar]
- Wichmann R, Fornari RV, Roozendaal B. (2012) Glucocorticoids interact with the noradrenergic arousal system in the nucleus accumbens shell to enhance memory consolidation of both appetitive and aversive taste learning. Neurobiol Learn Mem 98:197–205. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. (1993) Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci 107:955–962. [PubMed] [Google Scholar]
- Witter MP, Amaral DG. (2004) Hippocampal formation, in The Rat Nervous System (Paxinos G. ed) pp 635–704, Academic Press, Amsterdam. [Google Scholar]
- Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, Goetz JM, Fischer AM, Orr SP, Pitman RK. (2015) Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res 225:31–39. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. (2004) The ACTH response to dexamethasone in PTSD. Am J Psychiatry 161:1397–1403. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. (2007) Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56:19–32. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. (2002) The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA 99:2368–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Muller JF, McDonald AJ. (2013) Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience 228:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. (2007) Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci 27:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]